Preparation method of bromfenac sodium dimer impurity
A technology of bromfenac sodium and dimer, applied in the field of medicine, can solve the problems such as no literature report on the preparation method of bromfenac sodium dimer impurity, and achieve the effects of efficient purification and purification, improved purity and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 bromfenac
[0039] In a 50L reactor, add 1.80kg of 7-(4-bromobenzoyl)-1,3-dihydro-indol-2-one, add 20L of 20% by mass aqueous sodium hydroxide solution, and heat to reflux React for 2-h, cool down, add dichloromethane for extraction, adjust the pH of the aqueous layer to 7.0 with acetic acid, filter, and dry at 50°C to obtain 1.46 kg of bromfenac, the purity of bromfenac is 82.99%, and bromfenac sodium dimer The content of impurities was 13.3%.
Embodiment 2
[0040] The preparation of embodiment 2 bromfenac sodium dimer impurity crude product
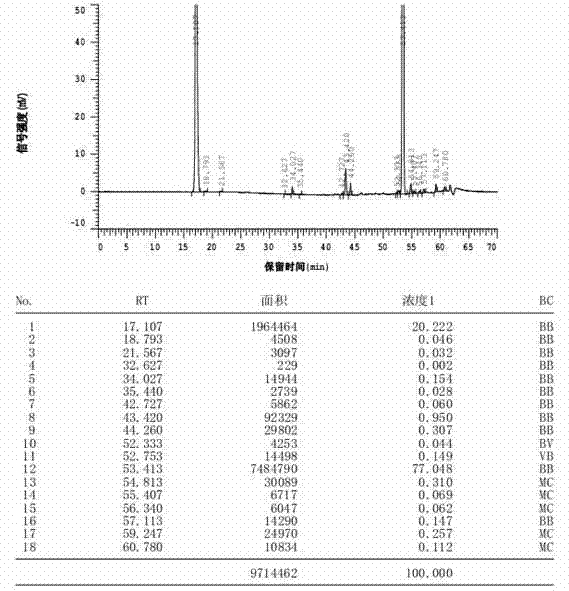
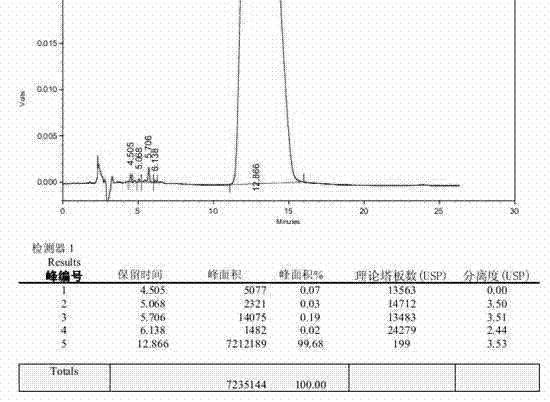
[0041] Take 100 g of bromfenac prepared in Example 1, add it to 400 mL of 50% by mass aqueous sodium hydroxide solution, heat up to 75° C. for 2 h, then continue to heat up to reflux for 3 h, then cool down to room temperature, and add acetic acid to adjust the pH to 6.5, crystallize, filter, wash the filter cake with 100mL of water, add to 200mL of ethyl acetate to make slurry for 1h, filter, collect the filtrate, concentrate under reduced pressure at 50°C, add acetonitrile to stir and crystallize, filter, and dry at 50°C to obtain 40.2g Bromfenac sodium dimer impurity crude product, the purity of bromfenac sodium dimer impurity is 77.05%, the HPLC figure of related substance is as follows figure 1 shown.
Embodiment 3
[0042] The preparation of embodiment 3 bromfenac sodium dimer impurity crude product
[0043] Take 100 g of bromfenac prepared in Example 1, add it to 350 mL of 45% by mass aqueous sodium hydroxide solution, heat up to 70°C for 3 hours, then continue to heat up to reflux for 4 hours, cool down to room temperature, and add acetic acid to adjust the pH to 5.5 , crystallization, filtration, the filter cake was washed with 100mL of water, added to 150mL of ethyl acetate for beating for 1h, filtered, the filtrate was collected, concentrated under reduced pressure at 50°C, added acetonitrile and stirred for crystallization, filtered, and dried at 50°C to obtain 39.6g of bromine Bromfenac sodium dimer impurity crude product, the purity of bromfenac sodium dimer impurity is 75.81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com