Preparation method and important intermediate of bromfenac sodium
A technology of bromfenac sodium and intermediates, which is applied in the field of drug synthesis, can solve the problems of affecting the therapeutic effect of products, less bromfenac sodium, and high toxicity, and achieve the effects of cheap and easy reagents, less environmental pollution, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
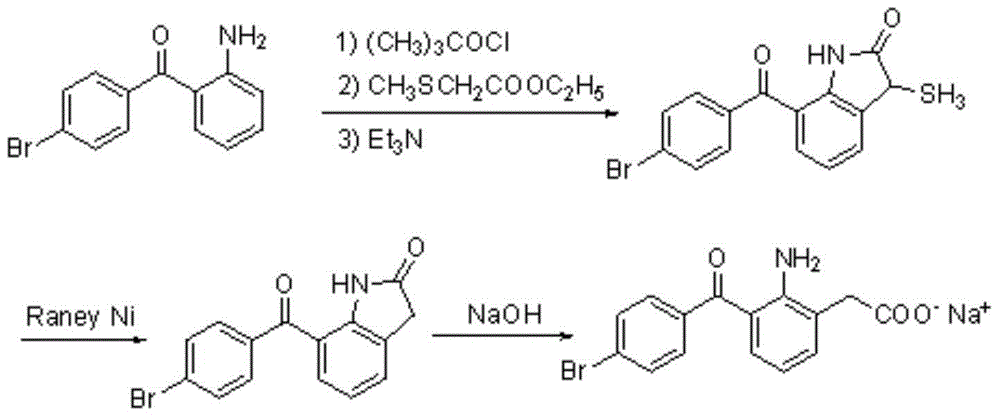
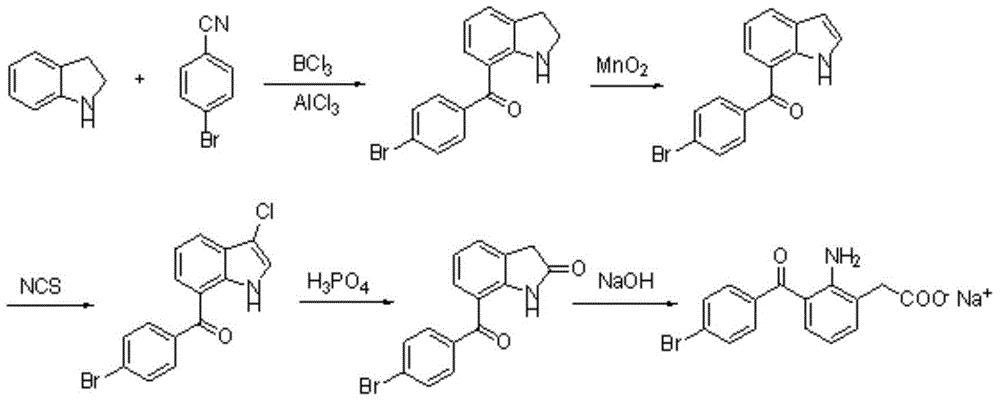
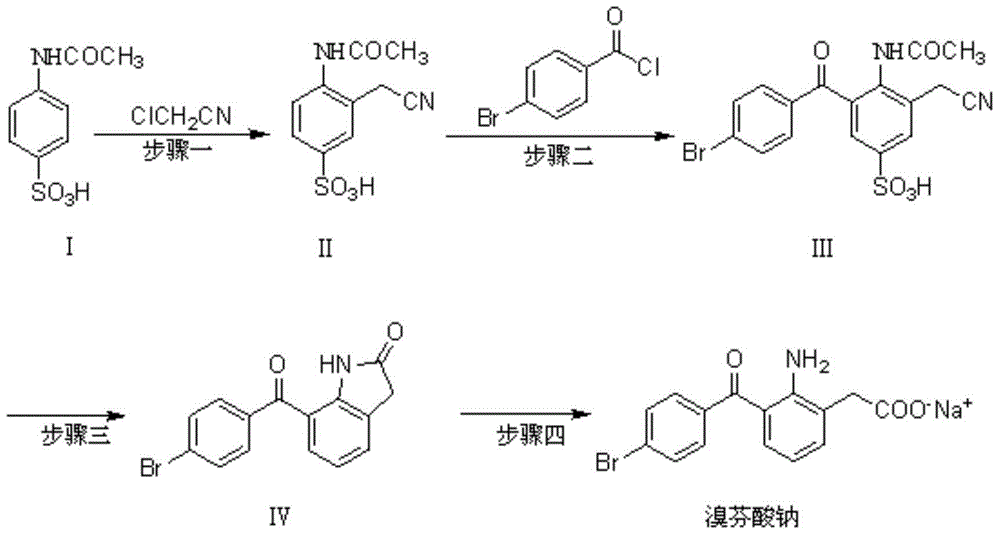
Image
Examples
Embodiment 1
[0028]
Embodiment 1-1
[0030] Under nitrogen protection, add 107g of acetaminobenzenesulfonic acid to a 1L reaction flask, add 537ml of dichloromethane to dissolve, add 41.5g of chloroacetonitrile, add 13.6g of zinc chloride in batches, heat to 40°C, reflux for 2 hours, and detect by TLC After the reaction is complete, cool to room temperature. Slowly add 3ml of water dropwise to the reaction solution, and control the temperature of the ice-salt bath to less than 20°C. The insoluble matter was removed by filtration, and the filtrate was washed with water until neutral, then concentrated to near dryness under reduced pressure. The obtained crude product was recrystallized from ethyl acetate to obtain 94 g of white solid, with a yield of 74%.
[0031] Examples 1-2 to 1-24 Refer to Example 1-1 for the amount of starting material and operation steps, and refer to the table below for other conditions. The experimental results are shown in the table below:
[0032]
[0033]
[0034] Compound II: m...
Embodiment 2
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com