Novel synthesis method of bromfenac sodium
Bromfenac sodium, a newly synthesized technology, applied in chemical instruments and methods, sulfonic acid preparation, carboxylic acid amide preparation and other directions, can solve the problems of excessive heavy metal, explosion or personnel poisoning, high toxicity, etc., and achieves low cost, avoidance of The effect of low moisture and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
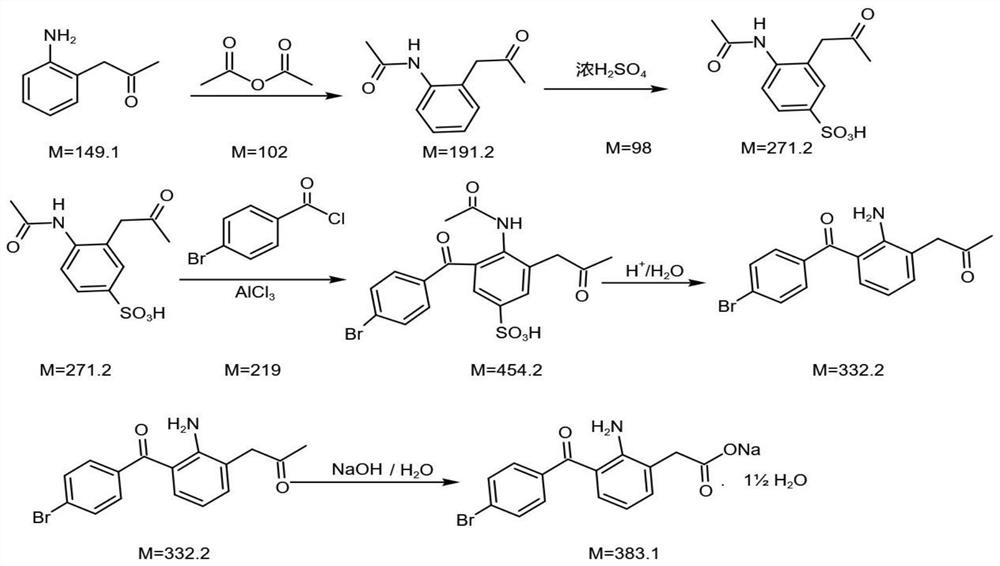
[0046] The new synthetic method of bromfenac sodium is characterized in that the preparation method comprises:
[0047] (1) Preparation of Intermediate I:
[0048] Using o-aminophenylacetic acid as the starting material, the intermediate I, i.e. o-acetamidophenylacetic acid, is obtained through acylation reaction;
[0049]
[0050] specifically:
[0051] Add anthranilic acid into solvent I (dichloromethane or toluene), add acetic anhydride to react at room temperature, and slowly add reaction auxiliary agent (triethylamine) to generate a mixed solution containing intermediate I; the mixed solution of intermediate I is depressurized After concentration, add ethanol and stir evenly, then add purified water to crystallize, filter with suction, and dry with hot air to obtain intermediate I, o-acetaminophenylacetic acid;
[0052] (2) Preparation of Intermediate II;
[0053] Intermediate Ⅰ is subjected to sulfonation reaction to obtain intermediate Ⅱ, namely 2-acetylamino-5 su...
Embodiment 1
[0074] (1) Preparation of Intermediate I:
[0075] ① Add 30g (0.20mol) of anthranilic acid and 300ml of dichloromethane into the reaction flask, start stirring, and slowly add 40.8g (0.4mol) of acetic anhydride at a temperature of 20-30°C, and control the dropping time at 1.5 -2h added;
[0076] ② After adding acetic anhydride dropwise for 30 minutes, add 40.8 g (0.40 mol) of triethylamine dropwise from another feeding port of the reaction bottle;
[0077] ③ After the dropwise addition is completed, keep the temperature for 1 hour;
[0078] 4. After the reaction is completed, distill under reduced pressure until the inside is viscous, stop the decompression, add 90g of ethanol, and stir evenly;
[0079] ⑤ Control the temperature of the reaction solution within 20-30°C, add 720g of purified water, and control the addition time within 1-1.5 hours;
[0080] ⑥ Keep stirring and crystallize for 1 hour, filter with suction, wash the filter cake once with 100ml purified water, and...
Embodiment 2
[0113] 1) Preparation of Intermediate I:
[0114] ①Add 15g (0.1mol) of anthranilic acid and 150ml of dichloromethane into the reaction flask, start stirring, and slowly add 20.4g (0.2mol) of acetic anhydride at a temperature of 20-30°C, and control the dropping time at 1.5 -2h added;
[0115] ②After acetic anhydride was added dropwise for 30 minutes, 20.4 g (0.20 mol) of triethylamine was added dropwise from another feeding port of the reaction flask.
[0116] ③ After the dropwise addition is completed, keep the reaction for 1 hour.
[0117] ④ After the reaction is completed, distill under reduced pressure until the inside is viscous, stop the decompression, add 90g of ethanol, and stir evenly.
[0118] ⑤ Control the temperature of the reaction solution within 20-30°C, add 360g of purified water, and control the addition time within 1-1.5 hours.
[0119] ⑥Insulate, stir and crystallize for 1 hour, filter with suction, wash the filter cake once with 50ml purified water, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com