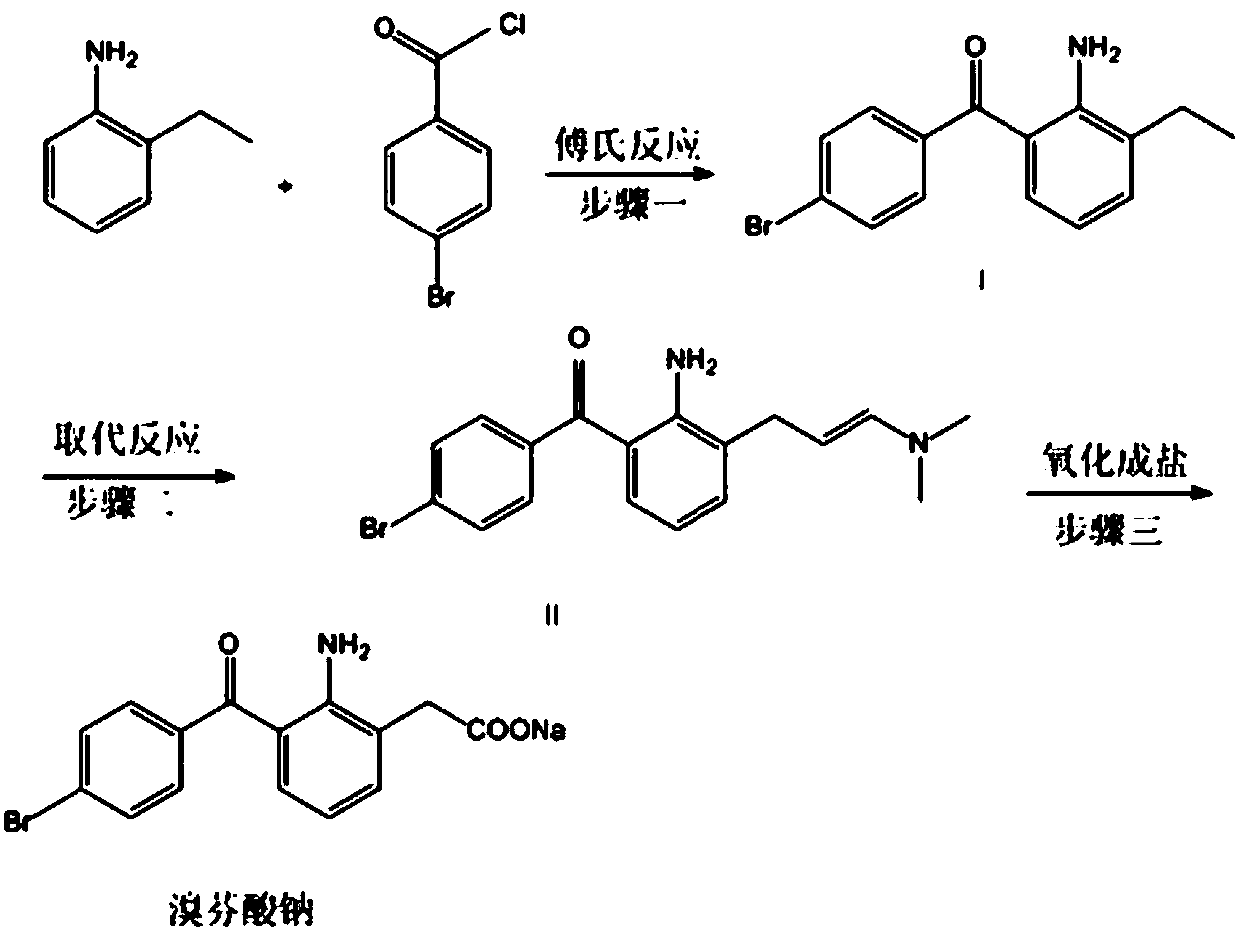
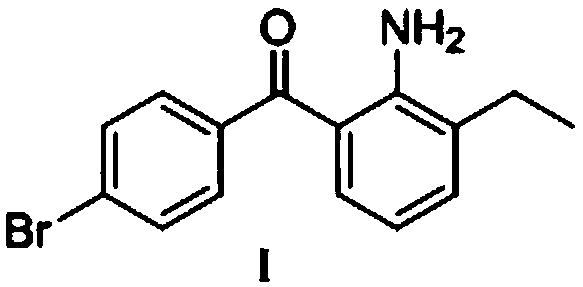
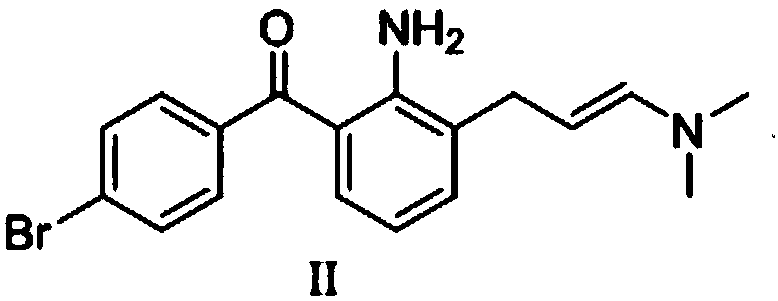
Preparation method of bromfenac sodium
A technology of bromfenac sodium and sodium salt is applied in the field of synthesis technology of raw material bromfenac sodium, can solve the problems of unsuitability for industrial production, harsh reaction conditions, weak selectivity and the like, and avoids catalytic hydrogenation and post-treatment. The effect of simplicity and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
Embodiment 1-1
[0032] Under nitrogen protection, add 36g of o-ethylaniline (0.3mol) and 79g of p-bromobenzoyl chloride (0.36mol) into a 1L reaction flask, add 400mL of toluene to dissolve, add 16g of aluminum trichloride (0.12mol) in batches within 30min, The ice-salt bath controls the temperature at 10-20°C. After the addition, it was slowly brought to room temperature, and the reaction was stirred for 4h. After the reaction was detected by TLC, 20 mL of 20% sodium sulfate aqueous solution was slowly added dropwise to the reaction solution under stirring, and the temperature of the ice-salt bath was controlled at 10-20°C. The insoluble matter was removed by filtration, and the filtrate was washed with water until neutral, then concentrated to near dryness under reduced pressure. The resulting crude product was recrystallized from dichloromethane / methanol to obtain 85 g (0.28 mol) of a yellow solid, with a mass yield of 95% and an HPLC purity of 96.5%.
Embodiment 1-2
[0034] Under nitrogen protection, add 36g of o-ethylaniline (0.3mol) and 65g of p-bromobenzoyl chloride (0.3mol) into a 1L reaction flask, add 400mL of chloroform to dissolve, add 20g of zinc chloride (0.15mol) in batches within 30min , the ice-salt bath controls the temperature at 0-10°C. After the addition, it was slowly brought to room temperature, and the reaction was stirred for 4h. After the completion of the reaction as detected by TLC, 20 mL of 20% sodium sulfate aqueous solution was slowly added dropwise to the reaction solution under stirring, and the temperature of the ice-salt bath was controlled at 0-10°C. The insoluble matter was removed by filtration, and the filtrate was washed with water until neutral, then concentrated to near dryness under reduced pressure. The resulting crude product was recrystallized from dichloromethane / methanol to obtain 83.73 g (0.28 mol) of a yellow solid, with a mass yield of 93% and an HPLC purity of 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com