Asymmetric bisamidation of malonic ester derivatives
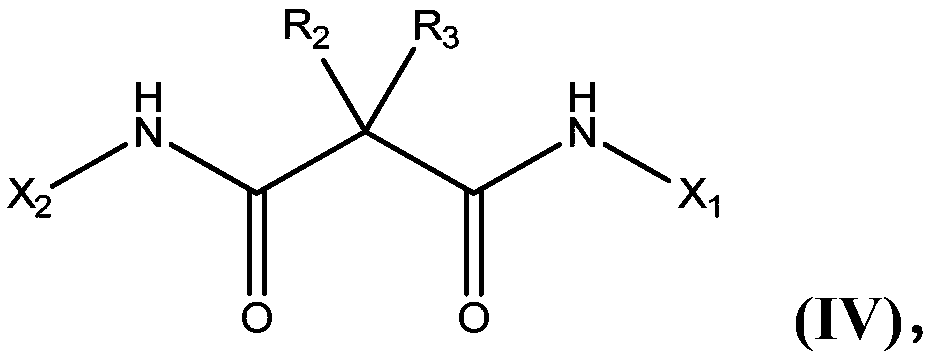
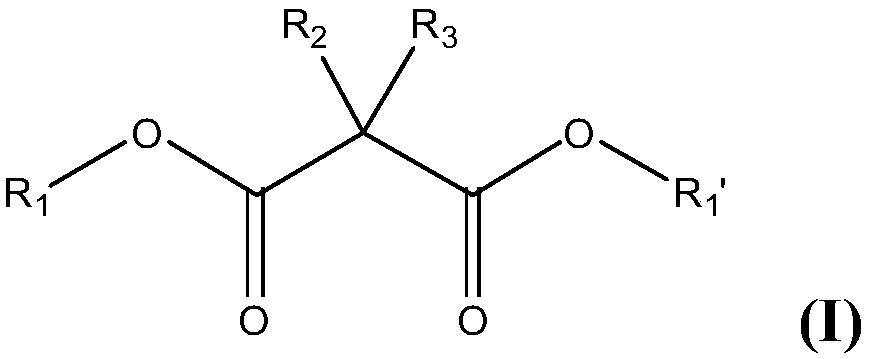
A malonic acid diamide, asymmetric technology, applied in the preparation of carboxylic acid amides, the preparation of organic compounds, organic chemistry, etc., can solve problems such as undisclosed asymmetric dianiline, and achieve the effect of excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
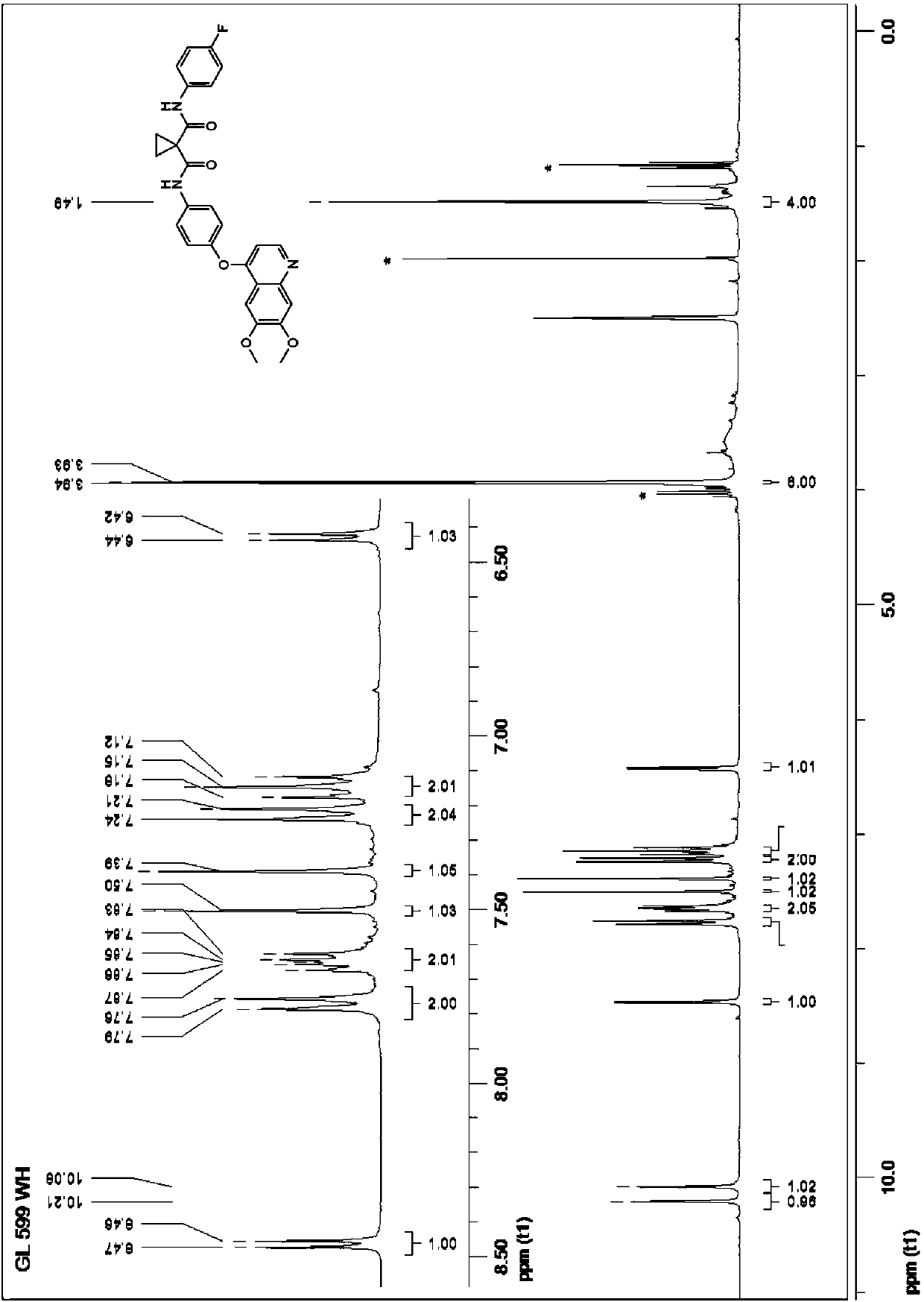
[0807] Embodiment 1: Preparation of 1-((4-fluorophenyl) carbamoyl) methyl cyclopropanecarboxylate
[0808] 4-Fluoroaniline (1.11 g, 10 mmol) was dissolved in toluene (12 mL), and sodium methoxide (0.54 g, 10 mmol) was added. The mixture was then stirred under an inert atmosphere for 30 minutes, with the bath temperature raised to 80 to 100° C., and about 2 mL of the azeotropic methanol / toluene mixture was evaporated. Then, a solution of dimethyl cyclopropane-1,1-dicarboxylate (1.58 g, 10 mmol) in toluene (5 mL) was added and the azeotropic distillation was continued for a further 30 minutes under stirring and inert gas flow. Upon cooling to room temperature, water (10 mL) and 1N hydrochloric acid (10 mL 1M) were added, and the mixture was stirred for 10 minutes, extracted with dichloromethane (10 mL). The organic layer was dried over magnesium sulfate and the solvent was evaporated. The residue (2.07 g) was extracted with isopentane (12 x 10 mL). Evaporation of the solvent ...
Embodiment 2
[0809] Example 2: N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phenyl)-N-(4-fluoro-phenyl)cyclo-propane-1,1 - Preparation of dimethylformamide (cabotinib)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com