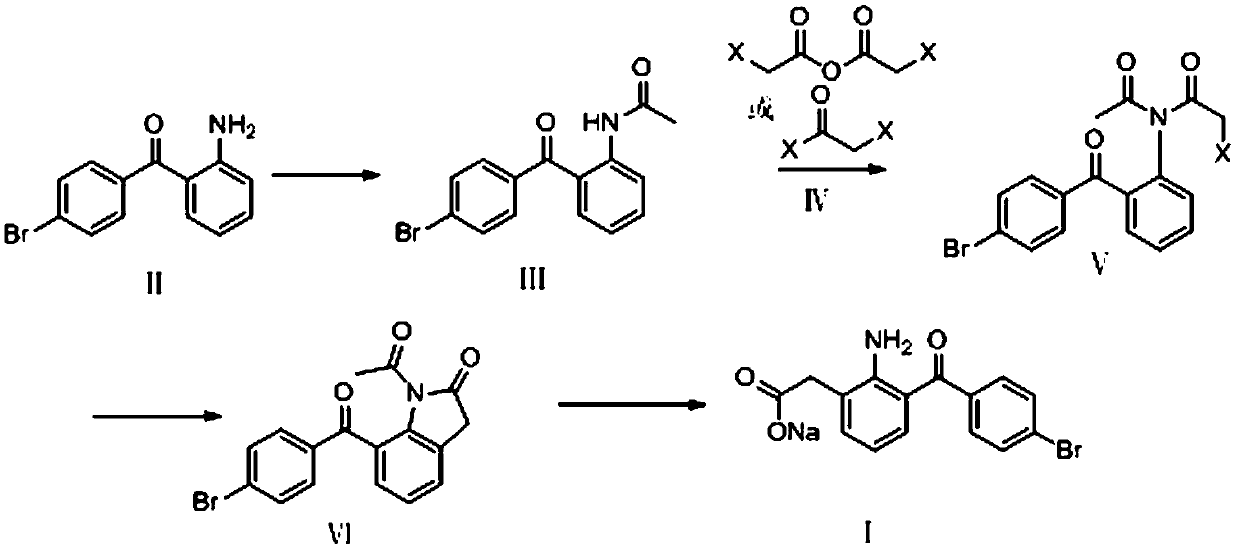
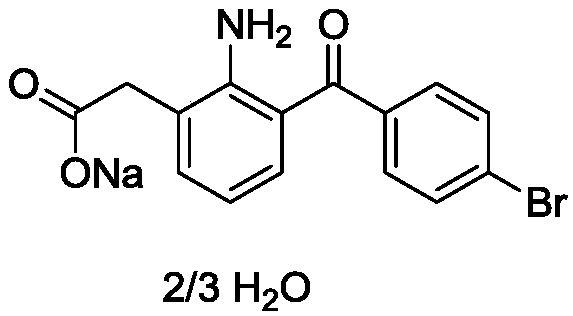
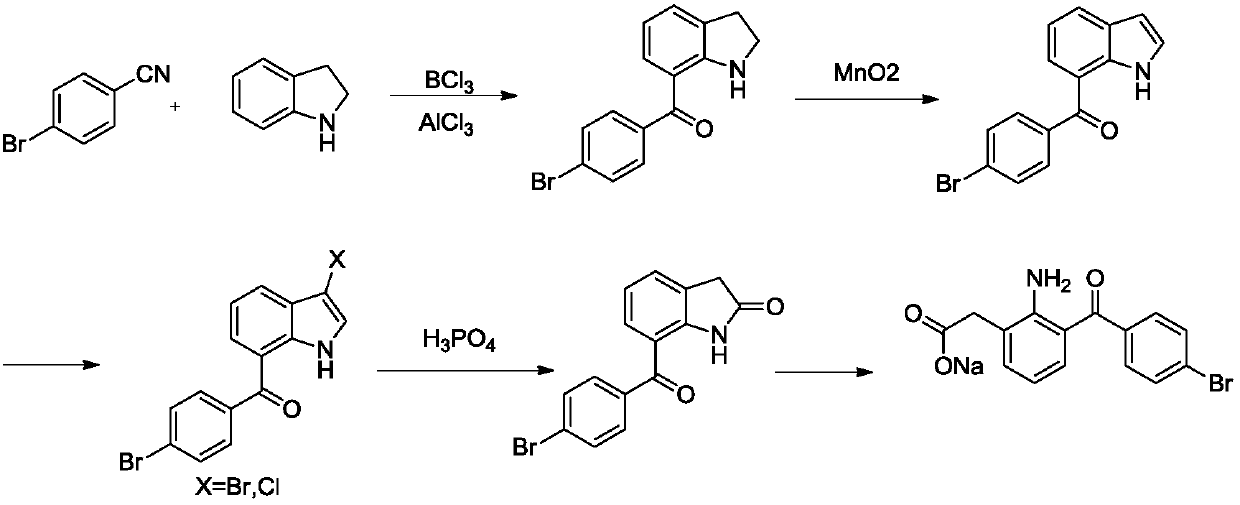
A kind of method of synthesizing bromfenac sodium
A technology of bromfenac sodium and its mixture, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve problems such as safety production threat, bad smell, restricted application, etc. Effects that are simple and inexpensive to process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of N-(2-(4'-bromobenzoyl)phenyl)acetamide (formula III)
[0041] Add 414g of 2-amino-4'-bromobenzophenone (formula II) and 150g of triethylamine into 2.5L of 2-methyltetrahydrofuran, control the temperature at 0-20°C, add 94g of acetyl chloride dropwise, and stir until After the reaction was complete, the organic phase was washed with water, dried and concentrated to obtain 470 g of N-(2-(4'-bromobenzoyl)phenyl)acetamide with a yield of 98.5%.
[0042] (2) N-acetyl-N-(2-(4'-bromobenzoyl)phenyl)-2-bromoacetamide (Formula V). Wherein X is the preparation of Br):
[0043] Get 450g of N-(2-(4'-bromobenzoyl)phenyl)acetamide, 220g of diisopropylethylamine and 22g of 4-dimethylaminopyridine into 5L of toluene, add 280g of bromoacetyl bromide dropwise, add dropwise After completion, the temperature was controlled at 90-100°C until the reaction was complete. After cooling down to room temperature, the organic phase was washed with water, dried, and concentrated...
Embodiment 2
[0049] (1) Preparation of N-(2-(4'-bromobenzoyl)phenyl)acetamide
[0050] Add 700g of 2-amino-4'-bromobenzophenone and 425g of diisopropylethylamine into 5L of dichloromethane, add 300g of acetic anhydride dropwise, stir until the reaction is complete, wash the organic phase with water, dry and concentrate to obtain N- (2-(4'-bromobenzoyl)phenyl)acetamide 766g, yield 95%.
[0051] (2) Preparation of N-acetyl-N-(2-(4'-bromobenzoyl)phenyl)-2-bromoacetamide
[0052] Get 750g of N-(2-(4'-bromobenzoyl)phenyl)acetamide, 357g of triethylamine and 35g of 4-dimethylaminopyridine into 10L of toluene, add 675g of bromoacetic anhydride dropwise, after the addition is complete, The temperature was raised to reflux until the reaction was complete, and after cooling down to room temperature, the organic phase was washed with water, dried, and concentrated under reduced pressure to recover the organic solvent to obtain 955 g of N-acetyl-N-(2-(4'-bromobenzoyl)phenyl)-2 -Bromoacetamide, yield...
Embodiment 3
[0058] (1) Preparation of N-(2-(4'-bromobenzoyl)phenyl)acetamide
[0059] 500g of 2-amino-4'-bromobenzophenone and 300g of diisopropylethylamine were added to 5L of toluene, the temperature was controlled at 10-20°C, 170g of acetyl chloride was added dropwise, the temperature was naturally raised and stirred until the reaction was complete, organic The phase was washed with water, dried and concentrated to obtain 543 g of N-(2-(4'-bromobenzoyl)phenyl)acetamide with a yield of 94.3%.
[0060] (2) Preparation of N-acetyl-N-(2-(4'-bromobenzoyl)phenyl)-2-chloroacetamide
[0061] Get 510g of N-(2-(4'-bromobenzoyl)phenyl)acetamide, 320g of triethylamine and 50g of 4-dimethylaminopyridine into 5L of toluene, add 270g of chloroacetyl chloride dropwise, after the dropwise addition, Control the temperature at 100-110°C until the reaction is complete. After cooling down to room temperature, the organic phase is washed with water, dried, and concentrated under reduced pressure to recover...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com