Patents
Literature
60 results about "Cabozantinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain types of cancer (including kidney, thyroid cancer).
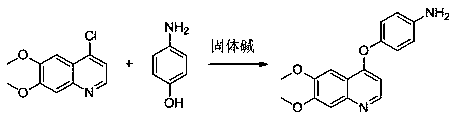
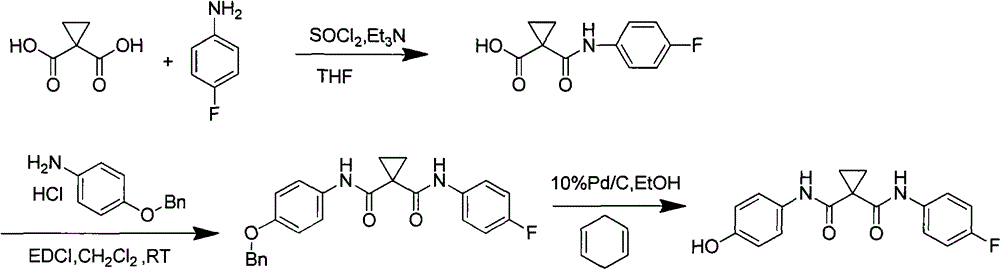
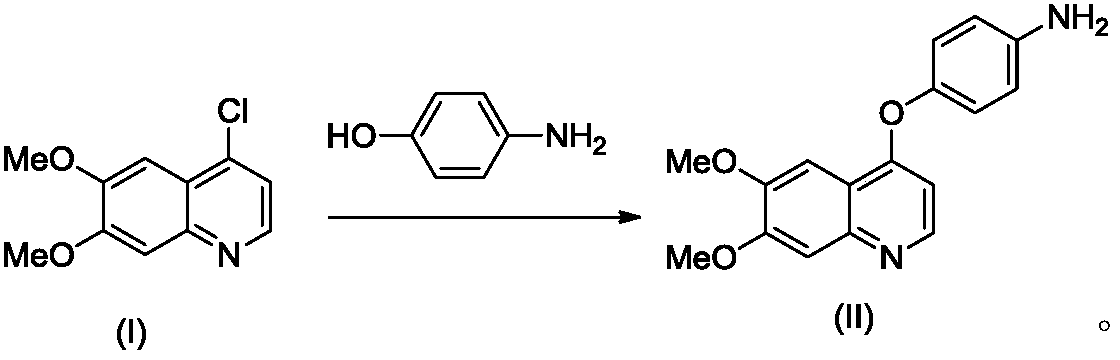
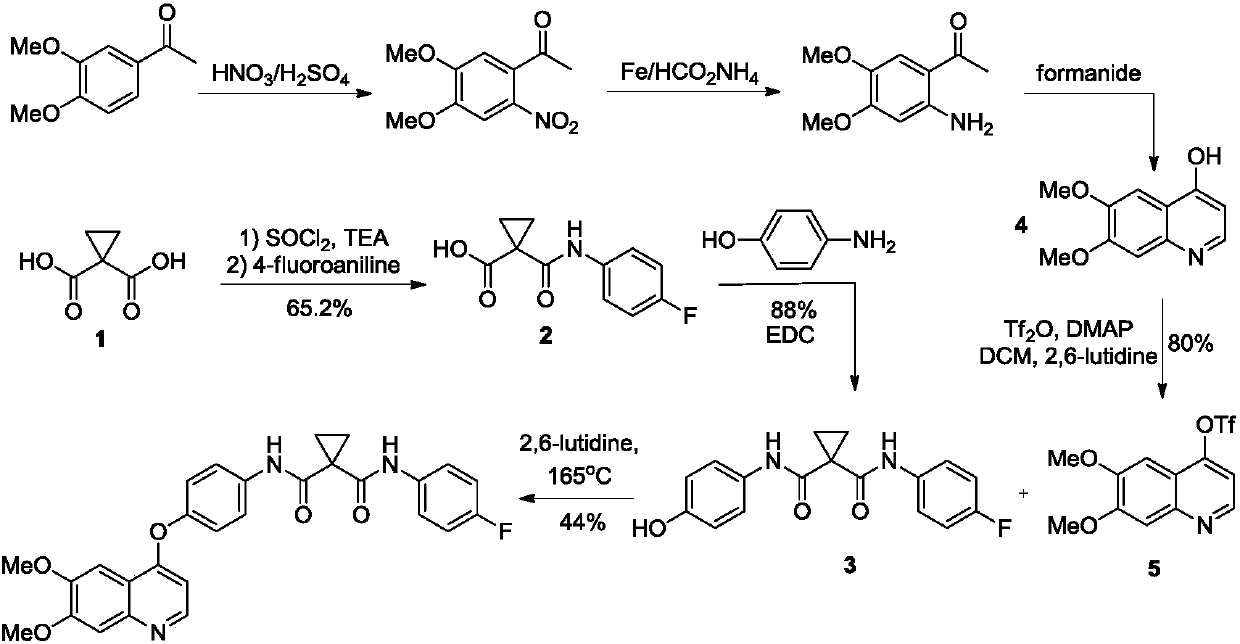
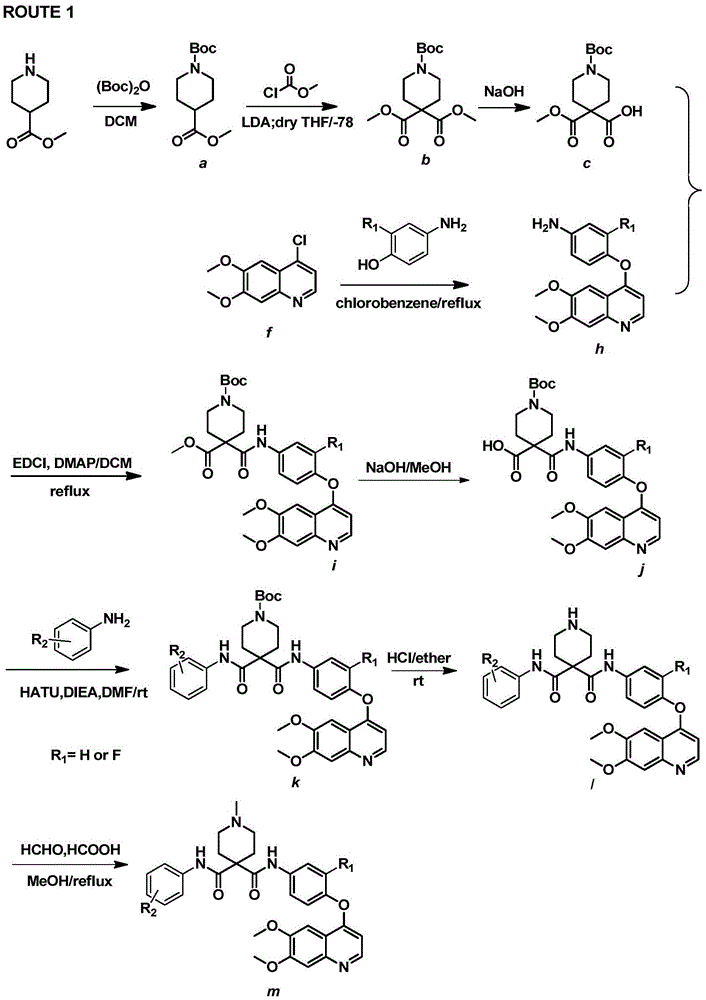
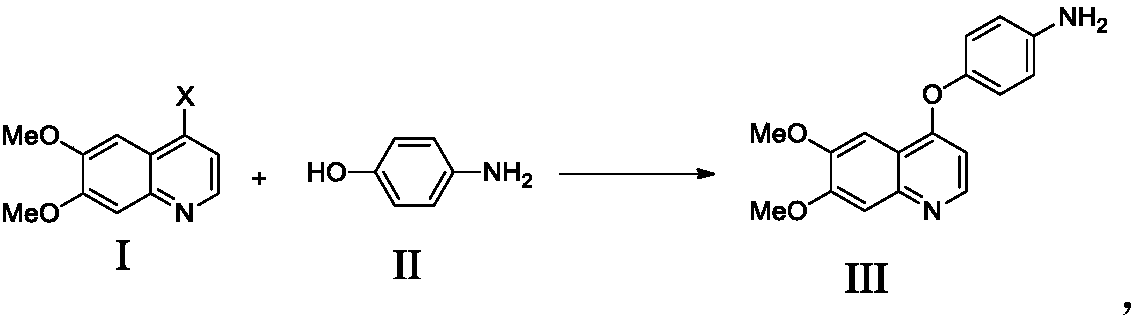
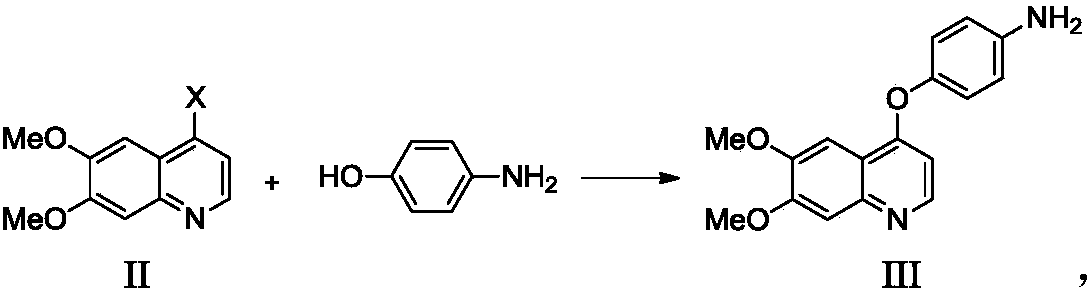
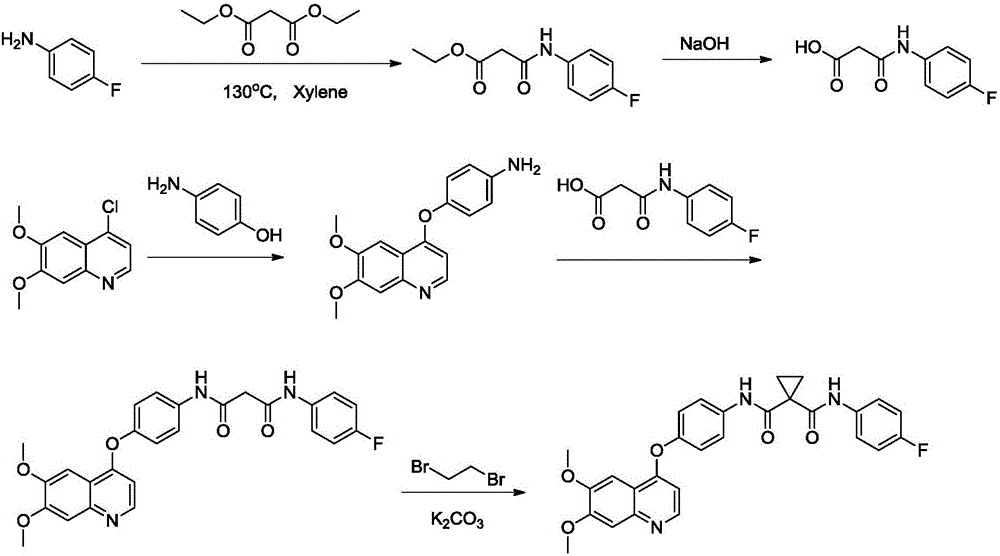
Preparation method for tyrosine kinase inhibitor and midbody thereof
ActiveCN103664776AMild reaction conditionsSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationTyrosine-kinase inhibitorQuinoline
The invention relates to a preparation method for a tyrosine kinase inhibitor and a midbody thereof. According to the method, a compound 1,1-cyclopropane dicarboxylic acid diester is taken as a raw material, and 1-((4-((6,7-dimethoxy quinoline-4-yl) oxy) phenyl) carbamoyl) cyclopropane formic ether is prepared by two ways and reacts with p-fluoro aniline after being hydrolyzed so as to prepare Cabozantinib. The reaction conditions of the preparation method are mild, the synthesis cost is lowered, and the preparation method is simple and convenient to operate and is applicable to industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Cabozantinib Dosage Form and Use in the Treatment of Cancer
This invention relates to a dosage form of cabozantinib and a method of employing the dosage form to treat cancer.
Owner:EXELIXIS INC
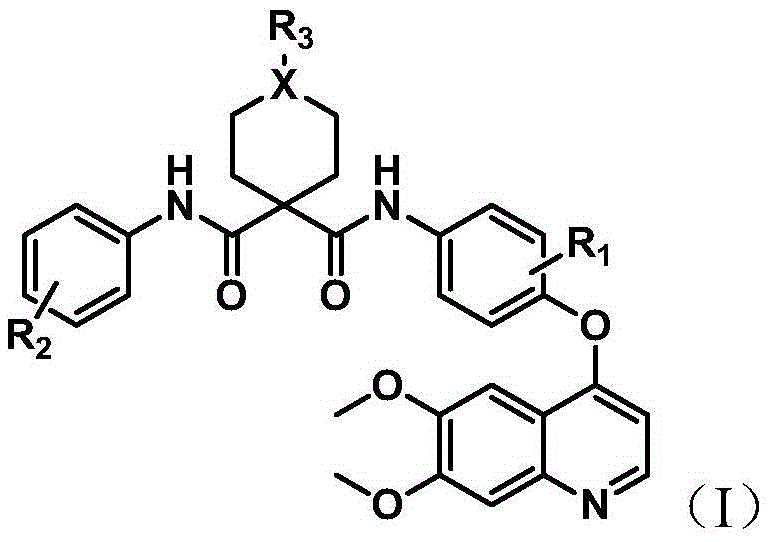
Quinoline multi-target kinase inhibitor with antitumor activity and preparation method thereof
InactiveCN105541798AStrong in vitro inhibitory activityStrong inhibitory activityOrganic chemistryAntineoplastic agentsPositive controlHuman gastric carcinoma
The invention relates to a quinoline multi-target kinase inhibitor with antitumor activity and a preparation method thereof. A general structural formula of the compound is shown in a formula (I) described in the specification. In vitro cell experiments verify that the compound provided by the invention has strong in vitro inhibitory activity on five common tumor cell lines, namely human thyroid carcinoma SW579, human hepatic carcinoma HepG2, human lung adenocarcinoma A549, human colorectal adenocarcinoma HCT116 and human gastric carcinoma MKN45, antitumor activities of most of target compounds are better than or equivalent to that of a positive control drug Cabozantinib, and the in vitro cell experiments verify that the compound provided by the invention has strong inhibitory activity on two kinases KDR and MET, so that the compound provided by the invention has a broad application prospect in preparation of a new antitumor drug.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Dosing of cabozantinib formulations
ActiveUS10159666B2Desired pharmacokineticDesired pharmacodynamic effectDigestive systemSkeletal disorderMetaboliteDepressant
The invention relates to administration of various pharmaceutical formulations of N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide, (cabozantinib) a c-Met inhibitor, and its metabolites, to achieve desirable pharmacokinetic and pharmacodynamic effects.
Owner:EXELIXIS INC
Dosing of Cabozantinib Formulations
The invention relates to administration of various pharmaceutical formulations of N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide, (cabozantinib) a c-Met inhibitor, and its metabolites, to achieve desirable pharmacokinetic and pharmacodynamic effects.
Owner:EXELIXIS INC
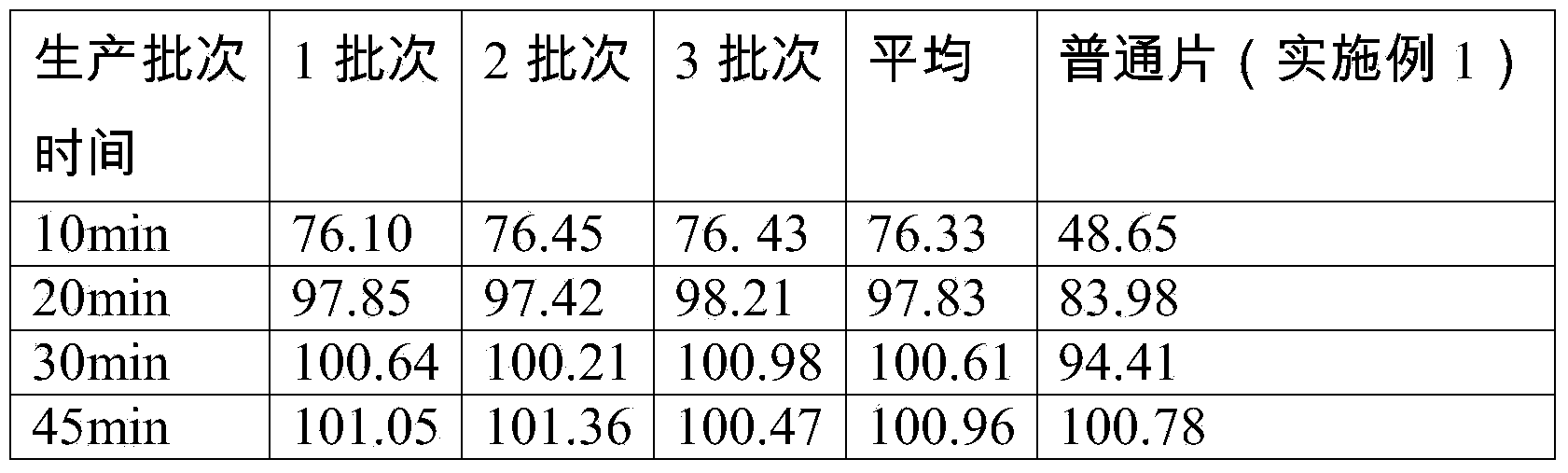
Cabozantinib dispersible tablets and preparation method thereof
The invention discloses cabozantinib dispersible tablets and a preparation method and application thereof. The cabozantinib dispersible tablets consist of the following components in percent by weight: 10-60 percent of cabozantinib, 10-40 percent of filler, 10-50 percent of disintegrating agent, 10-50 percent of acidifying agent, 0.1-10 percent of adhesive and 0.1-20 percent of lubricating agent and flow aids. Compared with common tablets, the dispersible tablets have the advantages that the cabozantinib dispersible tablets do not contain surfactants, are high in solubleness, dispersing property and disintegration property and can be completely disintegrated within 1 minute. The cabozantinib dispersible tablets prepared by the method are high in dissolution degree, high in bioavailability, rapid in in-vivo distribution, stable in quality, good in taste, simple and feasible in preparation method and suitable for industrial production.
Owner:QINGDAO TUMOR HOSPITAL
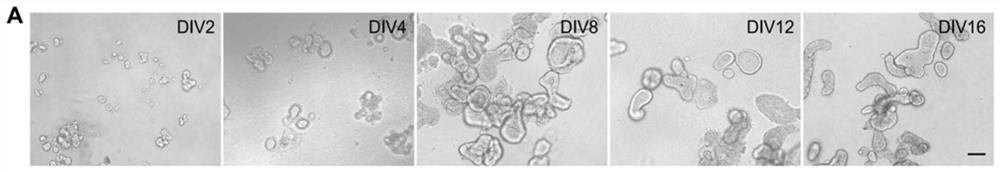
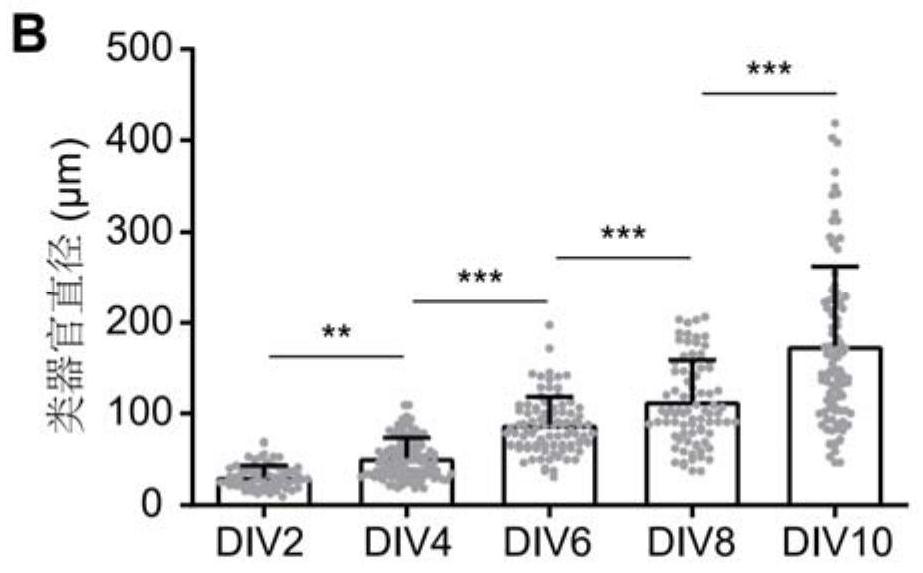
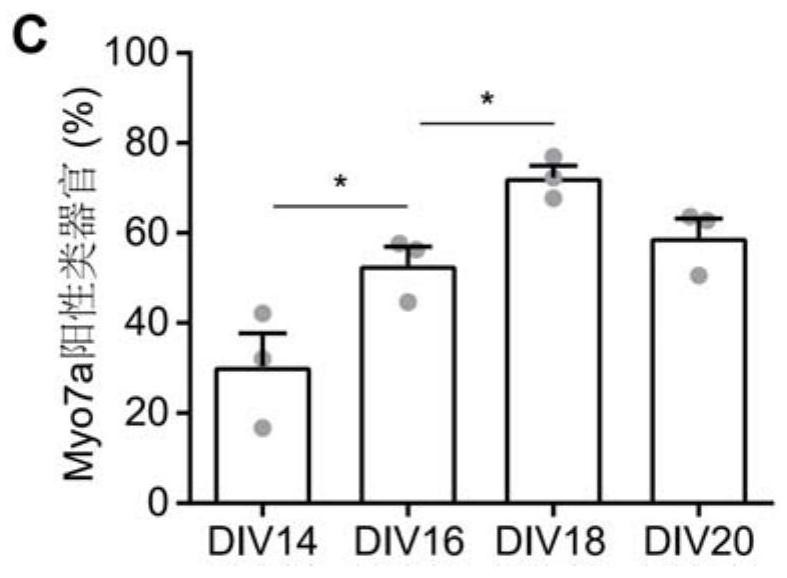
Composition for promoting hair cell regeneration and hearing recovery and application thereof
ActiveCN111643671APromote regenerationPromote maturitySenses disorderNervous system cellsHair cell differentiationRegorafenib
The invention belongs to the field of biological medicines, and discloses a composition for promoting hair cell regeneration and hearing recovery and an application thereof. The composition comprisesa Wnt agonist and one or more of the following reagents: (a) a VEGFR inhibitor; (b) a Tgfbr inhibitor; and (c) an ERG inhibitor. The VEGFR inhibitor comprises regorafenib, apatinib mesylate, cabozantinib, pazopanib hydrochloride and medicinal salts or derivatives of the regorafenib, the apatinib mesylate, the cabozantinib and the pazopanib hydrochloride. An organoid platform verifies that after any inhibitor in a signal axis of EGFR-TGFB1-ERG is combined with the Wnt agonist to prepare the composition, efficient hair cell differentiation, maturation and survival can be realized, and the composition has important value for hearing recovery.
Owner:NANJING UNIV
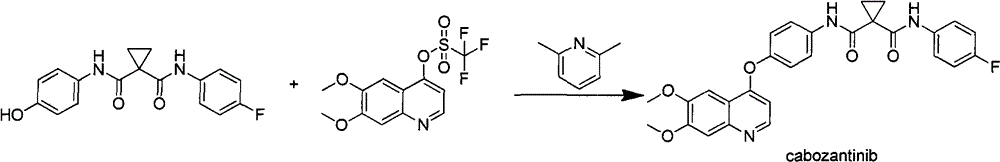
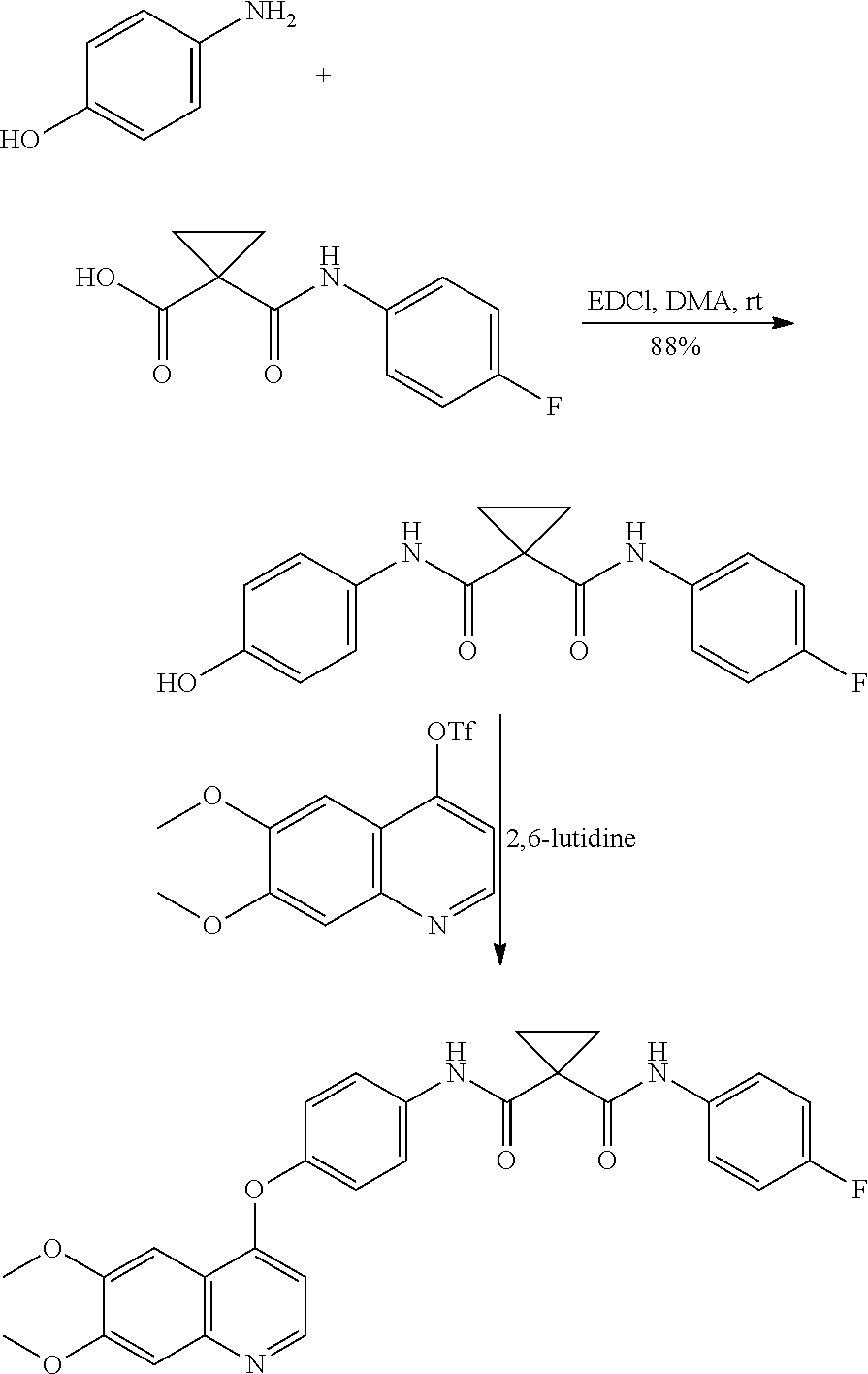
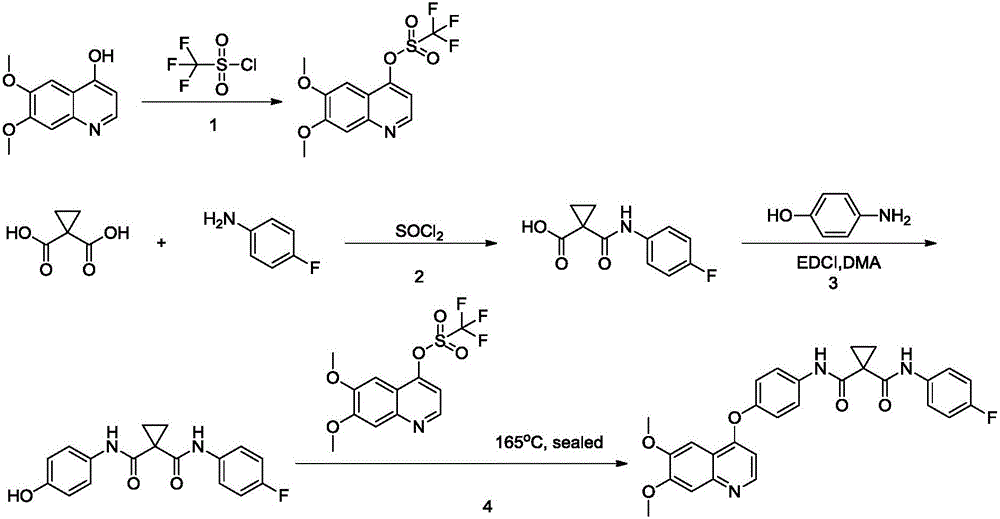
Synthetic method for cabozantinib
The invention provides a synthetic method for cabozantinib. The method comprises the following steps: 1,1-cyclopropanedicarboxylic acid is used a starting raw material, acylation is performed, condensation is performed on an acylation product and 4-[6,7-dimethoxy-4-quinolinyl]oxy]aniline, condensation is further performed on an obtained product and 4-fluoroaniline under effects of a polypeptide condensing agent, and therefore the cabozantinib is obtained. According to the method, the reaction conditions are mild, a chlorinating reagent is used just in one step, and the method is suitable for industrialized production.
Owner:深圳万乐药业有限公司
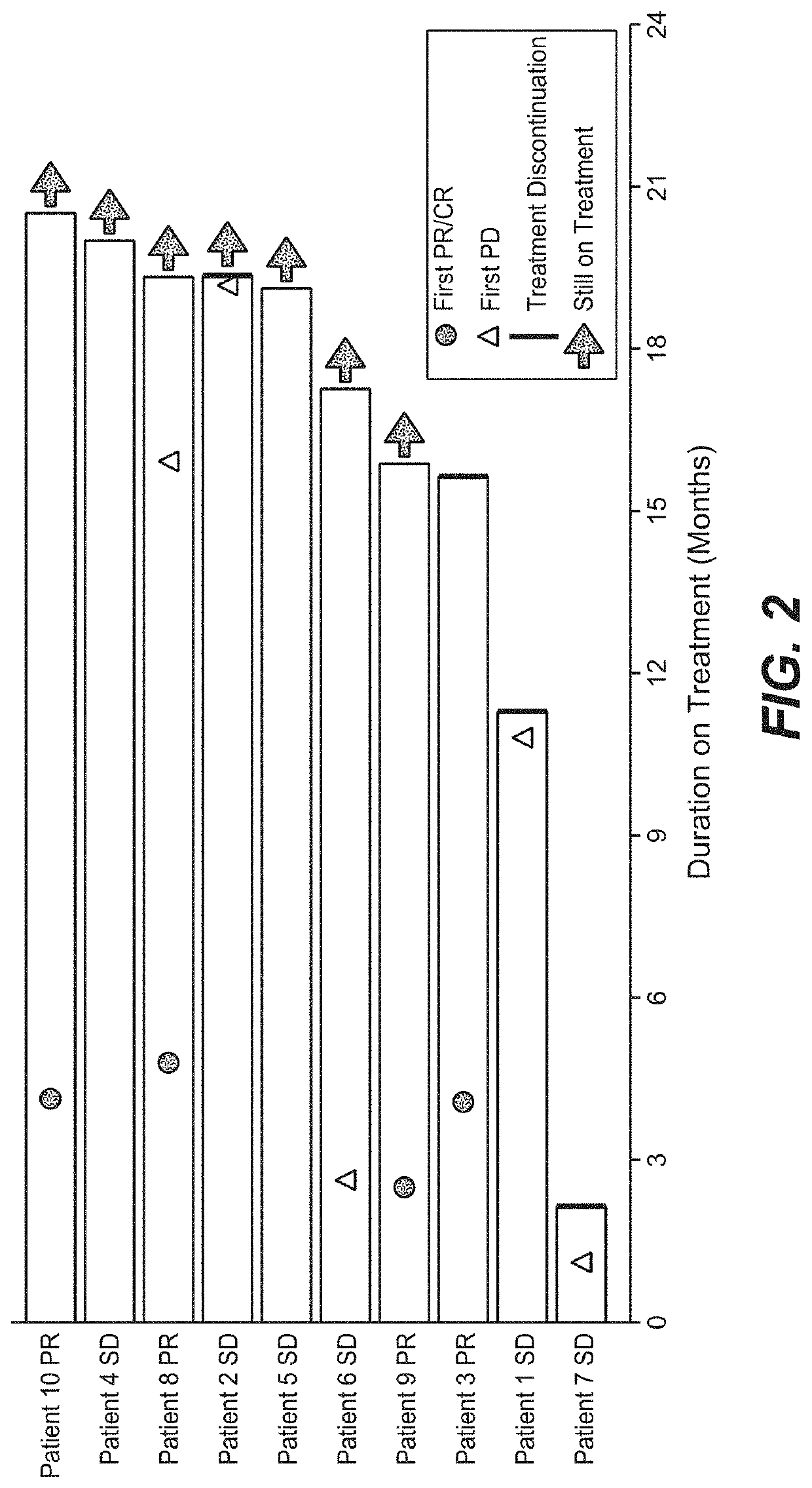
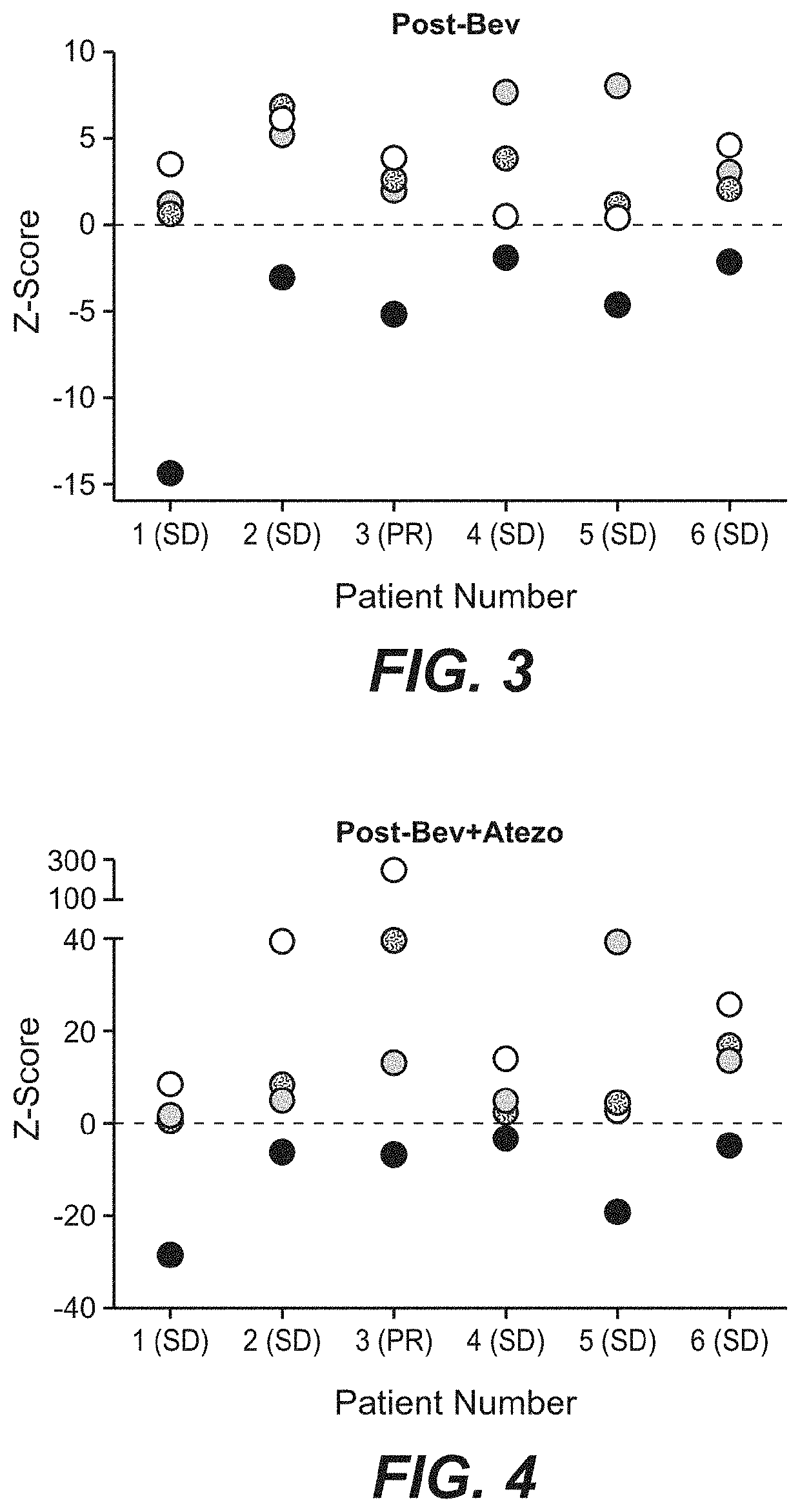
Diagnostic and therapeutic methods for cancer
ActiveUS20190369098A1Organic active ingredientsMicrobiological testing/measurementTyrosine kinaseKidney cancer
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., kidney cancer (e.g., renal cell carcinoma (RCC)), lung cancer (e.g., non-small cell lung cancer (NSCLC)), bladder cancer (e.g., urothelial bladder cancer (UBC)), liver cancer (e.g., hepatocellular carcinoma (HCC)), ovarian cancer, or breast cancer (e.g., triple-negative breast cancer (TNBC))). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a VEGF antagonist (e.g., an anti-VEGF antibody, (e.g., bevacizumab) or a VEGFR inhibitor (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib))) and a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)), or with an angiogenesis inhibitor (e.g., a VEGF antagonist (e.g., a VEGFR inhibitor, (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib)))).
Owner:GENENTECH INC
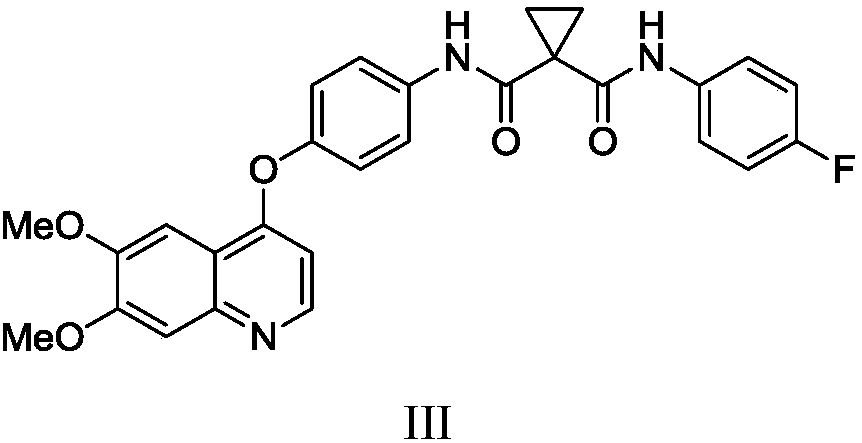
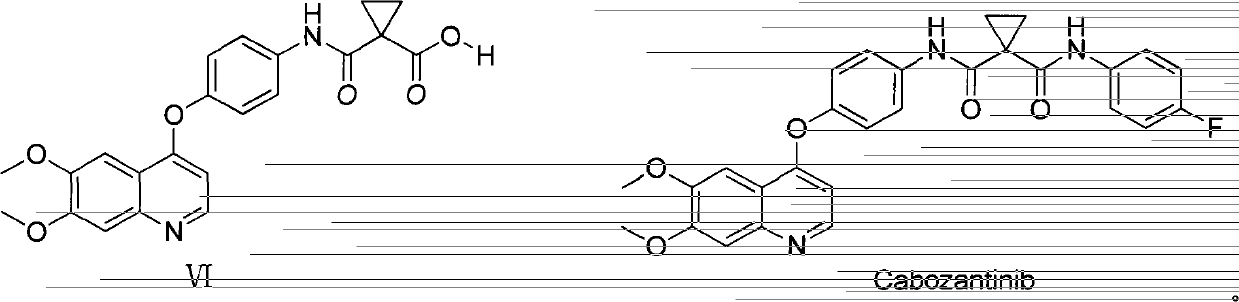
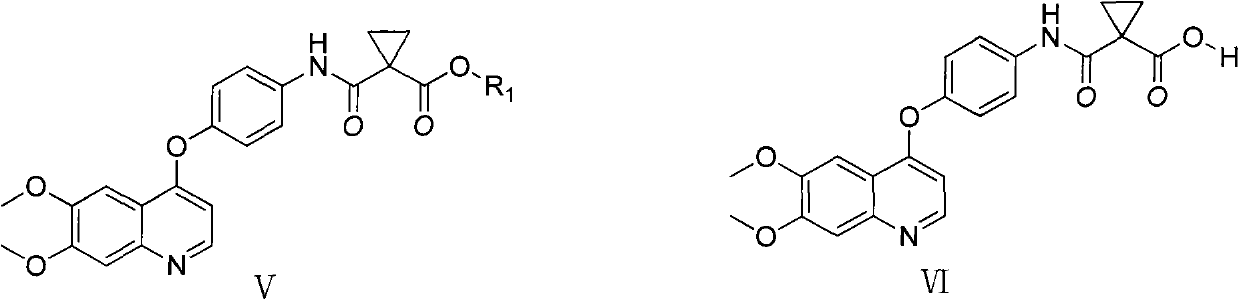
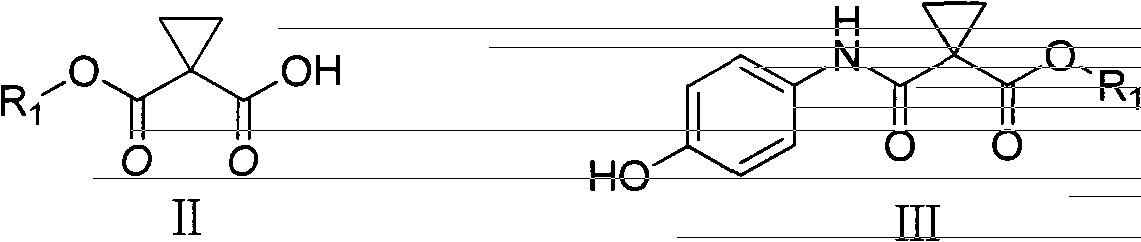
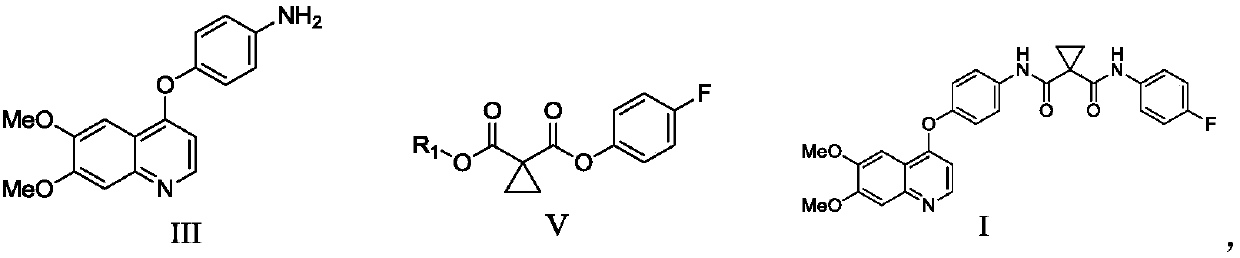
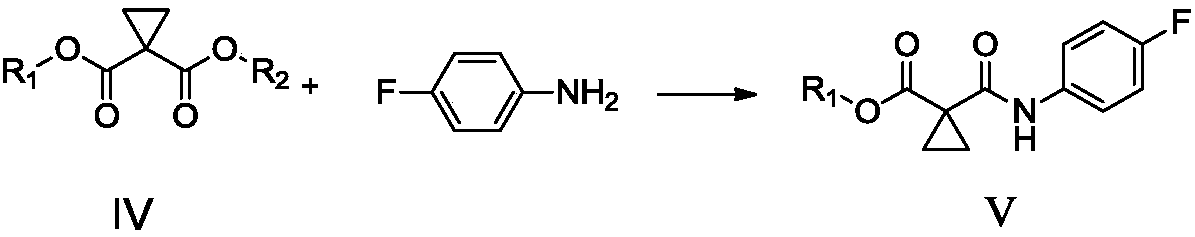
Preparation method of Cabozantinib
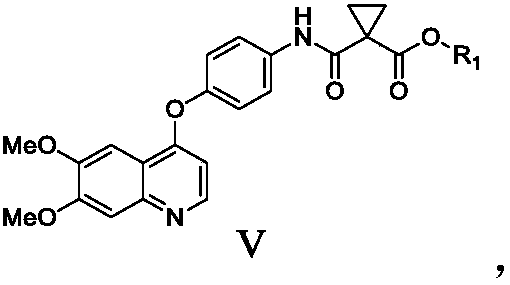
The invention relates to a preparation method of Cabozantinib, and belongs to the field of pharmaceutical chemistry. The Cabozantinib compound is prepared through two steps. The preparation method comprises following steps: 1, a compound III and a compound VI are subjected to condensation reaction to prepare a compound V; and 2, the compound V and p-fluoroaniline are subjected to condensation reaction to prepare Cabozantinib. The preparation method possesses following characteristics: operation is simple and convenient; reaction conditions are mild; yield is high; and industrial applications are convenient.
Owner:JIANGSU HANSOH PHARMA CO LTD
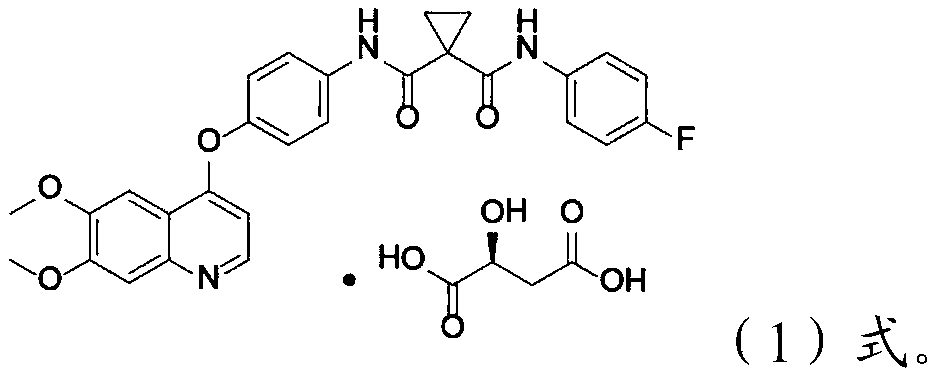
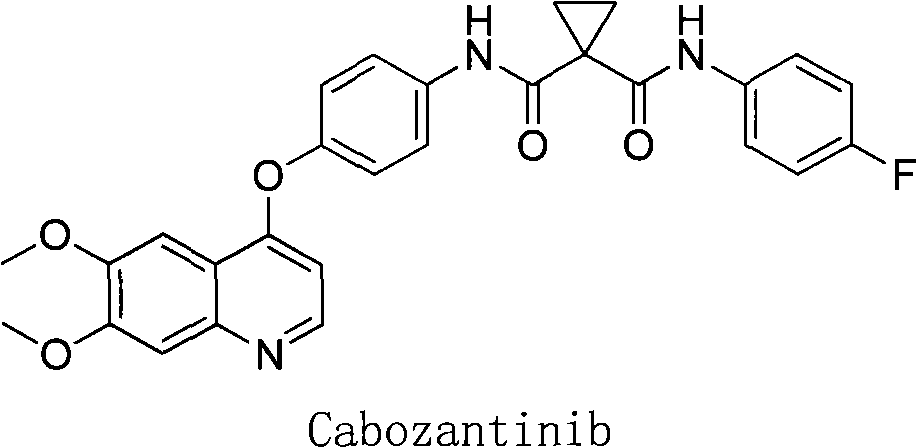
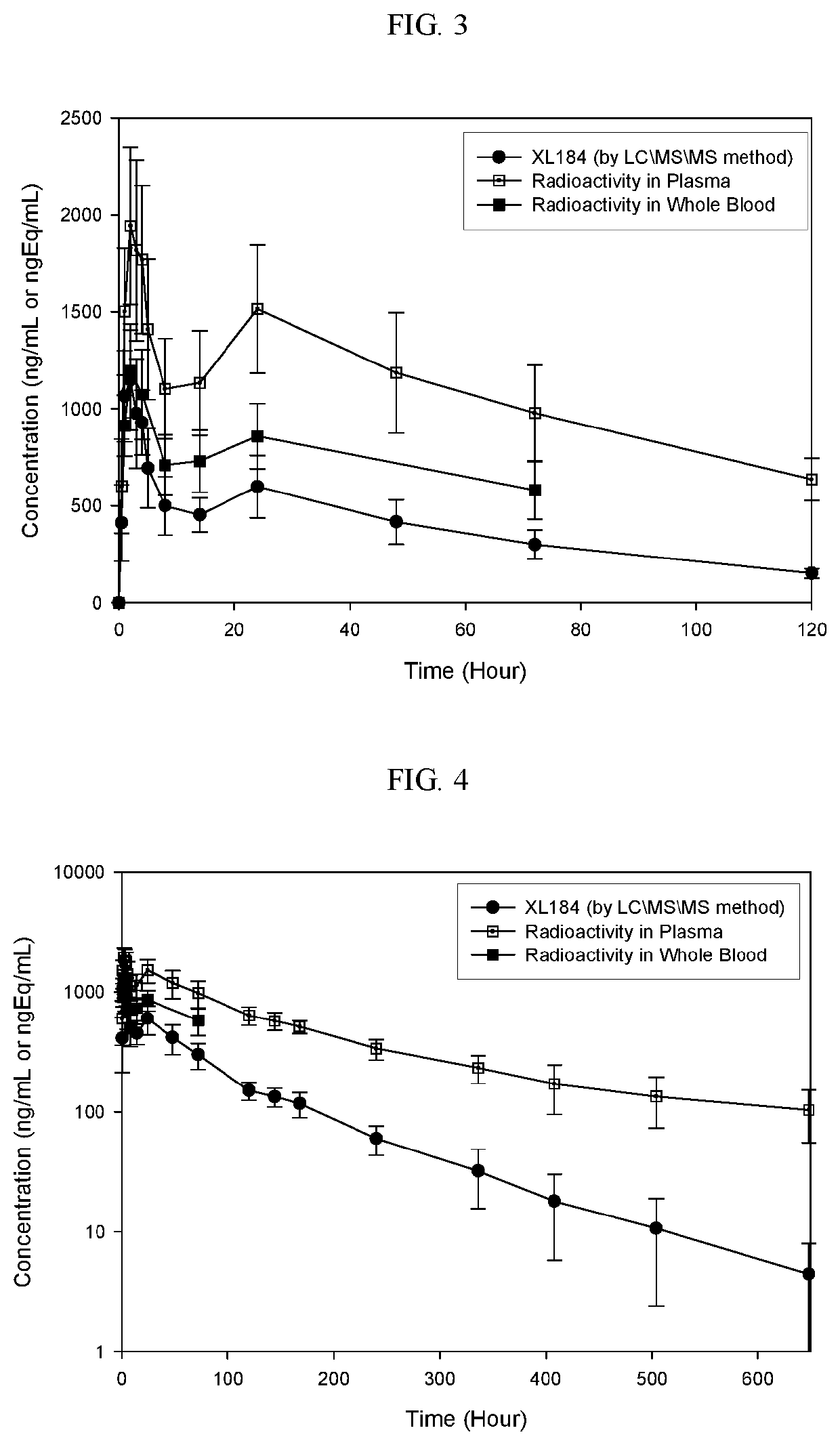
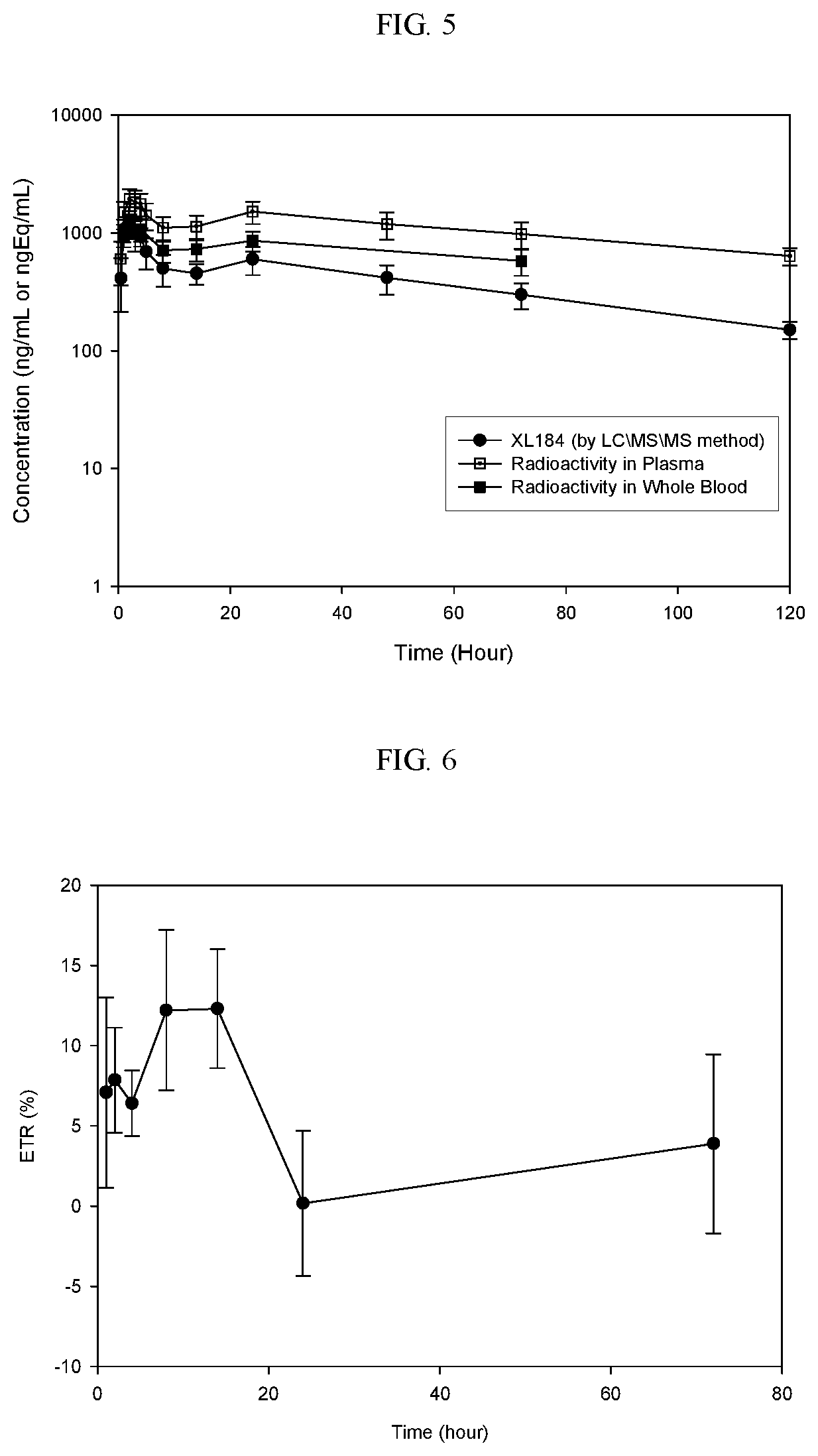
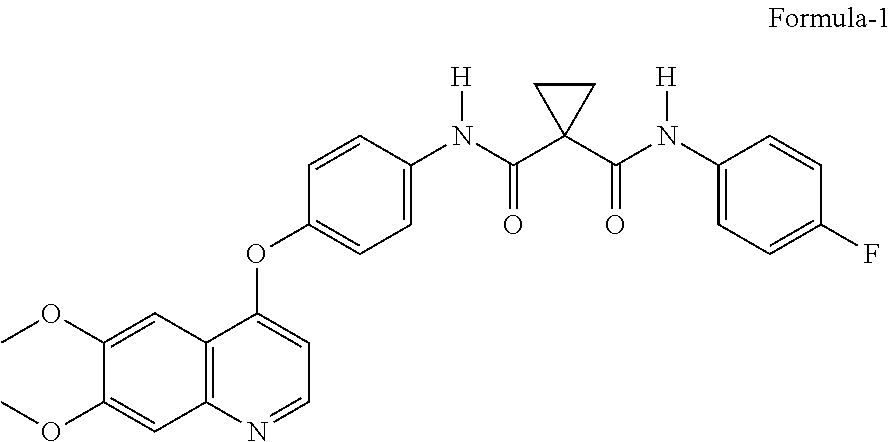
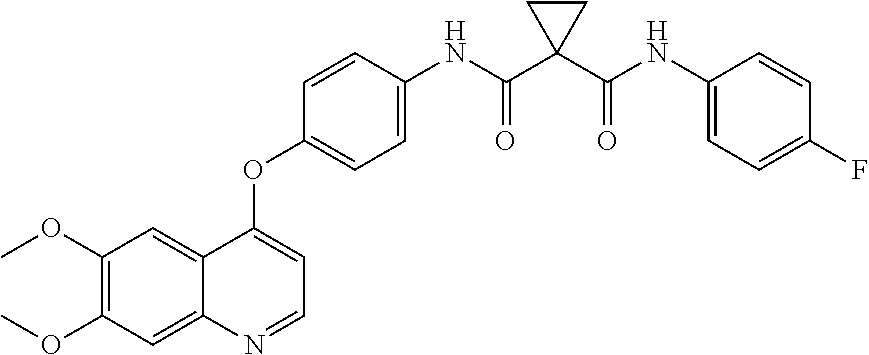
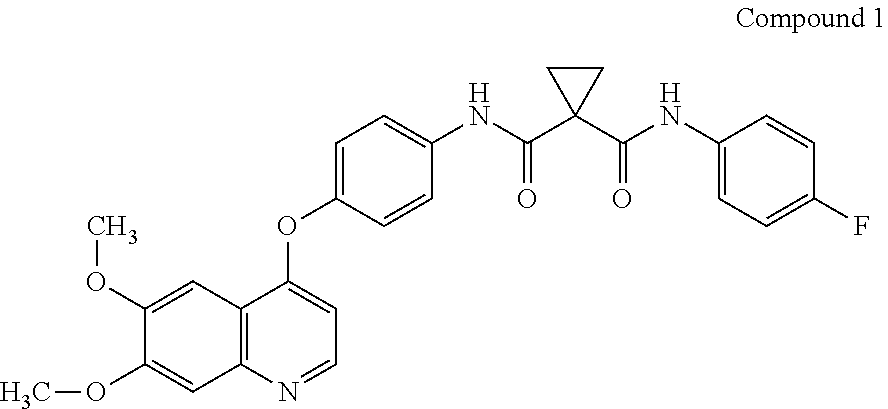
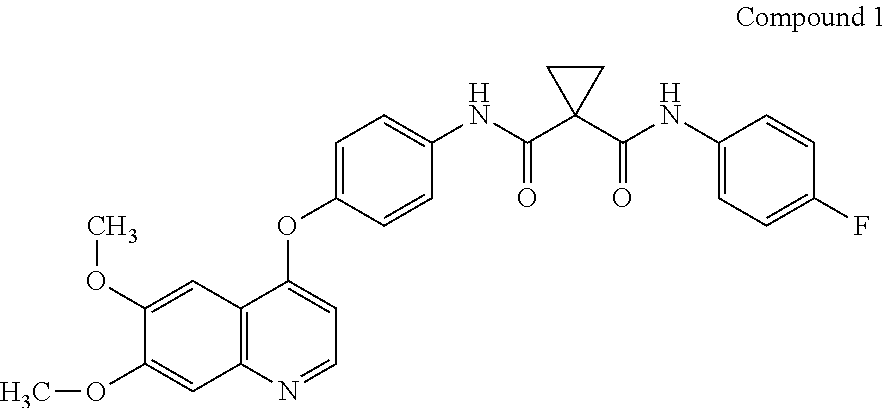
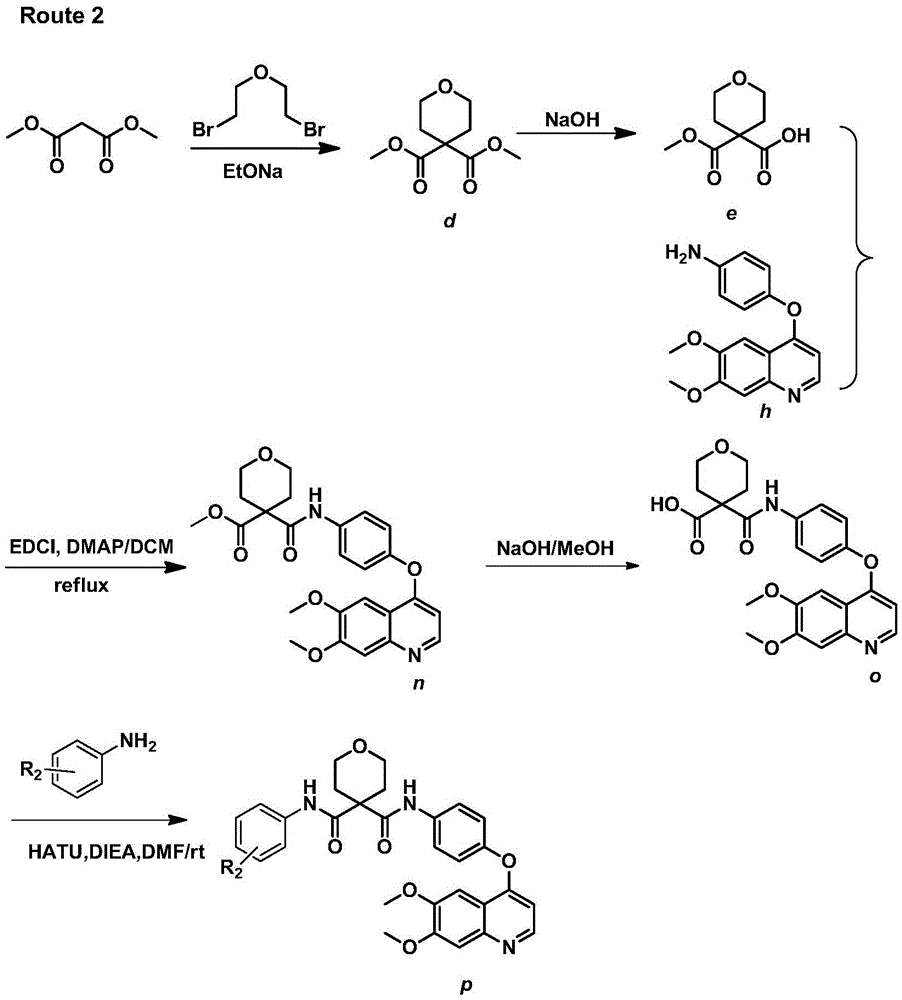
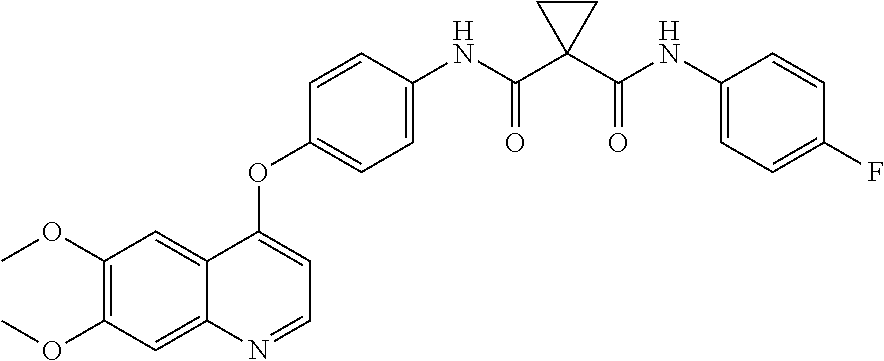
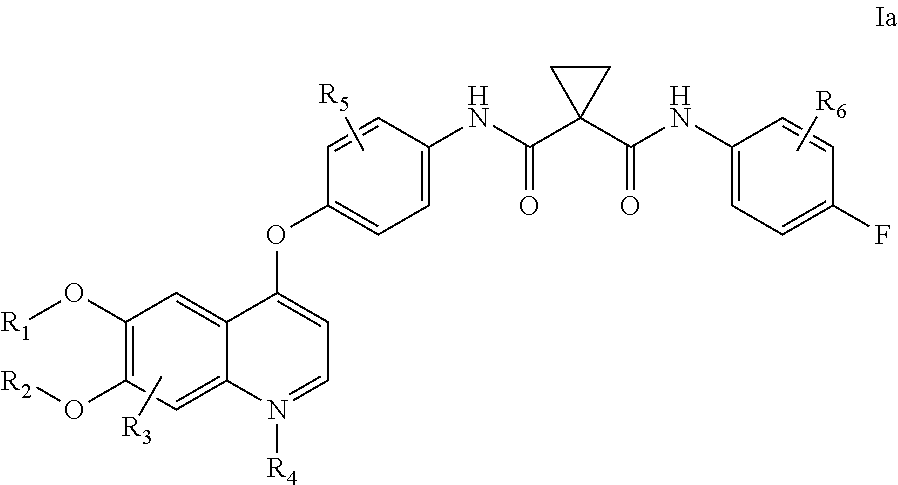
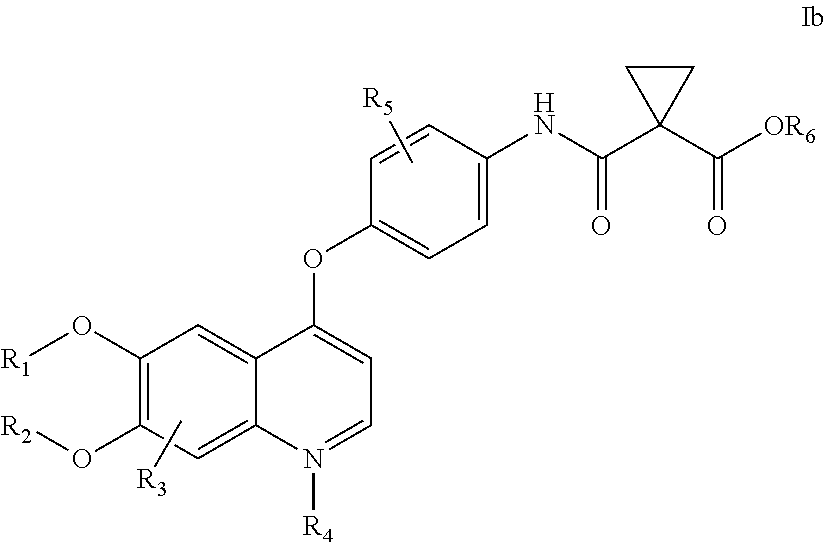
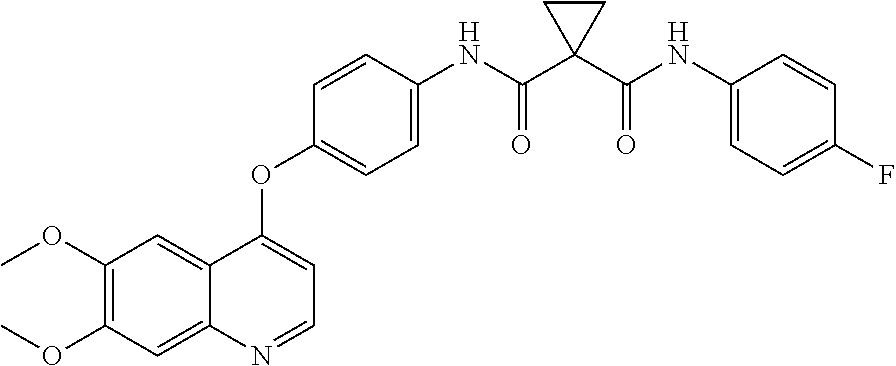
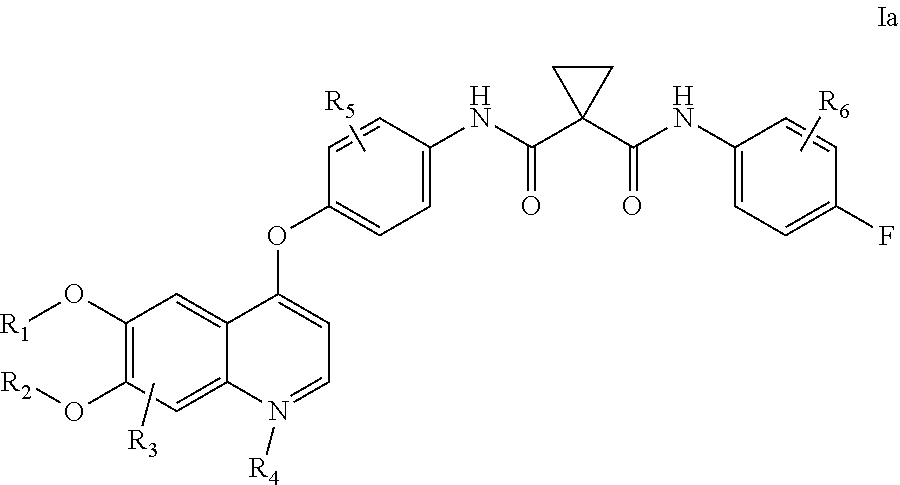
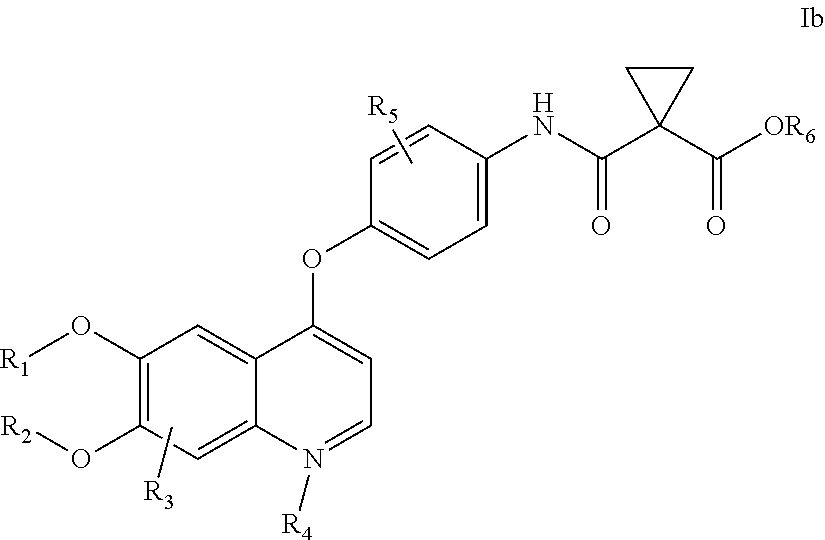
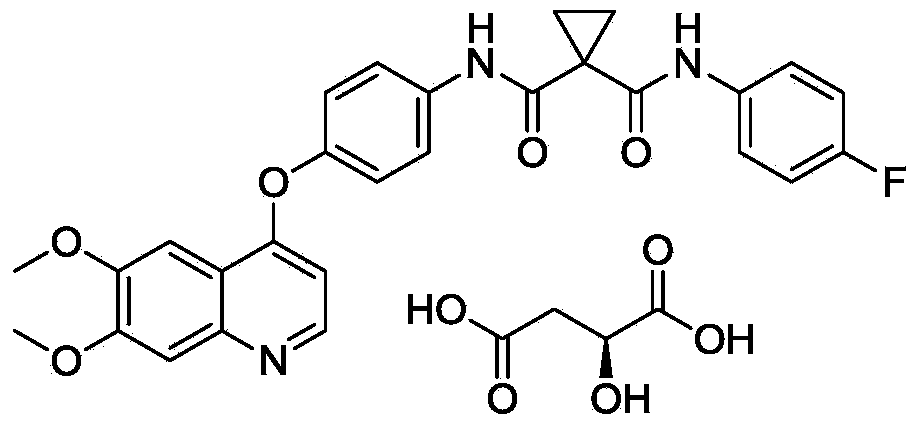
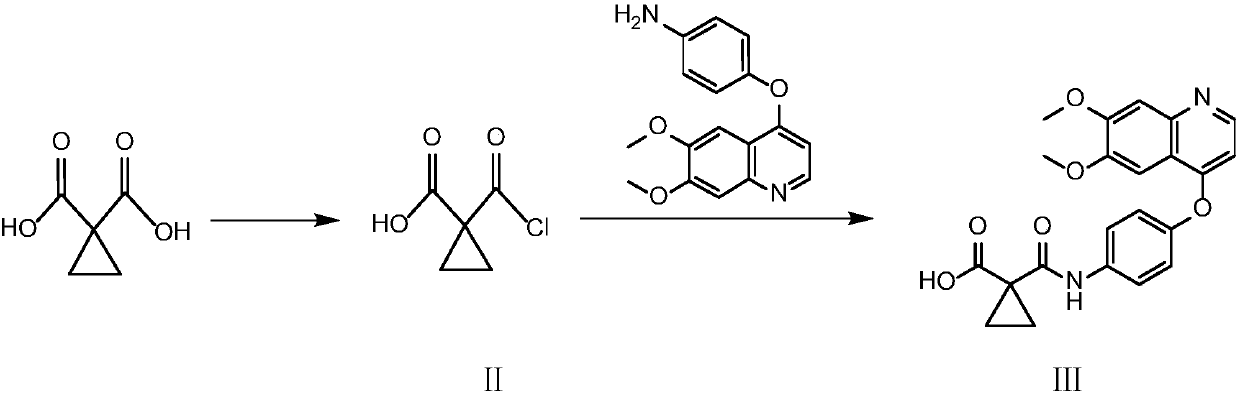
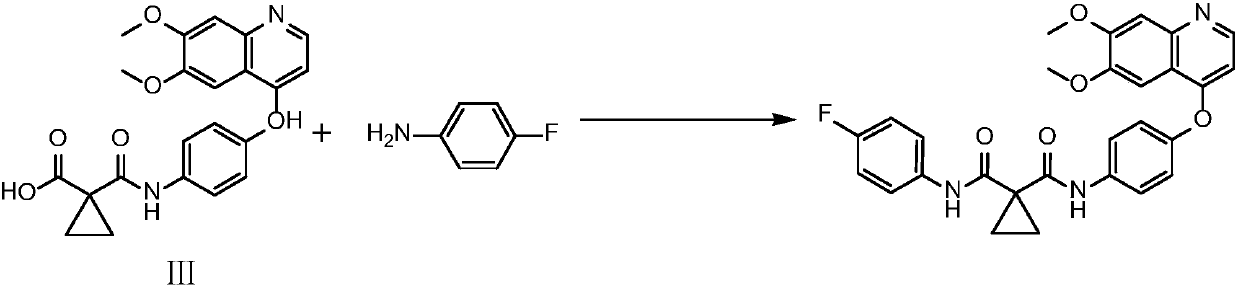
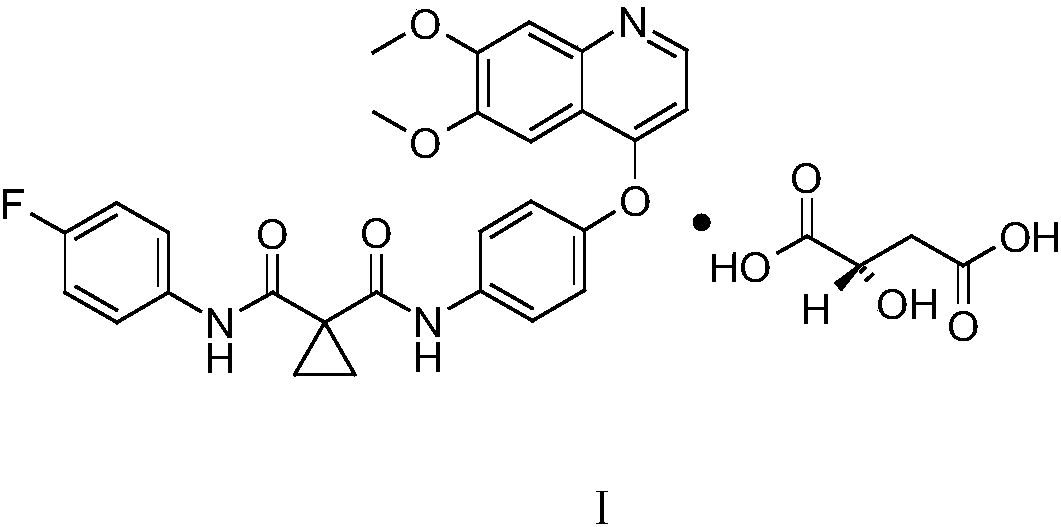
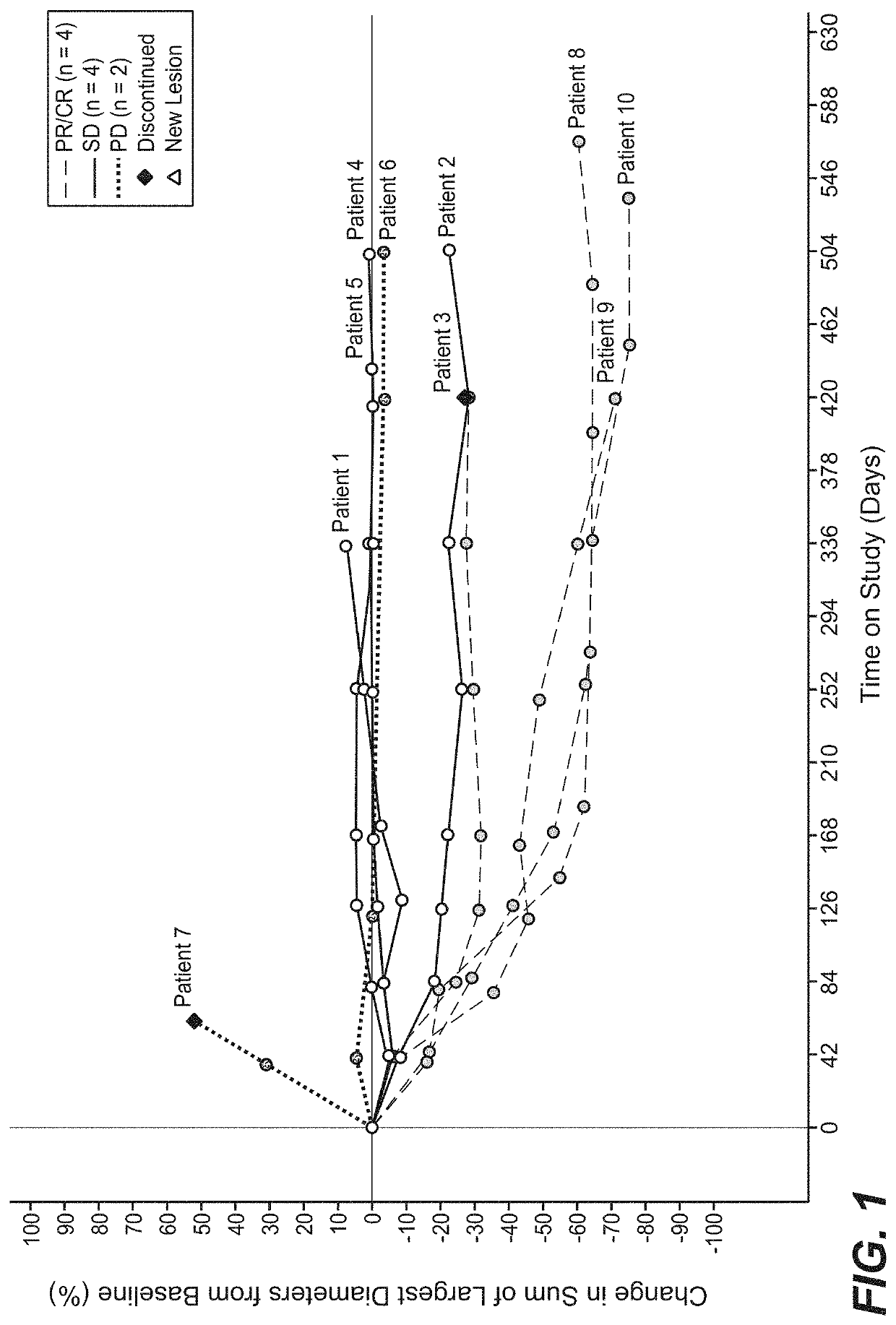
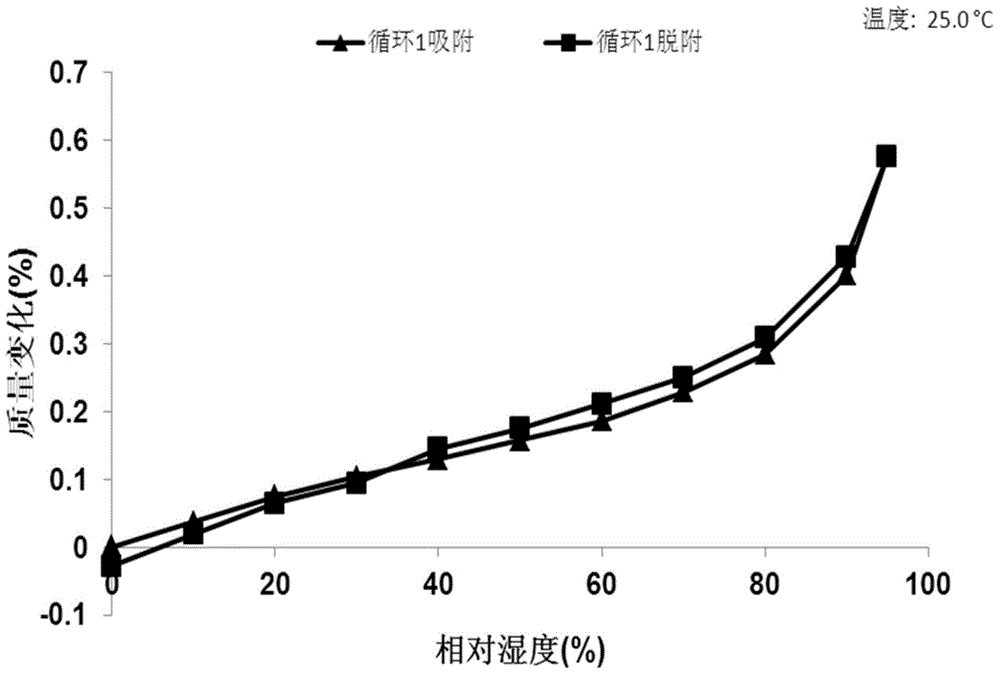
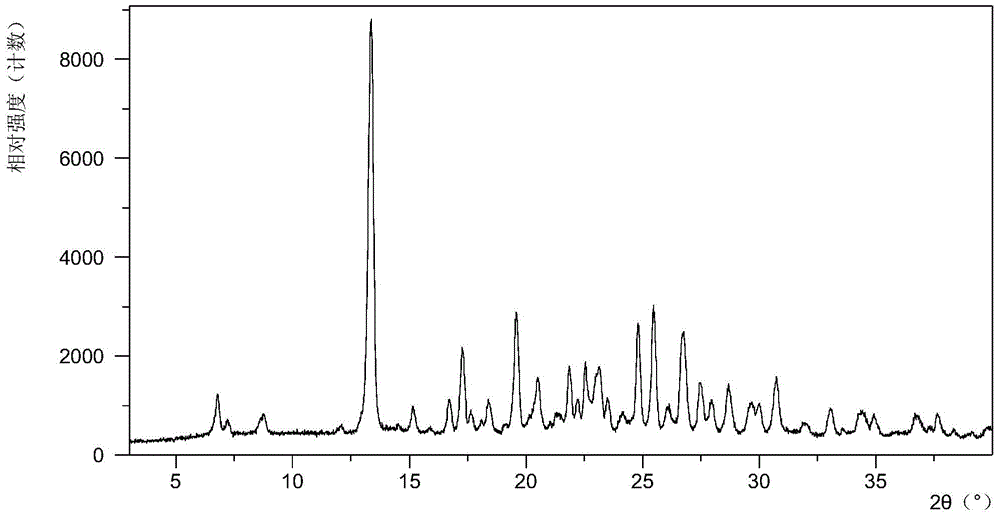
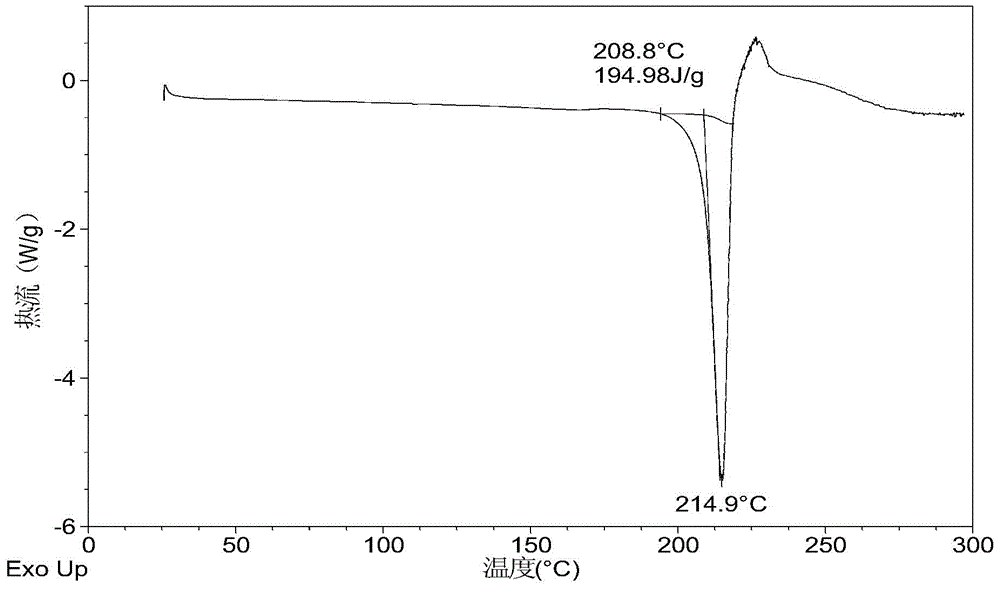
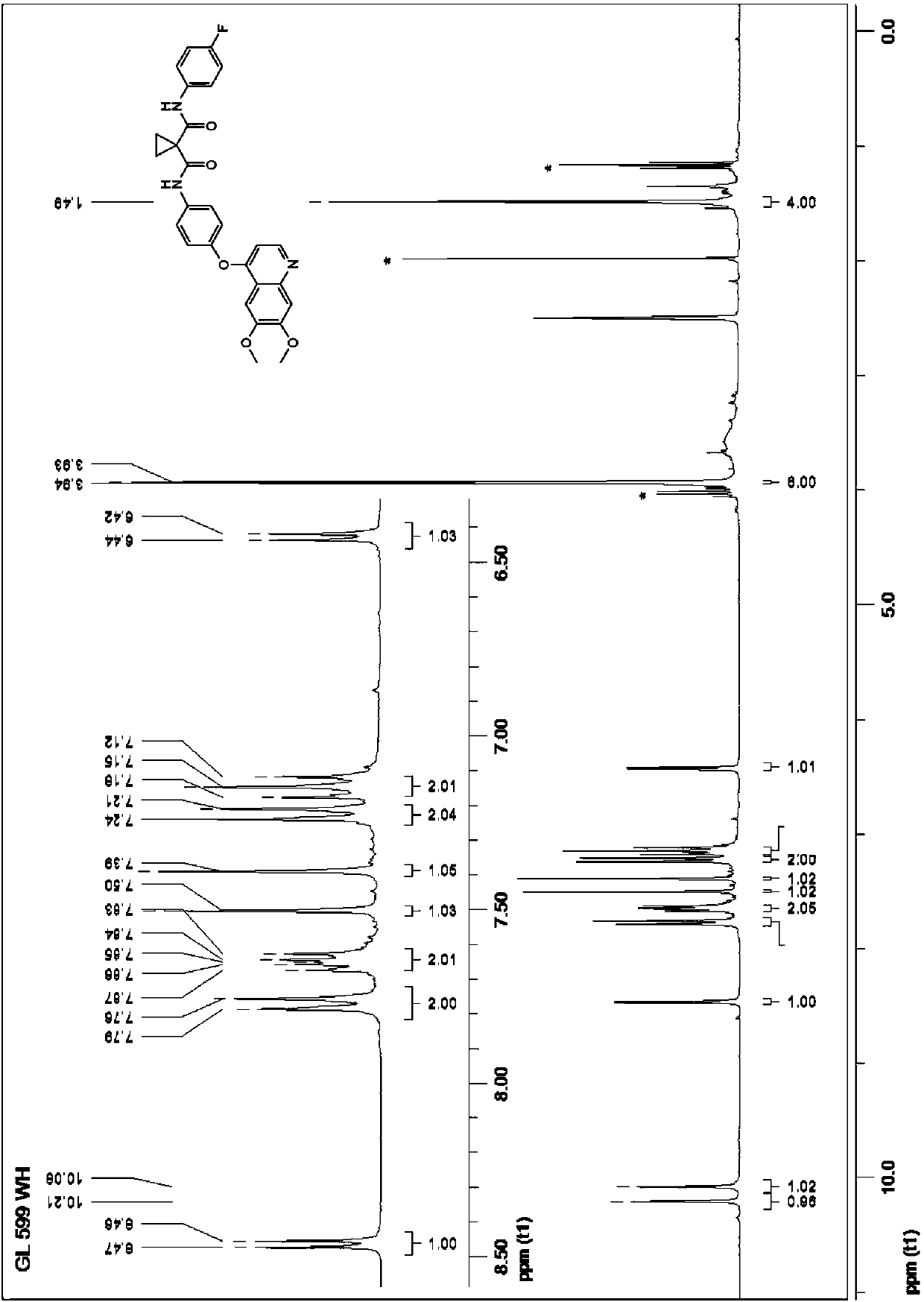
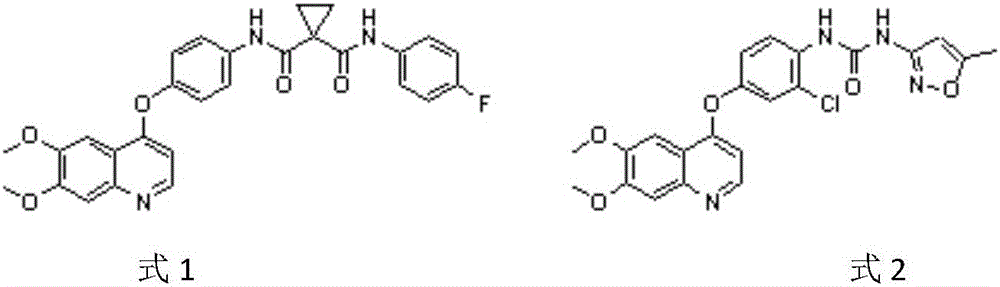
Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use
The invention relates to novel crystalline solid forms of the chemical compound N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide (Compound 1), and solvates thereof, including hydrates, that are useful for the treatment of cancer. Also disclosed are pharmaceutical compositions comprising the crystalline solid forms and processes for making the crystalline solid forms, as well as methods of using them for the treatment of cancer, particularly thyroid cancer, prostate cancer, hepatocellular cancer, renal cancer, and non-small cell lung carcinoma. The crystalline solid forms can be used to make the L-malate salt of cabozantinib.
Owner:EXELIXIS INC
Preparation method of Cabozantinib
The invention belongs to the field of pharmaceutical chemistry, and relates to a preparation method of Cabozantinib. The preparation method comprises following steps: 4-halogenated-6, 7-dimethoxyquinoline is reacted with 4-aminophenol to prepare a compound represented by formula III; cyclopropane-1, 1-dicarboxylic acid / ester and p-fluoroaniline are reacted to prepare a compound represented by formula V; and then condensation reaction is carried out to obtain the Cabozantinib compound. The preparation method is simple and convenient in operation, high in yield, low in cost, and promising in industrialization prospect.
Owner:JIANGSU HANSOH PHARMA CO LTD
Cabozantinib preparation method
ActiveCN106632028AReduce manufacturing costReduce usageOrganic chemistryEthylene DibromideCabozantinib
The invention discloses a cabozantinib preparation method. The target product cabozantinib is prepared by performing five-step reaction on diethyl malonate, 4-fluoroaniline, 4-chloro-6,7-dimethoxyquinoline, 4-aminophenol, 1,2-dibromoethane and the like used as raw materials. The preparation method is simple to operate and friendly to environment; the comprehensive yield is more than 50% and is obviously increased in comparison with the yield of 20% in the prior art; and the preparation method greatly lowers the existing medicine production cost, and is suitable for industrial large-scale production.
Owner:SHANGHAI ZAIQI BIO TECH
Pharmaceutical compositions of cabozantinib
Pharmaceutical compositions are provided, which comprise cabozantinib or pharmaceutically acceptable salts thereof, and at least one pharmaceutically acceptable excipient, wherein the inventive compositions exhibit enhanced bioavailability compared to the currently marketed or commercially available formulations. The present invention also provides manufacturing processes thereof and use of the said inventive compositions for the prevention, treatment or prophylaxis of disorders in human patients in need thereof. The present invention relates to oral pharmaceutical compositions of cabozantinib, methods for their administration, processes for their production, and use of these compositions for treatment of diseases treatable by cabozantinib.
Owner:SLAYBACK PHARMA LLC
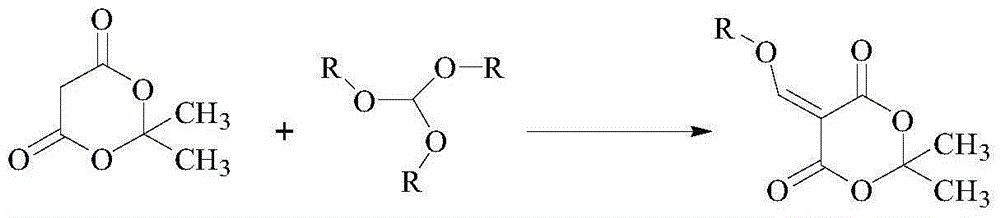
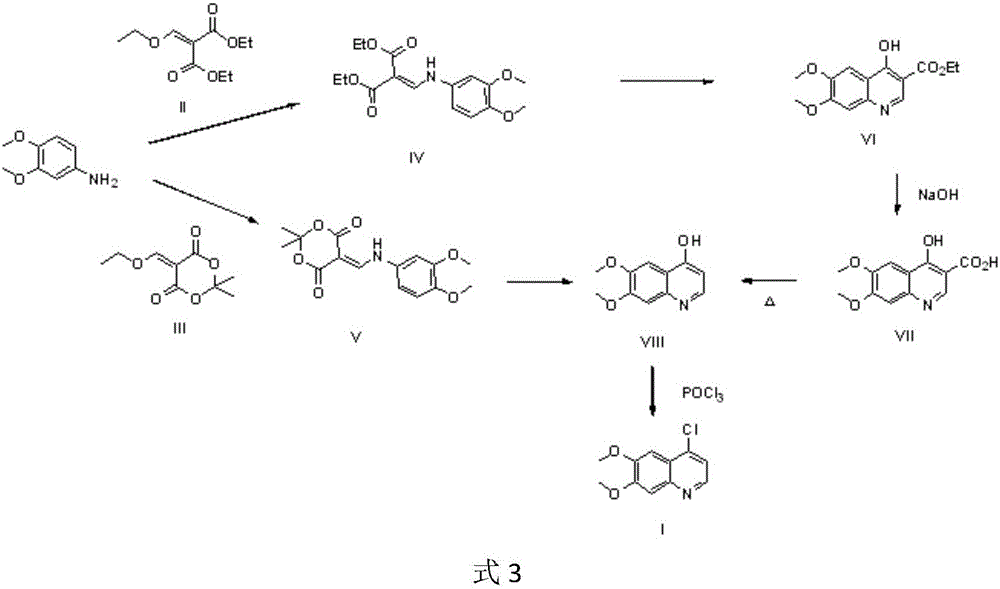
Cabozantinib intermediate 4-hydroxy-6,7-dimethoxyquinoline and preparation method thereof
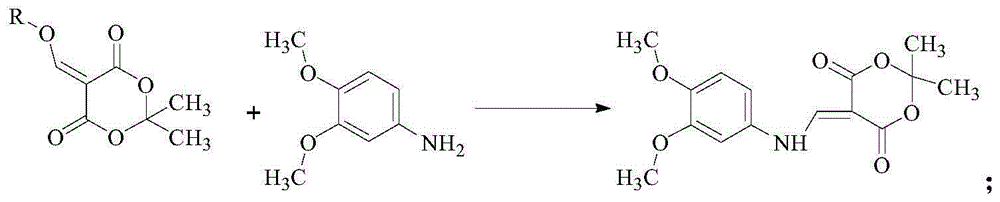
InactiveCN105111141AMild conditions for chemical reactionsHigh yieldOrganic chemistryChemical reactionMalonic acid
The invention provides a cabozantinib intermediate 4-hydroxy-6,7-dimethoxyquinoline and a preparation method thereof. According to the method, ortho-formate, isopropylidene malonate and 3,4-dimethoxyaniline used as raw materials are subjected to condensation and cyclization reaction to obtain the 4-hydroxy-6,7-dimethoxyquinoline. The chemical reaction conditions are milder; the yield for each reaction is up to 90% or so; and the total product yield is 71.3-79.3%. The method has the advantages of cheap and accessible raw materials, high safety, low toxicity, environment friendliness and simple preparation technique, and is suitable for industrial production.
Owner:苏州摩尔医药有限公司
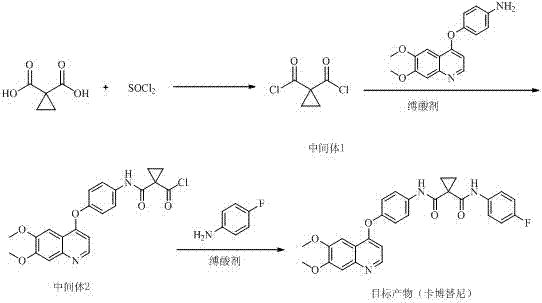
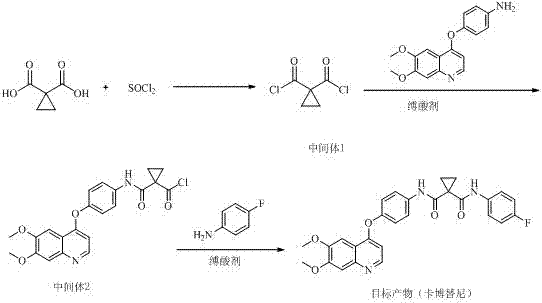
Method for preparing antitumor drug cabozantinib
The invention discloses a method for preparing an antitumor drug cabozantinib and belongs to the field of biological pharmacy. The method disclosed by the invention is realized through the following steps: taking 1,1-cyclopropanedicarboxylic acid and a chlorinating agent to react to generate 1,1-cyclopropyl diacyl chloride (an intermediate 1); and taking the 1,1-cyclopropyl diacyl chloride to react with 4-[(6,7-dimethoxy-4-quinolyl)oxyl]aniline and para-fluoroaniline in sequence to prepare the cabozantinib. Three-step reactions are carried out in the same reactor and an intermediate product does not need to be separated. Compared with the prior art, the method has the characteristics of few reaction steps, few byproducts, simplicity and convenience for operation, high yield and the like.
Owner:仁合熙德隆药业有限公司 +2
Cabozantinib mucate and crystal form there of
ActiveCN104961681AHigh Mucate SolubilityImprove bioavailabilityOrganic chemistry methodsAntineoplastic agentsSolubilityQuinoline
The invention relates to mucate of N-(4-{[6,7-bis(methoxy)quinoline-4-yl]oxy}phenyl)-N'-(4-fluorophenyl)cyclopropane-1,1-dimethanamide and a crystal form thereof, and a preparation method thereof. The solubility of the mucate of a compound as shown in a formula (I) which is described in the specification is higher than the solubility of malate of the compound, which is of important significance to improvement of bioavailability, curative effect and security of the compound.
Owner:CRYSTAL PHARMATECH CO LTD
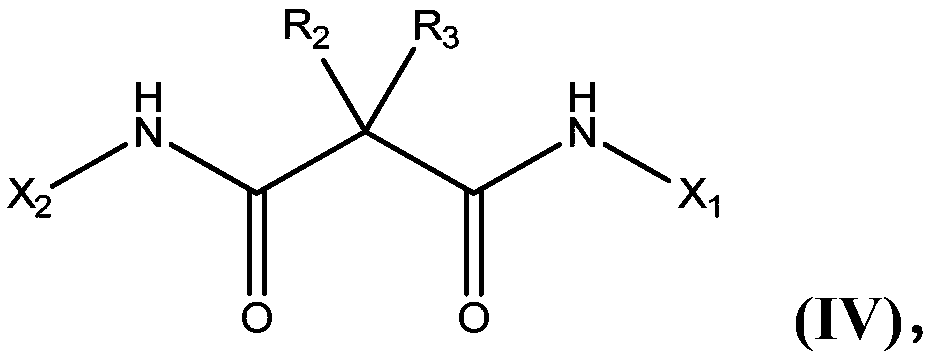
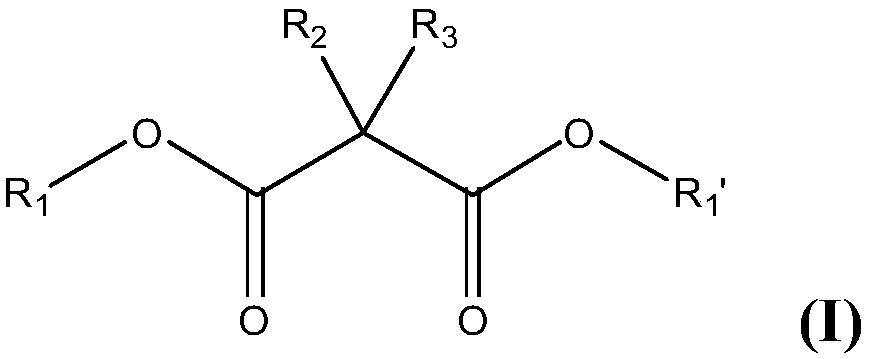
Asymmetric bisamidation of malonic ester derivatives
InactiveCN107646030AOrganic compound preparationCarboxylic acid amides preparationMalonic acidCombinatorial chemistry
The present invention relates to processes, process steps and intermediates useful in the asymmetric bisamidation of malonic ester derivatives wherein the new processes, process steps and intermediates are, for example, useful in the preparation of asymmetric malonic acid bisanilides such as cabozantinib.
Owner:SANDOZ LTD
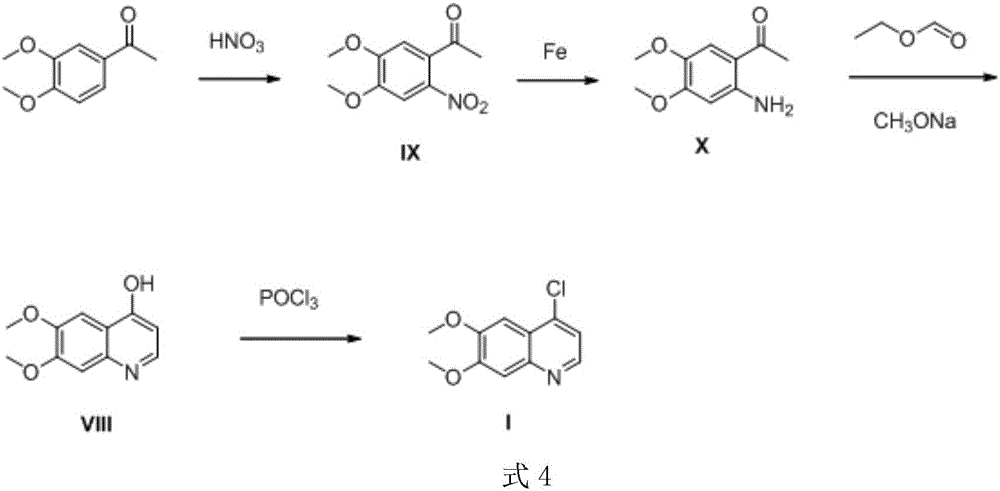
Preparation method of 4-chloro-6,7-dimethoxyquinoline
InactiveCN106008336ARaw materials are easy to getSimple processOrganic chemistryN dimethylformamideAfter treatment
The invention relates to the technical field of organic synthesis and preparation of bulk drug intermediates, particularly a preparation method of 4-chloro-6,7-dimethoxyquinoline. The compound is used as an intermediate for preparing antineoplastic drugs cabozantinib and tivozanib. The method comprises the following steps: (1) nitrification: by using 3,4-dimethoxyacetophenone as a raw material, carrying out nitrification to obtain 2-nitro-4,5-dimethoxyacetophenone; (2) condensation: carrying out condensation on the 2-nitro-4,5-dimethoxyacetophenone and N,N-dimethylformamide dimethyl acetal to obtain 1-(4,5-dimethoxy-2-nitrophenyl)-3-(dimethylamino)propenyl-1-one; (3) reduction cyclization: carrying out hydrogenation reaction on the 1-(4,5-dimethoxy-2-nitrophenyl)-3-(dimethylamino)propenyl-1-oneto obtain 4-hydroxy-6,7-dimethoxyquinoline through reduction cyclization of the molecules; and (4) chlorination: carrying out chlorination on the 4-hydroxy-6,7-dimethoxyquinoline to obtain the 4-chloro-6,7-dimethoxyquinoline. The synthesis route has the advantages of accessible raw materials, mild reaction conditions, simple and convenient after-treatment and high yield, and is suitable for scale-up preparation.
Owner:SHANGHAI UNIV OF ENG SCI
Pharmaceutical compositions of cabozantinib
PendingUS20220362235A1Improve bioavailabilityPowder deliverySolution deliveryDiseasePharmaceutical drug
Pharmaceutical compositions are provided, which comprise cabozantinib or pharmaceutically acceptable salts thereof, and at least one pharmaceutically acceptable excipient, wherein the inventive compositions exhibit enhanced bioavailability compared to the currently marketed or commercially available formulations. The present invention also provides manufacturing processes thereof and use of the said inventive compositions for the prevention, treatment or prophylaxis of disorders in human patients in need thereof. The present invention relates to oral pharmaceutical compositions of cabozantinib, methods for their administration, processes for their production, and use of these compositions for treatment of diseases treatable by cabozantinib.
Owner:SLAYBACK PHARMA LLC
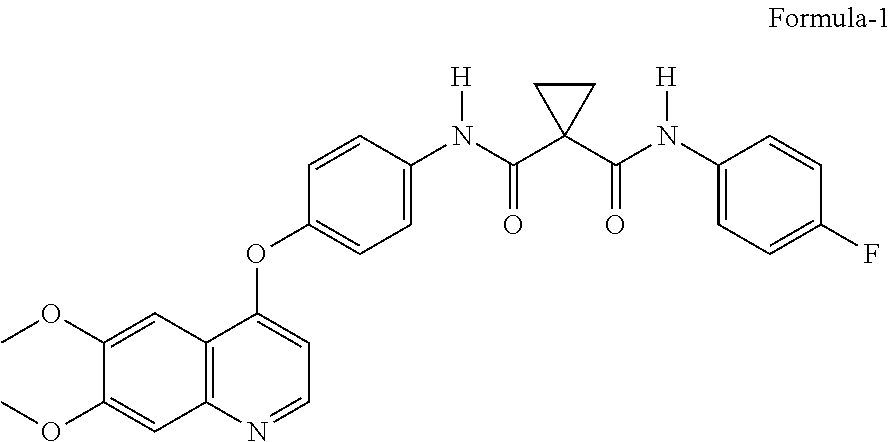
Crystalline Solid Forms of N-{4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, Processes for Making, and Methods of Use
The invention relates to novel crystalline solid forms of the chemical compound N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide (Compound 1), and solvates thereof, including hydrates, that are useful for the treatment of cancer. Also disclosed are pharmaceutical compositions comprising the crystalline solid forms and processes for making the crystalline solid forms, as well as methods of using them for the treatment of cancer, particularly thyroid cancer, prostate cancer, hepatocellular cancer, renal cancer, and non-small cell lung carcinoma. The crystalline solid forms can be used to make the L-malate salt of cabozantinib.
Owner:EXELIXIS INC
Method for preparing antitumor drug cabozantinib intermediate by solid base catalysis
InactiveCN108658856AAvoid it happening againNo pollution in the processOrganic chemistryPhysical/chemical process catalystsChemical industryM-aminophenol
The invention belongs to the technical field of catalytic materials, and in particular relates to a method for preparing an antitumor drug cabozantinib intermediate by solid base catalysis. Accordingto the method provided by the invention, Zr is used as a third metal element to modify Mg / Al bimetal, a Zr-doped Mg / Al composite metal hydroxide is prepared by a co-precipitation method, then ion exchange is carried out through potassium carbonate, high-temperature calcination is carried out, and finally reforming is carried out in water to finally prepare a Zr-doped Mg / Al composite metal oxide solid base with a three-dimensional structure. The solid base prepared by the method provided by the invention can replace sodium hydroxide to catalyze the preparation of 4-chloro-6,7-dimethoxyquinolinewhich is condensed with 4-aminophenol to prepare 4-(4-aminophenoxy)-6,7-dimethoxyquinoline, an antitumor drug cabozantinib intermediate. The catalytic system provided by the invention is green and pollution-free, avoids the generation of alkaline waste water and has a high yield and meets the requirements of green chemical industry.
Owner:张淑华
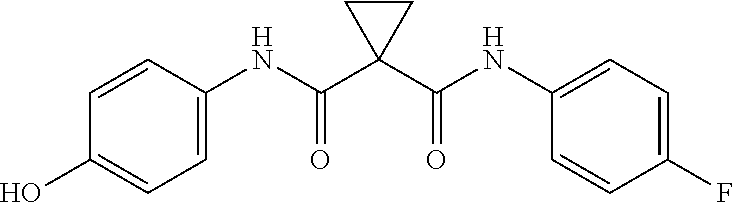
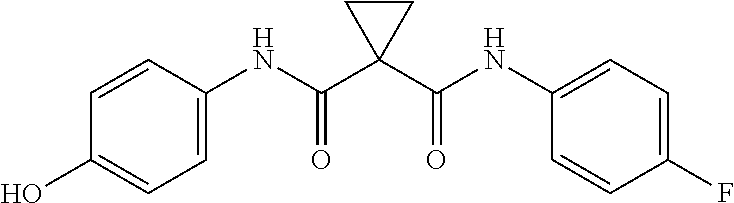
Anti-tumor drug cabozantinib impurity, preparation method thereof and application thereof
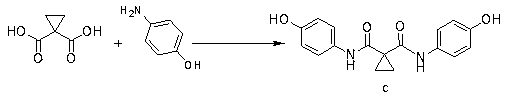
The invention discloses an antitumor drug cabozantinib impurity, a preparation method and application thereof and belongs to the technical field of biological medicine. The structural formula of the anti-tumor drug cabozantinib impurity is shown in the specification. Meanwhile, the invention also provides a synthesis method of a compound. The method comprises the following synthesis steps: (1) dissolving cyclopropane-carboxylic acid and an acid-binding agent in a solvent, activating by a condensing agent, adding p-aminophenol, and carrying out a condensation reaction to obtain a compound c; and (2) dissolving the compound c in the solvent, adding alkali to form a salt with the compound c, adding a compound d, and carrying out a substitution reaction to obtain a target compound II.
Owner:LEPU PHARMACEUTICAL CO LTD
Deuterated cabozantinib derivative, preparation method and application thereof, and intermediate of deuterated cabozantinib derivative
ActiveCN104788372AExtended half-lifeExtended stayOrganic chemistryAntineoplastic agentsSide effectHalf-life
The invention belongs to the technical field of pharmaceutical chemistry, and discloses a deuterated cabozantinib derivative, a preparation method and an application thereof, and an intermediate of the deuterated cabozantinib derivative. The deuterated cabozantinib derivative is a compound represented by formula I and may be a pharmaceutically acceptable salt, a crystalline hydrate, a solvate, a prodrug, or a monocrystalline of polymorphic substance. The decomposition speed of the deuterated cabozantinib derivative in vivo is slow, so that the poor metabolism of the medicine is reduced; the half life of the medicine is prolonged; the blood concentration of the medicine is increased; and a better therapeutic effect is achieved. Furthermore, the dosage of the medicine is decreased while keeping the therapeutic effect, and the toxic and side effect of the medicine is further reduced.
Owner:河南英诺唯医药科技有限公司
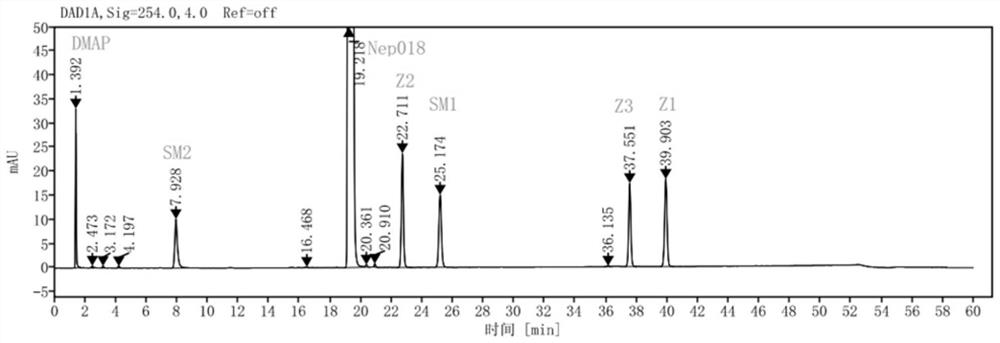
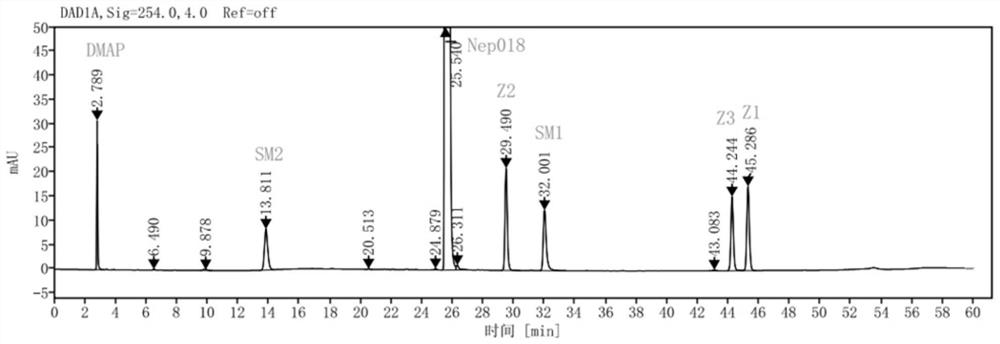
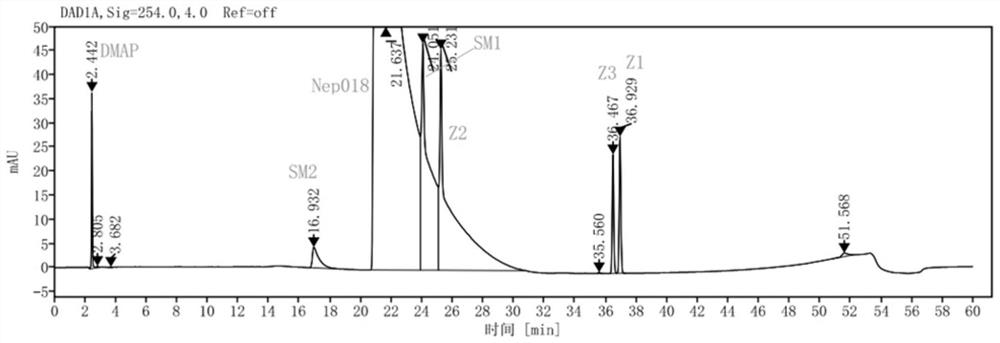
Method for simultaneously determining cabozantinib analogue and related substances thereof
ActiveCN114354789AEfficient separationHigh sensitivityComponent separationBulk chemical productionChromatography columnOctadecane
The invention discloses a high performance liquid chromatography analysis method for simultaneously determining cabozantinib analogues and related substances thereof, and the chromatographic conditions are as follows: a chromatographic column is an octadecyl silane bonded silica gel column, acetate buffer solution-acetonitrile (70: 30) is used as a mobile phase A, acetonitrile is used as a mobile phase B, gradient elution is performed, the concentration of the acetate buffer solution is 0.005-0.01 mol / L, the pH value is 8.5-9.5, the column temperature is 25-35 DEG C, the flow velocity is 0.9-1.1 ml / min, the column temperature is 25-35 DEG C, the column temperature is 25-35 DEG C, the column temperature is 25-35 DEG C, the column temperature is 25-35 DEG C, the column temperature is 25-35 DEG C, the column temperature is 25-35 DEG C, the column temperature is 25-35 DEG C, and the column temperature is the detection wavelength is 254 nm, the temperature of a sample injector is 2-20 DEG C, and the sample injection amount is 10 microliters. The determination method provided by the invention can rapidly and accurately detect the cabozantinib analogue and the related substances thereof, is simple to operate, good in reproducibility and high in sensitivity, can better control the quality of a cabozantinib analogue bulk drug product, and provides a guarantee for optimization of a synthesis process.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
A kind of preparation method of tyrosine kinase inhibitor and its intermediate
ActiveCN103664776BMild reaction conditionsSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationQuinolineTyrosine-kinase inhibitor
The invention relates to a preparation method for a tyrosine kinase inhibitor and a midbody thereof. According to the method, a compound 1,1-cyclopropane dicarboxylic acid diester is taken as a raw material, and 1-((4-((6,7-dimethoxy quinoline-4-yl) oxy) phenyl) carbamoyl) cyclopropane formic ether is prepared by two ways and reacts with p-fluoro aniline after being hydrolyzed so as to prepare Cabozantinib. The reaction conditions of the preparation method are mild, the synthesis cost is lowered, and the preparation method is simple and convenient to operate and is applicable to industrial production.
Owner:正大天晴(广州)医药有限公司
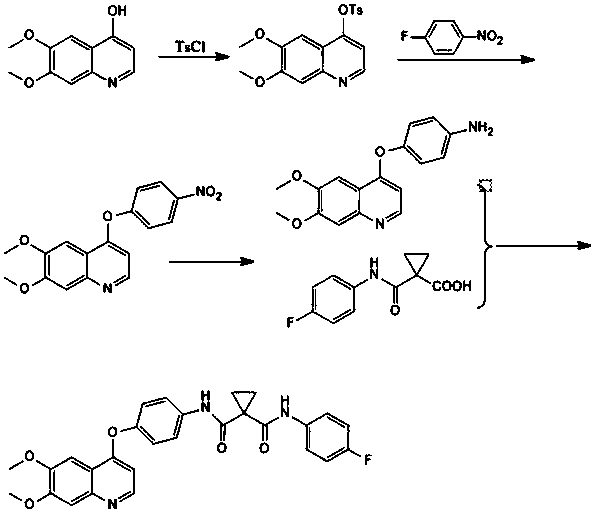
Preparation method of cabozantinib
InactiveCN108264482AHigh reaction yieldImprove response qualityOrganic chemistryP-fluoronitrobenzeneCarboxylic acid
The invention relates to a preparation method of cabozantinib. The method comprises the following steps: taking 5,6-dimethoxy-4-hydroxyquinoline, TsCl, fluoronitrobenzene, and 1-(4-fluorophenyl)amino-carbonyl cyclopropane carboxylic acid as raw materials, and performing steps of a hydroxy protection reaction, a coupling reaction, a reduction reaction, and a condensation reaction to obtain the cabozantinib. The preparation method of cabozantinib has the advantages of high yield, low cost, less three wastes, easy operation, and safety, and is suitable for industrialization.
Owner:南京法恩化学有限公司
Liquid Dosage Forms to Treat Cancer
ActiveUS20200268737A1Dispersion deliveryPharmaceutical non-active ingredientsRenal Cell CancersPharmaceutical drug
This invention relates to a liquid pharmaceutical composition comprising cabozantinib to treat locally advanced or metastatic solid tumors, particularly advanced urothelial cancer or renal cell carcinoma in patients in need thereof.
Owner:EXELIXIS INC
Preparation method of multi-receptor tyrosine kinase inhibitor and intermediate thereof
PendingCN109836381AOrganic chemistryReceptor tyrosine kinase inhibitorReceptor Tyrosine Kinase Inhibitors
The invention relates to a preparation method of cabozantinib. The method includes: taking 4-chloro-6, 7-dimethoxyquinoline (I) as the starting raw material, and conducting substitution and condensation to obtain cabozantinib. Compared with other preparation methods, the preparation method provided by the invention has the advantages of cheap and easily available raw materials, mild reaction conditions, high total yield and high product purity, at the same time avoids high temperature production, reduces risk, simplifies operation, and is more beneficial to industrial production.
Owner:连云港恒运药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
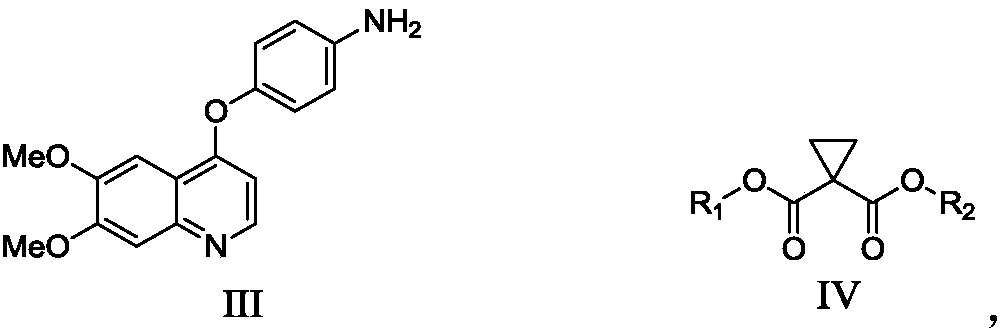
![Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use](https://images-eureka.patsnap.com/patent_img/3ea48ff4-46e9-4662-9819-cf9dff241981/US10501418-D00001.png)
![Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use](https://images-eureka.patsnap.com/patent_img/3ea48ff4-46e9-4662-9819-cf9dff241981/US10501418-D00002.png)
![Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use Crystalline solid forms of N-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N′-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, processes for making, and methods of use](https://images-eureka.patsnap.com/patent_img/3ea48ff4-46e9-4662-9819-cf9dff241981/US10501418-D00003.png)
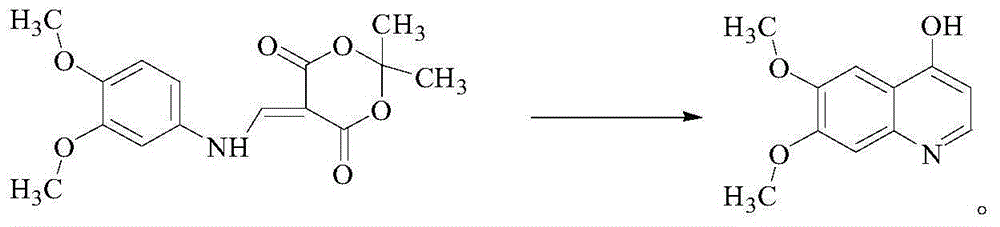
![Crystalline Solid Forms of N-{4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, Processes for Making, and Methods of Use Crystalline Solid Forms of N-{4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, Processes for Making, and Methods of Use](https://images-eureka.patsnap.com/patent_img/db80cd24-b2c0-421d-9b1d-0f4d969407da/US20210206722A1-D00001.png)
![Crystalline Solid Forms of N-{4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, Processes for Making, and Methods of Use Crystalline Solid Forms of N-{4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, Processes for Making, and Methods of Use](https://images-eureka.patsnap.com/patent_img/db80cd24-b2c0-421d-9b1d-0f4d969407da/US20210206722A1-D00002.png)
![Crystalline Solid Forms of N-{4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, Processes for Making, and Methods of Use Crystalline Solid Forms of N-{4-[(6,7-Dimethoxyquinolin-4-yl)oxy]phenyl}-N'-(4-fluorophenyl) cyclopropane-1,1-dicarboxamide, Processes for Making, and Methods of Use](https://images-eureka.patsnap.com/patent_img/db80cd24-b2c0-421d-9b1d-0f4d969407da/US20210206722A1-D00003.png)