Preparation method of bromfenac sodium sesquihydrate
A technology of sesquihydrate and bromfenac sodium, which is applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve the risk of inability to obtain bromfenac sodium sesquihydrate and organic solvent residues Large, unfavorable industrial production and other problems, to achieve the effect of simple and easy preparation method, lower production cost, and less discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
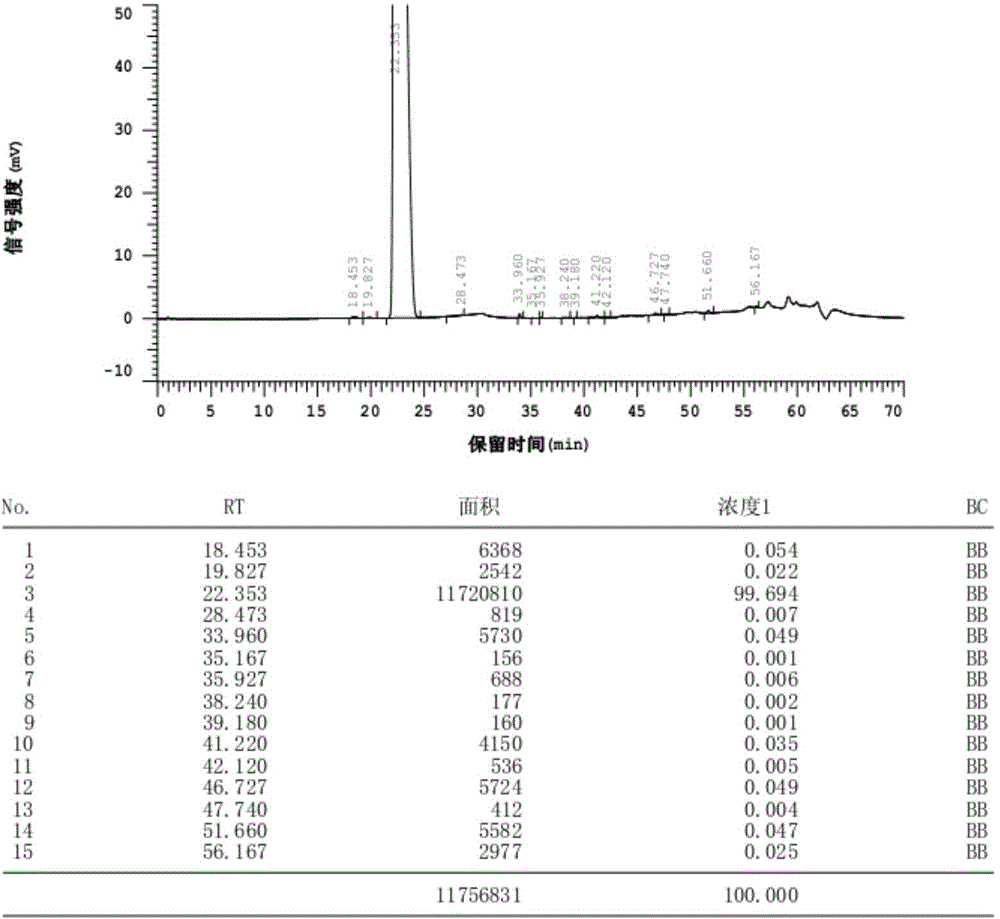
[0044]In a 5L reaction flask, add 500g of bromfenac sodium anhydrate and 0.75g of sodium sulfite into a mixed solvent of 500mL of water and 500mL of ethanol. After heating and dissolving, add acetic acid to adjust the pH value to 9.0, then add 1500mL of ethanol, and cool to 30°C, add 15g of bromfenac sodium sesquihydrate as a seed crystal, stir evenly, cool to 5°C, stand for crystallization for 1h, collect crystals by centrifugation, and vacuum dry at 50°C, -0.095MPa~-0.090MPa After 15 hours, 341.5 g of bromfenac sodium sesquihydrate was obtained, with a mass yield of 68.3%, a purity of 99.69%, a single largest impurity content of 0.054%, and a moisture content of 6.97%.
[0045] The HPLC figure of the bromfenac sodium sesquihydrate related substance prepared by the present embodiment is as follows figure 1 shown.
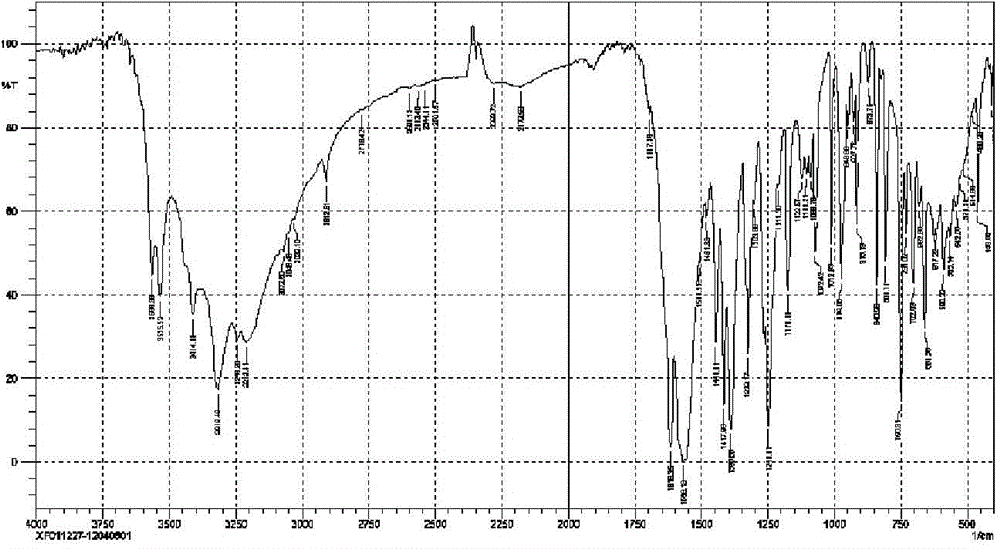
[0046] The infrared spectrogram of the bromfenac sodium sesquihydrate prepared by the present embodiment is as follows figure 2 shown.
[0047] The X-ray powd...
Embodiment 2
[0051] In a 1L reaction flask, add 100g of bromfenac sodium anhydrate and 0.20g of sodium thiosulfate into a mixed solvent of 100mL of water and 100mL of methanol, heat to dissolve, add acetic acid to adjust the pH to 10.0, and then add 250mL of methanol , cooled to 35°C, added 3.0g of bromfenac sodium monohydrate as a seed crystal, stirred evenly, cooled to 15°C, stirred and crystallized for 2 hours, collected crystals by centrifugation, and kept at 47°C, -0.095MPa~-0.090MPa After vacuum drying for 14 hours, 67.2 g of bromfenac sodium sesquihydrate was obtained, with a mass yield of 67.2%, a purity of 99.67%, a single largest impurity content of 0.073%, and a water content of 7.04%.
Embodiment 3
[0053] In a 1L reaction flask, add 100g of bromfenac sodium monohydrate and 0.10g of sodium thiosulfate into a mixed solvent of 100mL of water and 100mL of isopropanol, heat to dissolve, add acetic acid to adjust the pH to 8.0, and then add 300mL isopropanol, cooled to 32°C, added 1.0g bromfenac sodium monohydrate and 1.0g bromfenac sodium sesquihydrate as seed crystals, stirred evenly, cooled to 0°C, stirred and crystallized for 1h, centrifuged The crystals were collected and dried under vacuum at 47°C and -0.095MPa to -0.090MPa for 16h to obtain 65.8g of bromfenac sodium sesquihydrate, with a mass yield of 65.8%, a purity of 99.75%, and a single largest impurity content of 0.048 %, the moisture content is 6.49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com