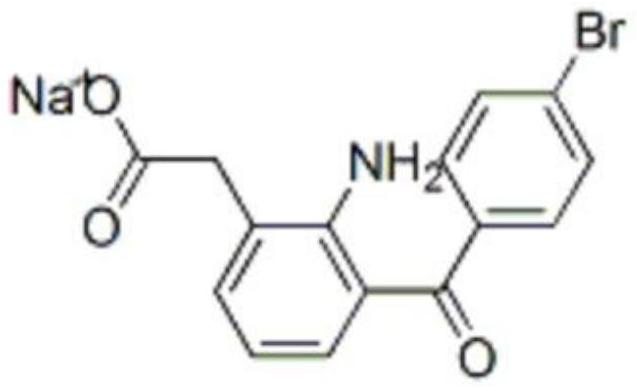
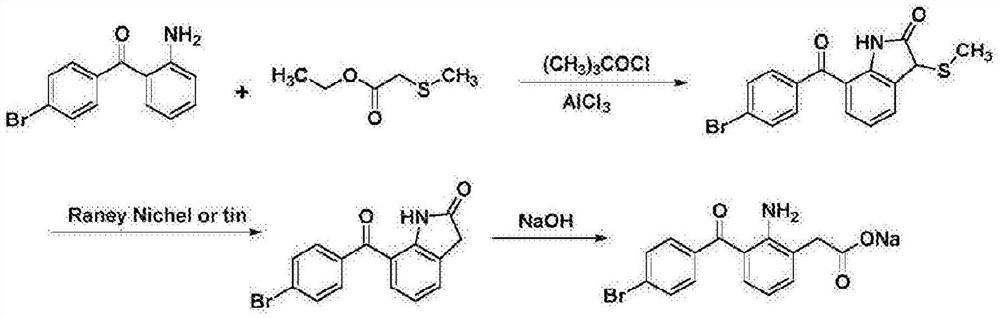
Synthesis method of bromfenac sodium
A technology of bromfenac sodium and its synthesis method, which is applied in the field of drug synthesis, can solve problems such as explosion or personnel poisoning, high toxicity, and excessive heavy metals, and achieve the effects of less environmental pollution, good environmental protection, and mild and controllable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
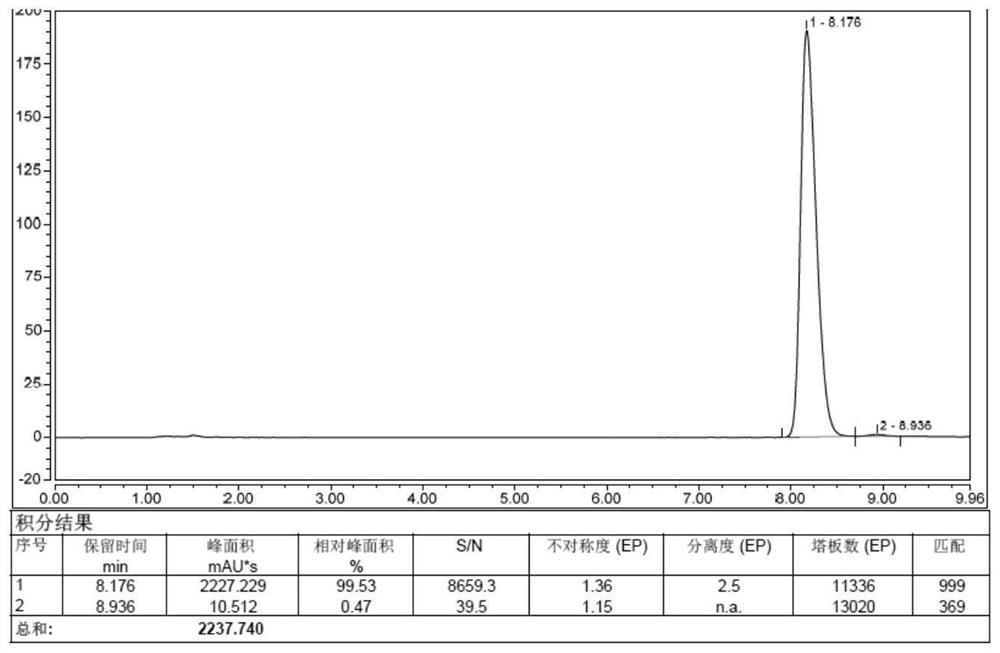
Image
Examples
Embodiment 1
[0053] The synthetic method of bromfenac sodium of the present invention, the detailed steps of this synthetic method are as follows:
[0054] a. Preparation of 2-(9-fluorenemethoxycarbonylamino)benzoic acid:
[0055] First, add solvent isopropyl ether 1600g, triethylamine 111g and anthranilic acid 137g (1mol) into a 5L reaction flask for stirring and dissolving; then cool down to 0°C, take 284.6g of fluorene methoxycarbonyl chloride and dissolve in 1000g of isopropyl ether , the obtained fluorene methoxycarbonyl chloride isopropyl ether solution was slowly added dropwise to the reaction flask, which lasted for 30min; after the dropwise addition was completed, the reaction was continued to stir at 0°C for 2h; after the reaction was completed, the filtrate was filtered, and 80% of the solvent was removed under reduced pressure, and the residue 2 kg of water was added to the mixture, and the temperature was kept at 0°C for 2 hours for crystallization. After crystallization, filt...
Embodiment 2
[0065] The synthetic method of bromfenac sodium of the present invention, the detailed steps of this synthetic method are as follows:
[0066] a. Preparation of 2-(9-fluorenemethoxycarbonylamino)benzoic acid:
[0067] First, 1370 g of isopropyl ether, 101 g of triethylamine and 137 g (1 mol) of anthranilic acid were added to a 5L reaction flask for stirring and dissolving, and the temperature was lowered to 0°C. Fluorene methoxycarbonyl chloride isopropyl ether solution was slowly added dropwise to the reaction flask, which lasted 30 min; after the dropwise addition was completed, the reaction was continued to stir at 0 °C for 2 h; after the reaction was completed, the filtrate was filtered, and 80% of the solvent was removed from the filtrate under reduced pressure. 2kg of water was incubated at 0°C for 2h for crystallization, and filtered after crystallization, and the obtained solid was dried at 60°C for 3h to obtain 294.8g (0.82mol) of 2-(9-fluorenylmethoxycarbonylamino)be...
Embodiment 3
[0077] The synthetic method of bromfenac sodium of the present invention, the detailed steps of this synthetic method are as follows:
[0078] a. Preparation of 2-(9-fluorenemethoxycarbonylamino)benzoic acid:
[0079] First, 2055g of isopropyl ether, 121g of triethylamine and 137g (1mol) of anthranilic acid were added to a 5L reaction flask for stirring and dissolving, and the temperature was lowered to 0°C; The fluorene methoxycarbonyl chloride isopropyl ether solution was slowly added dropwise to the reaction flask, which lasted 30 min; after the dropwise addition, the reaction was continued at 0 °C for 2 h; after the reaction was completed, the filtrate was filtered, and 80% of the solvent was removed from the obtained filtrate under reduced pressure. Add 2kg of water, keep at 0°C for 2h for crystallization, filter after crystallization, and dry the obtained solid at 60°C for 3h to obtain 310g (0.86mol) of 2-(9-fluorenylmethoxycarbonylamino)benzoic acid;
[0080] b, prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com