A kind of preparation method of bromfenac sodium intermediate
An intermediate, phosphoric acid technology, applied in organic chemistry methods, organic chemistry and other directions, can solve problems such as affecting yield and post-processing, and achieve the effects of improving purity, simplifying production procedures, and not easy to decompose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0027] Preparation Example 1 Preparation of 3-bromo-7-p-bromobenzoyl indole (intermediate III):
[0028] Add 90g of 7-(4-bromobenzoyl)indole into 4500mL of dichloromethane, lower the temperature to -10℃~-5℃, dissolve 55.8g of NBS in 2000mL of dichloromethane solution, and then Add it dropwise to the above solution at 10~-5°C. After the dropwise addition, keep at -5~0°C to continue the reaction for 2 hours. After the reaction is completed, add cold water to quench the reaction, wash the organic layer with purified water, collect the organic layer, and add Dry with sulfuric acid in water for 2-3 hours, filter, remove the desiccant, concentrate dichloromethane to obtain a solid, add 200 mL of cold ethyl acetate to make a slurry, then filter with suction, dry at 40-50°C for 2 hours to obtain intermediate III, the yield is 91.29% .
[0029] 1 H-NMR (400MHz, CDCl 3 )δ10.377(s,1H), 7.890~7.870(m,1H), 7.680~7.603(m,5H), 7.403~7.397(m,1H), 7.263~7.224(m,1H); MS(m / z):[M-H] - 377.9...
Embodiment 1
[0030] Example 1 Preparation of 7-p-bromobenzoyl-1,3-dihydro-2H-indol-2-one (intermediate II)
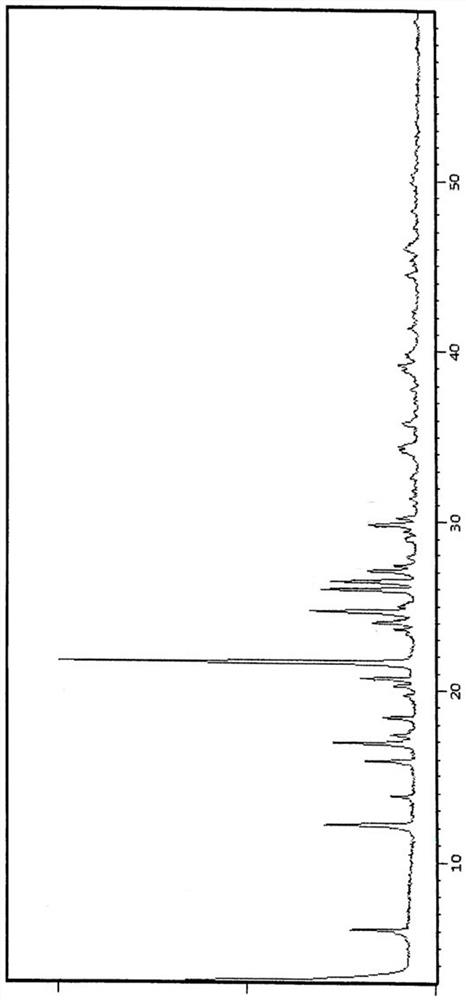
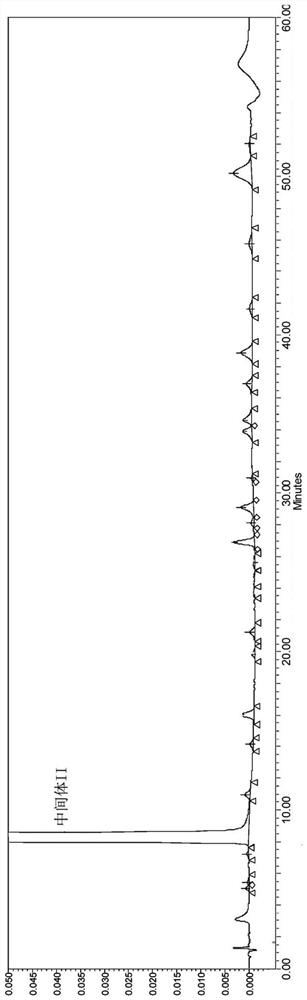
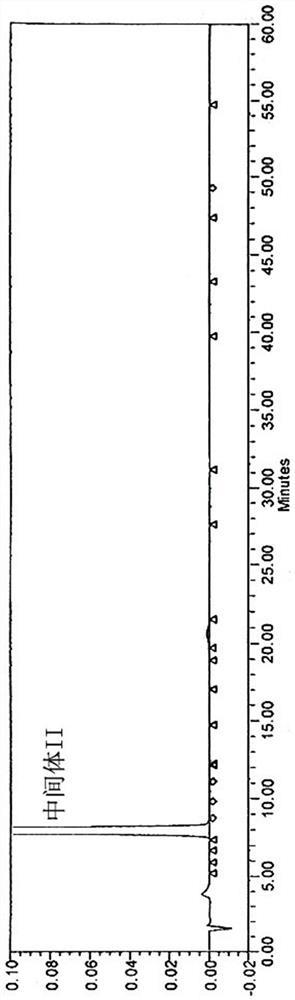
[0031] Add 100g of Intermediate III to 450mL of glacial acetic acid, heat to 90-95°C, then add 1800mL of phosphoric acid, react at 120-125°C for 6 hours, cool down to 80-85°C, add 1700mL of purified water to the reaction solution to cool down and analyze crystallized, filtered, washed, and dried to obtain the crude product of intermediate II, and its HPLC was as follows: figure 2 As shown; the obtained Intermediate II crude product was added to 250 mL of ethyl acetate and stirred for 0.5-2 hours, filtered and dried to obtain 75.2 g of Intermediate II product with a total yield of 90.2%. After testing, the X-RPD collection of illustrative plates of gained intermediate II product is as follows figure 1 As shown, its HPLC spectrum is as image 3 As shown, the HPLC purity was 99.2%, and impurity X was not detected.
[0032] 1 H-NMR (400MHz, CDCl 3 )δ9.520(s,1H), 7.660~7.586(m,4H),...
Embodiment 2
[0034] Example 2 Preparation of 7-p-bromobenzoyl-1,3-dihydro-2H-indol-2-one (intermediate II)
[0035] Add 100g of intermediate III to a mixed solvent composed of 450mL of glacial acetic acid and 1800mL of phosphoric acid, react at 120-125°C for 5h, cool down to 80-85°C, add 1600mL of purified water to the reaction solution to cool down and crystallize, filter and wash , dried, added 250mL ethyl acetate and stirred for 0.5-2h, filtered and dried to obtain 70.43g of intermediate II with a yield of 84.51%; the X-RPD pattern of the obtained intermediate II is as follows figure 1 As shown, its HPLC spectrum is as image 3 As shown, the HPLC purity was 99.3%, and impurity X was not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com