A kind of preparation method of bromfenac sodium
A technology of bromfenac sodium and hydrochloric acid, which is applied in the field of medicine and chemical industry, can solve the problems of non-recyclable solvents and high cost of environmental protection treatment, and achieve the effects of increased product yield, low disposal cost and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
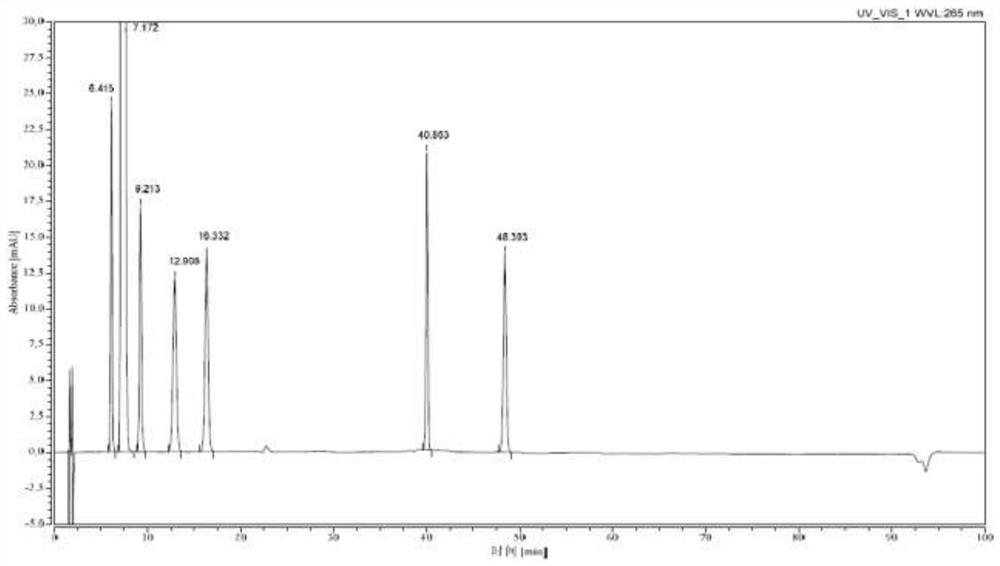
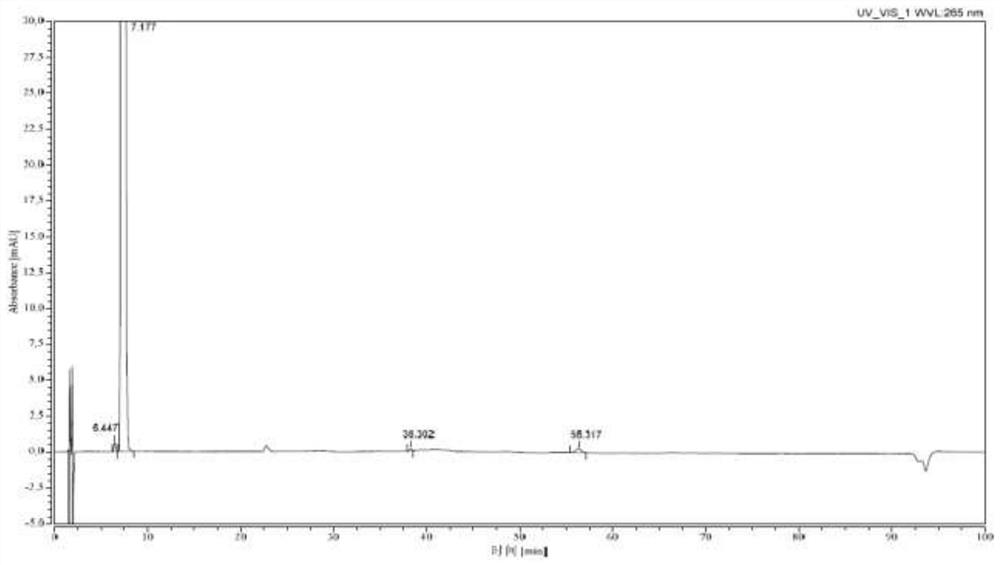
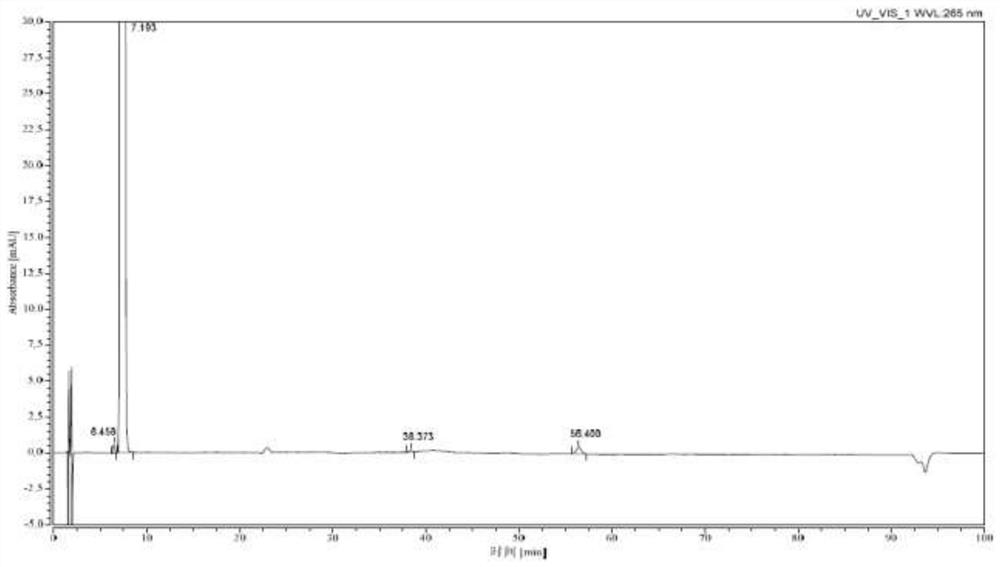
[0033] 30L of 60% methanol, 2.5kg (62.5mol) of sodium hydroxide, and 10kg of 7-(4-bromobenzoyl)-1,3-dihydro-2H-indol-2-one were successively put into the pressurized reactor (31.6mol), reacted at 90°C for 5 hours, cooled to 20-30°C, added dropwise hydrochloric acid to adjust the pH to 11, added 500g of activated carbon, stirred and decolorized under normal pressure for 30min, filtered while hot, and cooled the filtrate to 15-20°C , add seed crystals and stand for crystallization for 2h, then stand for crystallization at 0-10°C for 2h, -10-0°C for 3h, filter, and dry the solid in vacuum at 40-50°C for 6h to obtain orange-yellow Bromophenolate sodium 10kg, yield 88.9%, purity 100% (HPLC method).
Embodiment 2
[0035] Into the pressurized reactor, 36L of 70% methanol, 1.9kg (47.5mol) of sodium hydroxide, and 10kg of 7-(4-bromobenzoyl)-1,3-dihydro-2H-indol-2-one were successively put into the pressurized reactor (31.6mol), reacted at 110°C for 3h under closed stirring, cooled to 20-30°C, added dropwise hydrochloric acid to adjust the pH to 11, added 500g of diatomaceous earth, stirred and decolorized under normal pressure for 5min, filtered while it was hot, and cooled the filtrate to 15- 20°C, add seed crystals and stand for crystallization for 3 hours, then stand for crystallization at 0-10°C for 3 hours, -10-0°C for 4 hours, filter, and dry the solid in vacuum at 40-50°C for 8 hours to obtain Orange-yellow sodium bromophenolate 10.2kg, yield 90.6%, purity 100% (HPLC method).
Embodiment 3
[0037] Put 40L of 80% methanol, 1.9kg (47.5mol) of sodium hydroxide, and 10kg of 7-(4-bromobenzoyl)-1,3-dihydro-2H-indol-2-one into the pressurized reactor in sequence (31.6mol), react at 100°C for 4h under closed stirring, cool down to 20-30°C, add hydrochloric acid dropwise to adjust the pH to 11, add 500g of neutral alumina, stir and decolorize under normal pressure for 10min under reflux, filter while hot, and cool the filtrate to 15 ~20°C, add seed crystals and stand for crystallization for 2h, then stand for crystallization at 0~10°C for 3h, -10~0°C for 4h, filter, and dry the solid in vacuum at 40~50°C for 6h, that is 9.9 kg of orange-yellow sodium bromophenolate was obtained, with a yield of 88.0% and a purity of 100% (HPLC method).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com