Pharmaceutical composition of bromfenac sodium
A technology of bromfenac sodium and a composition, applied in the field of pharmaceutical compositions of bromfenac sodium, can solve the problems of reducing the bacteriostatic effect of benzalkonium chloride, increasing, etc., to increase residence time, improve drug efficacy, and ensure The effect of bacteriostatic effect
Active Publication Date: 2020-10-09
TIANJIN PHARMA GROUP CORP
View PDF16 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0009] Through the published information and our development process of bromfenac sodium eye drops, it is found that the combination of polysorbate 80 in the original prescription and the preservative benzalkonium chloride will reduce the antibacterial effect of benzalkonium chloride, which has a certain safety risk; bromfenac sodium and thickener povidone are prone to produce a polymer impurity U-II impurity, which increases with the prolongation of imitation time and the increase of temperature. The content of this impurity in the product is about 3%
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
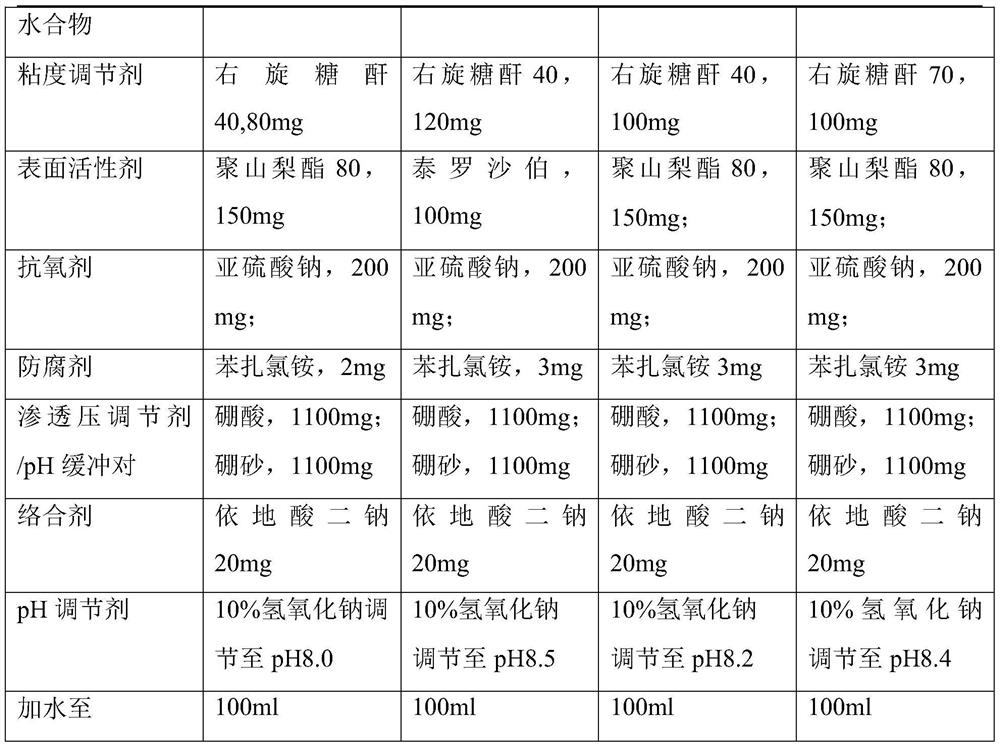
[0071] A pharmaceutical composition of bromfenac sodium, 40ml, comprising
[0072] Bromfenac Sodium Sesquihydrate, 41.4mg
[0073] Dextran 40, 40mg;
[0074] Polysorbate 80, 60mg;
[0076] Benzalkonium chloride 1mg;
[0077] Boric acid, 440mg;
[0078] Borax, 440mg;
[0079] Edetate disodium, 8mg;
[0080] Add water to 40ml
[0081] 10% sodium hydroxide to adjust the pH to 8.3.
Embodiment 2- Embodiment 5
[0083]
[0084]
Embodiment 6~ Embodiment 8
[0086]
[0087]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a pharmaceutical composition of bromfenac sodium, which comprises the following components in percentage by weight: 0.01-0.5% of bromfenac sodium sesquihydrate and pharmacologically acceptable salt thereof; 0.01%-1% by weight of a viscosity modifier dextran; 0.01%-1% by weight of a surfactant; and 0.01%-0.5% by weight of an antioxidant; and optional other auxiliary materialsacceptable in pharmacology. The bromfenac sodium eye drops have the beneficial effects that when the bromfenac sodium eye drops contain dextran 40 or dextran 70, impurities U-II can be effectively prevented from being generated, and the condition that the addition of dextran can also ensure the antibacterial effect of benzalkonium chloride, prolong the intraocular residence time and improve the drug effect can be accidentally found.
Description
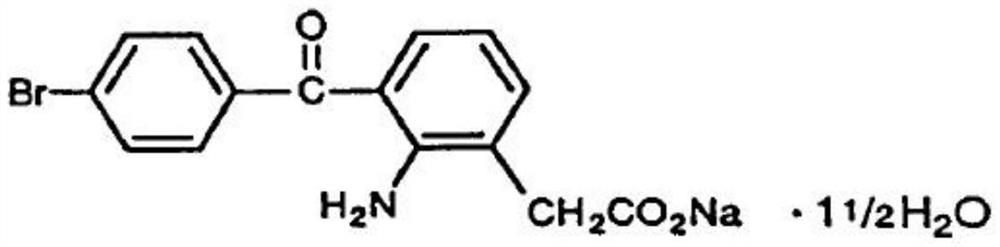
technical field [0001] The invention belongs to the field of medicine, in particular to a pharmaceutical composition of bromfenac sodium. Background technique [0002] The chemical name of bromfenac sodium: 2-amino-3-(4-bromobenzoyl)phenylacetic acid (sodium), the structural formula is as follows: [0003] [0004] Bromfenac sodium is a non-steroidal anti-inflammatory drug with anti-inflammatory activity. Its mechanism of action is to prevent the synthesis of prostaglandins by inhibiting cyclooxygenase 1 and 2. Prostaglandins are mediators of some intraocular inflammation. Prostaglandins can destroy the blood-aqueous humor barrier, dilate blood vessels, increase vascular permeability, increase leukocytes, and increase intraocular pressure. [0005] Bromfenac sodium ophthalmic solution is developed by Japanese Senju company, listed in Japan in 2000, trade name ブロナック / eye solution (BRONUCK OPHTHALMIC SOLUTION), is the 0.1% bromfenac sodium ophthalmic solution (with bromfen...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/08A61K31/196A61K47/26A61P27/02A61P29/00
CPCA61K9/08A61K31/196A61K47/26A61K9/0048A61P27/02A61P29/00Y02A50/30
Inventor 张成飞韩昆颖李秀娟
Owner TIANJIN PHARMA GROUP CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com