Separation and detection method of isomer impurities in 3-halogenated-7-(4-bromobenzoyl)-1H-indole and application
A bromobenzoyl and detection method technology, which is applied in the field of separation and detection of isomer impurities in 3-halo-7--1hydro-indole, to improve safety and effectiveness, strong specificity, and separation good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 specificity test
[0059] LD-Z1 reference substance solution: Accurately weigh an appropriate amount of LD-Z1 reference substance, dissolve and dilute with the sample solution to make a solution containing 0.4mg per 1mL, and use it as the LD-Z1 reference substance stock solution. Precisely measure 0.5mL of LD-Z1 reference substance stock solution, put it in a 100mL measuring bottle, dilute to the mark with sample solution, shake well, and use it as LD-Z1 reference substance solution.
[0060] Chlorinated reference substance solution: Accurately weigh an appropriate amount of chlorinated reference substance, dissolve and dilute the sample solution to make a solution containing 0.4 mg per 1 mL, and use it as a chlorinated reference substance stock solution. Precisely measure 1mL of the chlorinated substance reference substance stock solution, put it in a 100mL measuring bottle, dilute to the mark with the sample solution, shake well, and use it as the chlorin...
Embodiment 1-1
[0064] Chromatographic conditions:
[0065] Column: IA (4.6mm×250mm, 5μm);
[0066] Mobile phase: n-hexane-isopropanol is used as the mobile phase, and the volume ratio of the two is 95:5;
[0067] Detection wavelength: UV detector, the detection wavelength is 220nm;
[0068] Flow rate: 1.0mL / min;
[0069] Column temperature: 30°C;
[0070] Injection volume: 20μL;
[0071] Isocratic elution.
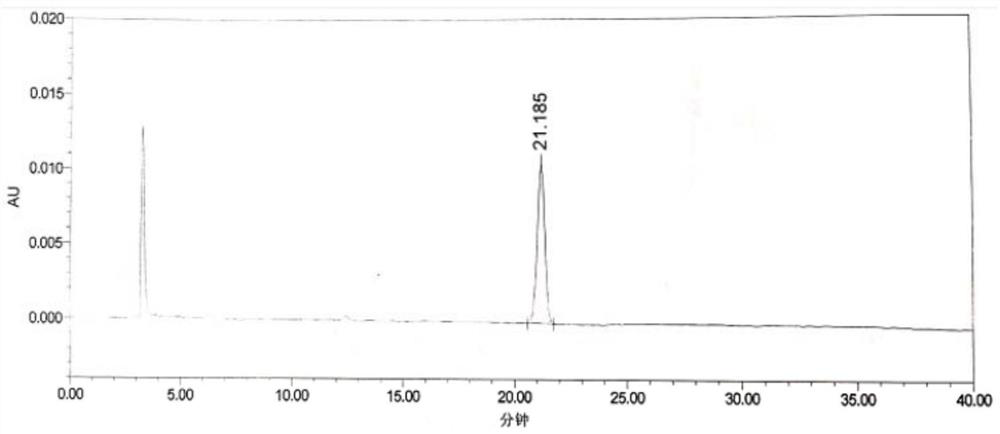
[0072] The HPLC spectrogram of LD-Z1 reference substance solution is as follows figure 1 shown.
[0073] Depend on figure 1 It can be seen that the retention time of LD-Z1 is 21.185min.
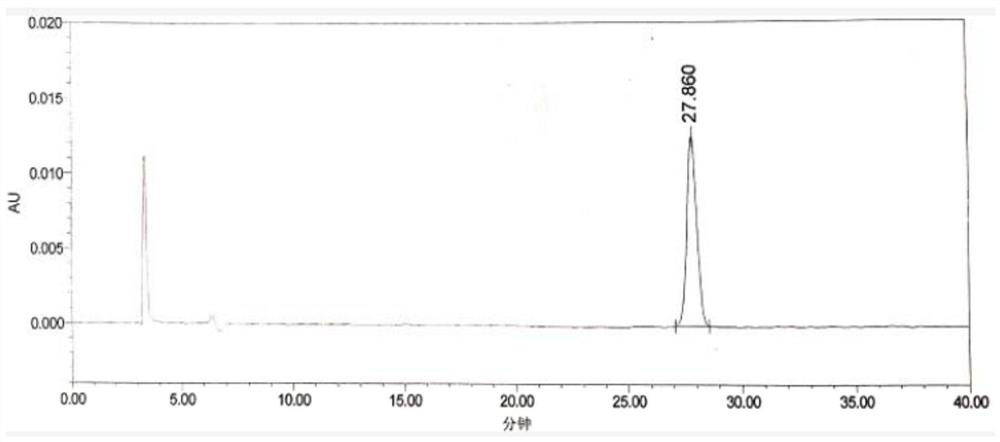
[0074] The HPLC spectrogram of chloride reference substance solution is as follows figure 2 shown.
[0075] Depend on figure 2 It can be seen that the retention time of chloride is 27.860min.
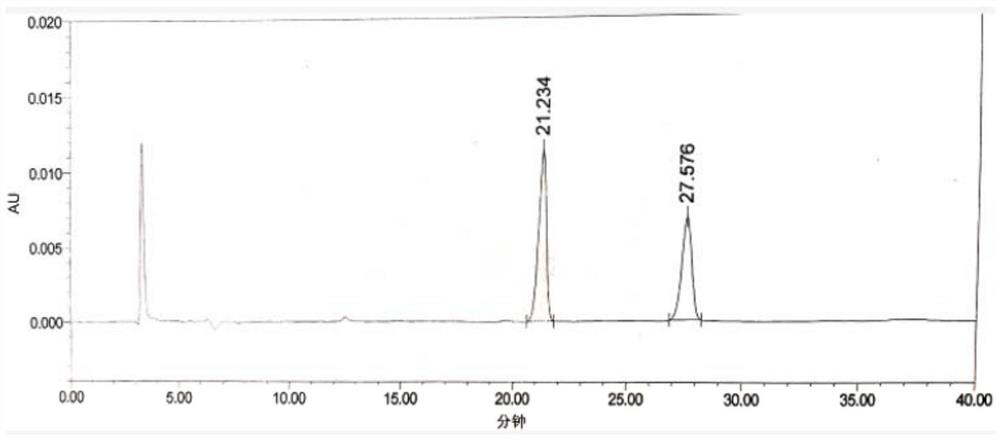
[0076] The HPLC spectrogram of LD-Z1+chloride mixed solution is as follows image 3 As shown, the detection data are shown in Table 1.
[0077] Table 1 Test results of LD-Z1+ chloride mixed solu...
Embodiment 1-2
[0081] Chromatographic conditions:
[0082] Column: IA (4.6mm×250mm, 5μm);
[0083] Mobile phase: n-hexane-isopropanol is used as the mobile phase, and the volume ratio of the two is 98:2;
[0084] Detection wavelength: UV detector, the detection wavelength is 215nm;
[0085] Flow rate: 1.1mL / min;
[0086] Column temperature: 35°C;
[0087] Injection volume: 20μL;
[0088] Isocratic elution.
[0089] The detection data of LD-Z1+ chloride mixed solution are shown in Table 2.
[0090] Table 2 Detection results of LD-Z1+ chloride mixed solution
[0091]
[0092]
[0093] It can be seen from Table 2 that the resolution between LD-Z1 and chlorinated compounds is greater than 2, which meets the requirements, and the detection method under the chromatographic conditions of the present invention can completely separate LD-Z1 and chlorinated compounds without mutual interference.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com