Method for synthesizing bromfenac sodium impurity standard substance
A synthesis method, the technology of bromfenac sodium, is applied in the field of medicine to achieve the effects of short synthesis period, low synthesis cost and quality control of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
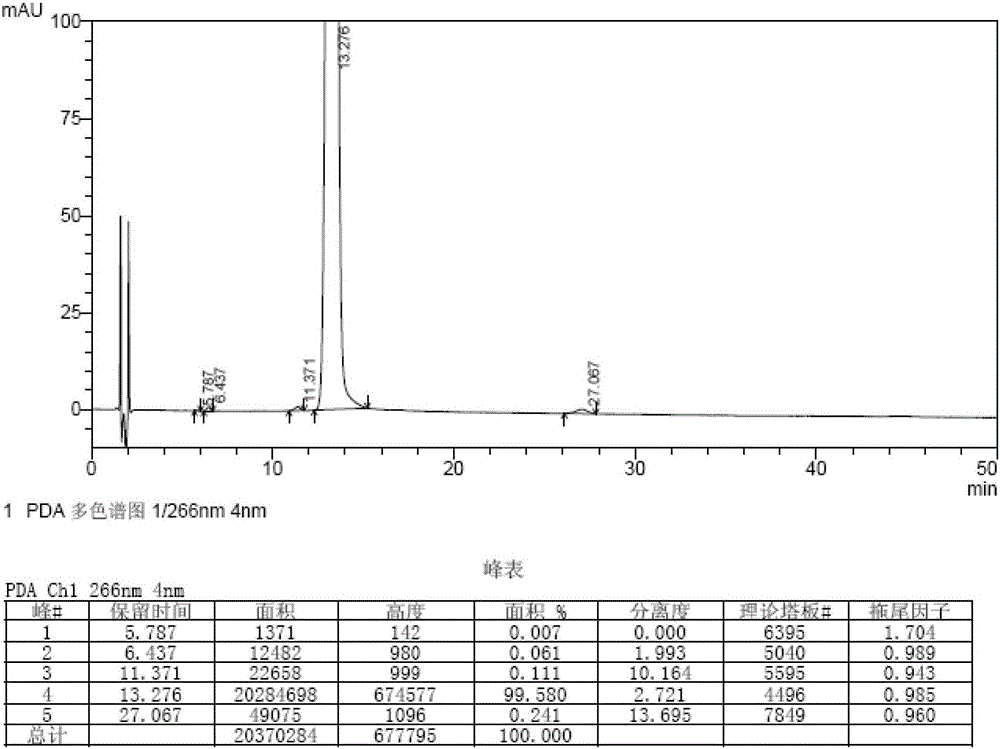
Embodiment 1
[0046] The synthetic method of bromfenac sodium impurity standard substance in the present embodiment is as follows:
[0047] 1. Stir and dissolve 10 g (31.6 mmol) of 7-(4-bromobenzoyl) indolin-2-one in 280 ml of ethyl acetate, add 28.4 g (127.2 mmol) of copper bromide, and ℃, reflux and stir for 3 hours, lower the temperature to 20-30 ℃, wash the reaction solution with water equal to the volume of ethyl acetate and saturated aqueous sodium chloride solution, and concentrate the organic phase to dryness under reduced pressure at 50-60 ℃ to obtain the intermediate.
[0048] 2. Add 300ml of methanol / water mixed solution (volume ratio of methanol to water: 4:1) to the intermediate obtained in step 1, reflux and stir at 70-80°C for 2.5h, cool down to 20-30°C, filter, and filter cake Wash with water and methanol in turn, add 250ml of methanol to the filter cake, reflux at 65-70°C for 30 minutes, filter while hot, cool the filtrate to -5-0°C, stir and crystallize for 1 hour, filter,...
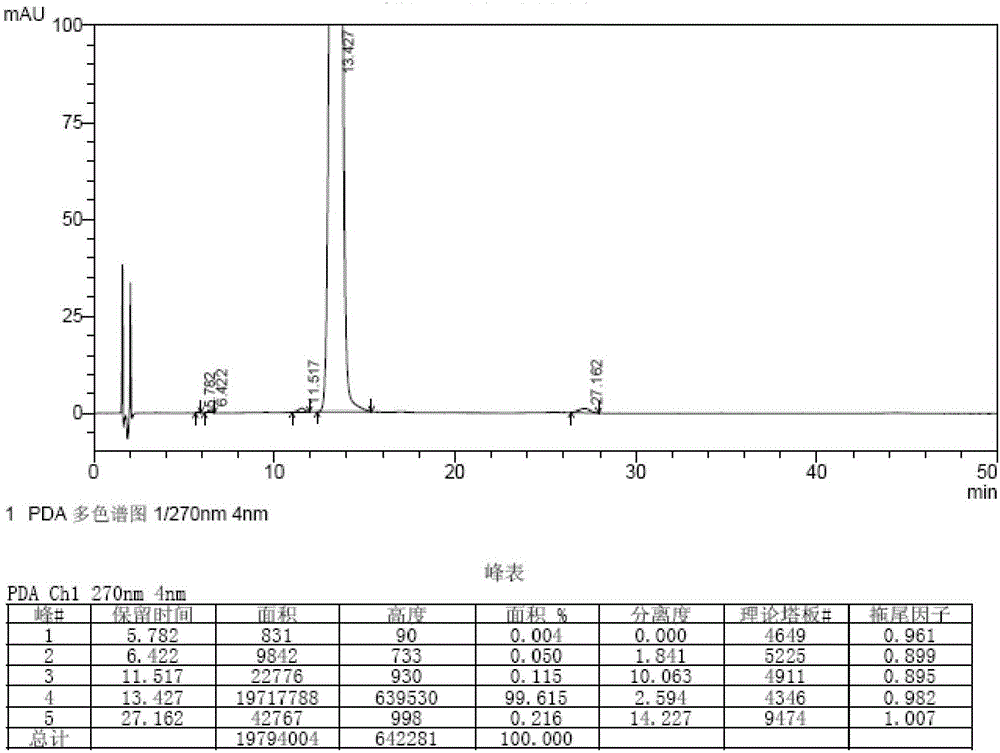
Embodiment 2
[0059] The synthetic method of bromfenac sodium impurity standard substance in the present embodiment is as follows:
[0060] 1. Stir and dissolve 10g (31.6mmol) of 7-(4-bromobenzoyl)indolin-2-one in 300ml of ethyl acetate, add 28.4g (127.2mmol) of copper bromide, and ℃ reflux and stir for 3 hours, lower the temperature to 20-30 ℃, wash the reaction solution with water equal to the volume of ethyl acetate and saturated aqueous sodium chloride solution, and concentrate the organic phase to dryness at 50-60 ℃ under reduced pressure to obtain the intermediate.
[0061] 2. Add 250ml of methanol / water mixed solution (volume ratio of methanol to water: 4:1) to the intermediate obtained in step 1, reflux and stir at 70-80°C for 2 hours, cool down to 20-30°C, filter, filter Wash the cake with appropriate amount of water and methanol in turn, add 200ml of methanol to the filter cake, reflux at 65-70°C for 30min, filter while hot, cool the filtrate to -5-0°C, stir and crystallize for 2h...
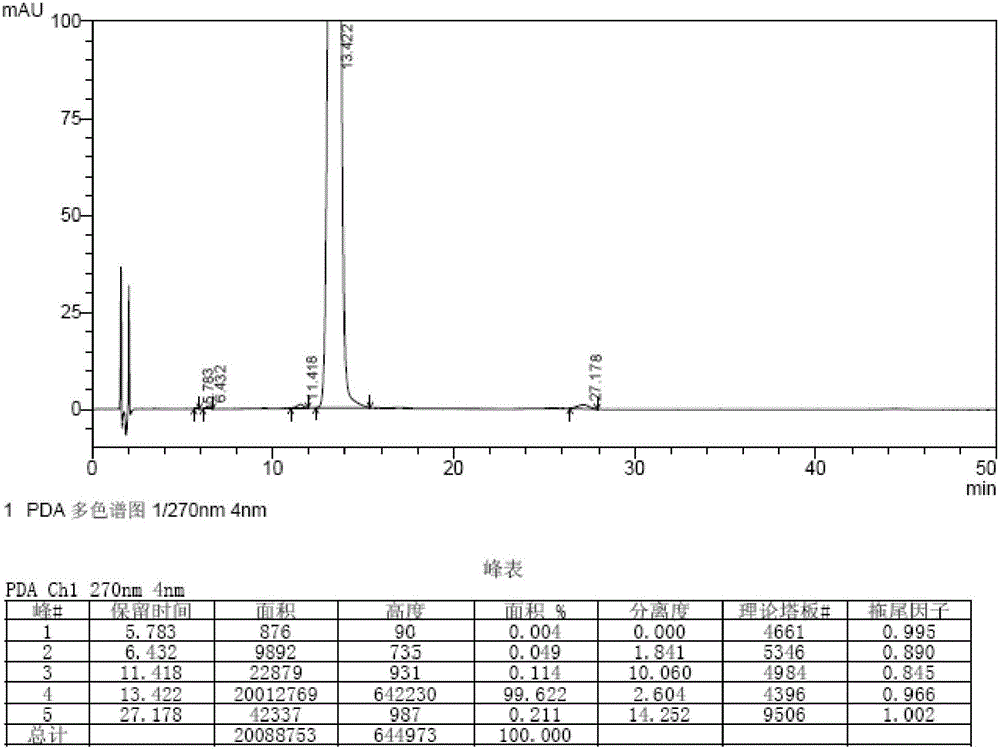
Embodiment 3
[0066] The synthetic method of bromfenac sodium impurity standard substance in the present embodiment is as follows:
[0067] 1. Stir and dissolve 25g (79mmol) of 7-(4-bromobenzoyl)indolin-2-one in 750ml of ethyl acetate, add 88.2g (395mmol) of copper bromide, and reflux at 78-85°C Stir the reaction for 4 hours, lower the temperature to 20-30°C, wash the reaction solution with water equal to the volume of ethyl acetate and saturated aqueous sodium chloride solution, and concentrate the organic phase to dryness at 50-60°C under reduced pressure to obtain the intermediate.
[0068] 2. Add 700ml of methanol / water mixed solution (volume ratio of methanol to water: 4:1) to the intermediate obtained in step 1, reflux and stir at 70-80°C for 3 hours, cool down to 20-30°C, filter, and filter cake in turn Wash with water and methanol, add 500ml of methanol to the solid, reflux at 65-70°C for 30min, filter while hot, cool the filtrate to -5-0°C, stir and crystallize for 2h, filter, dry ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com