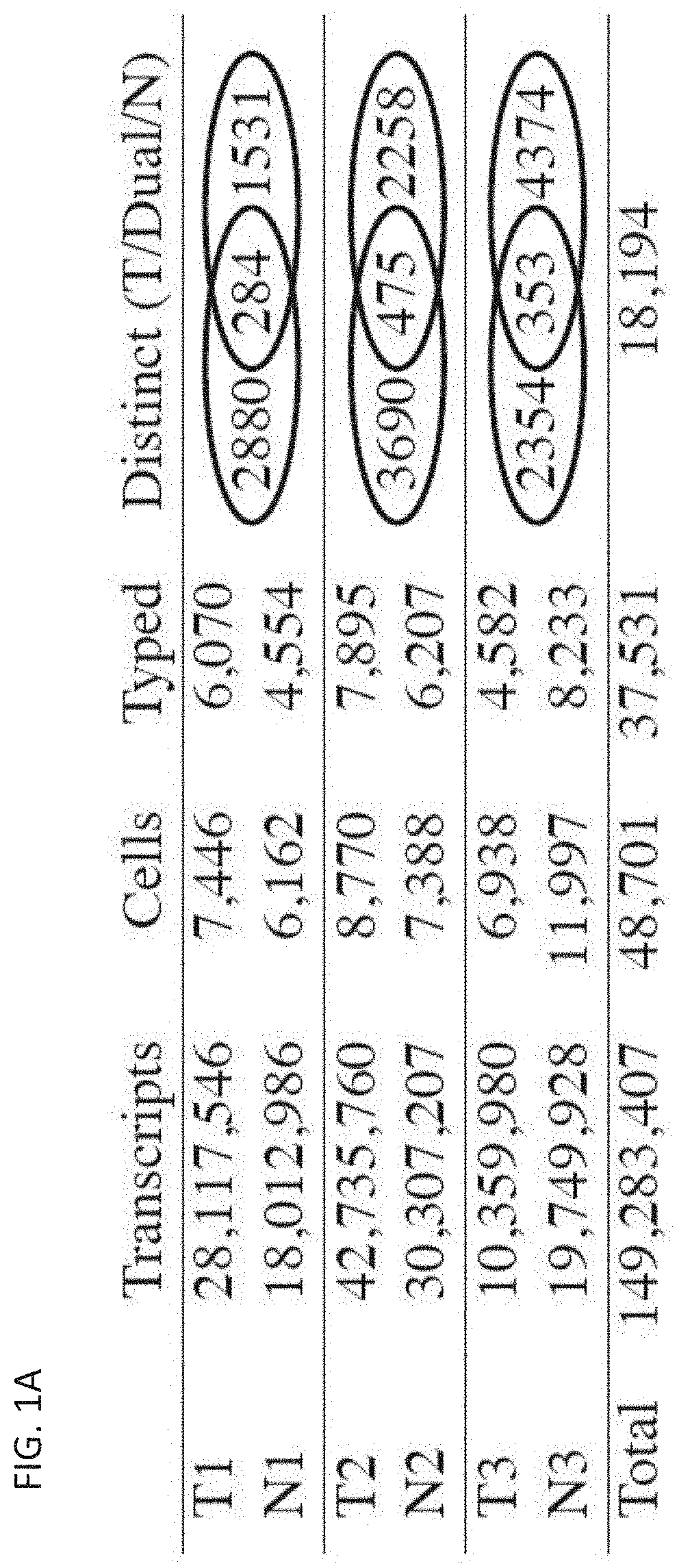
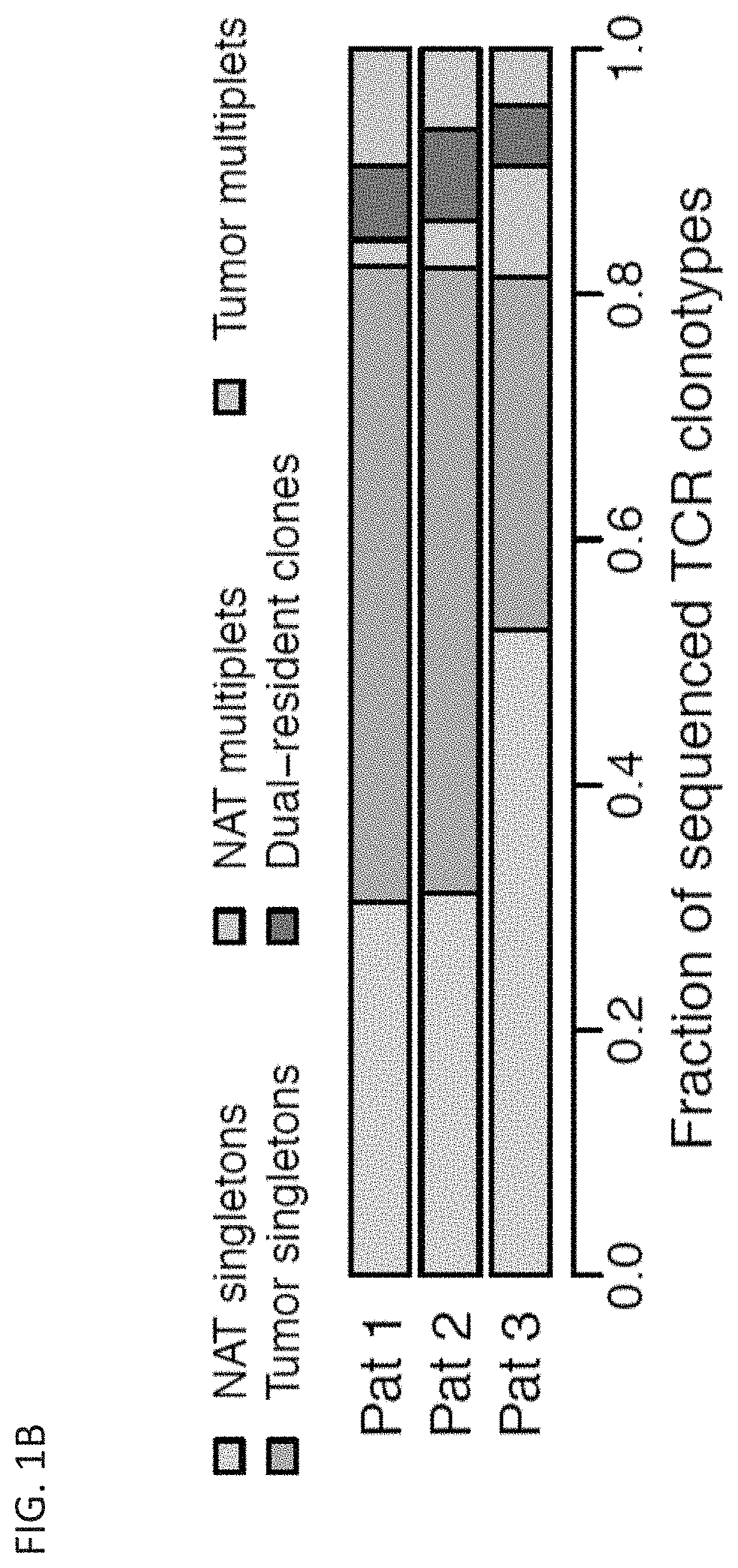
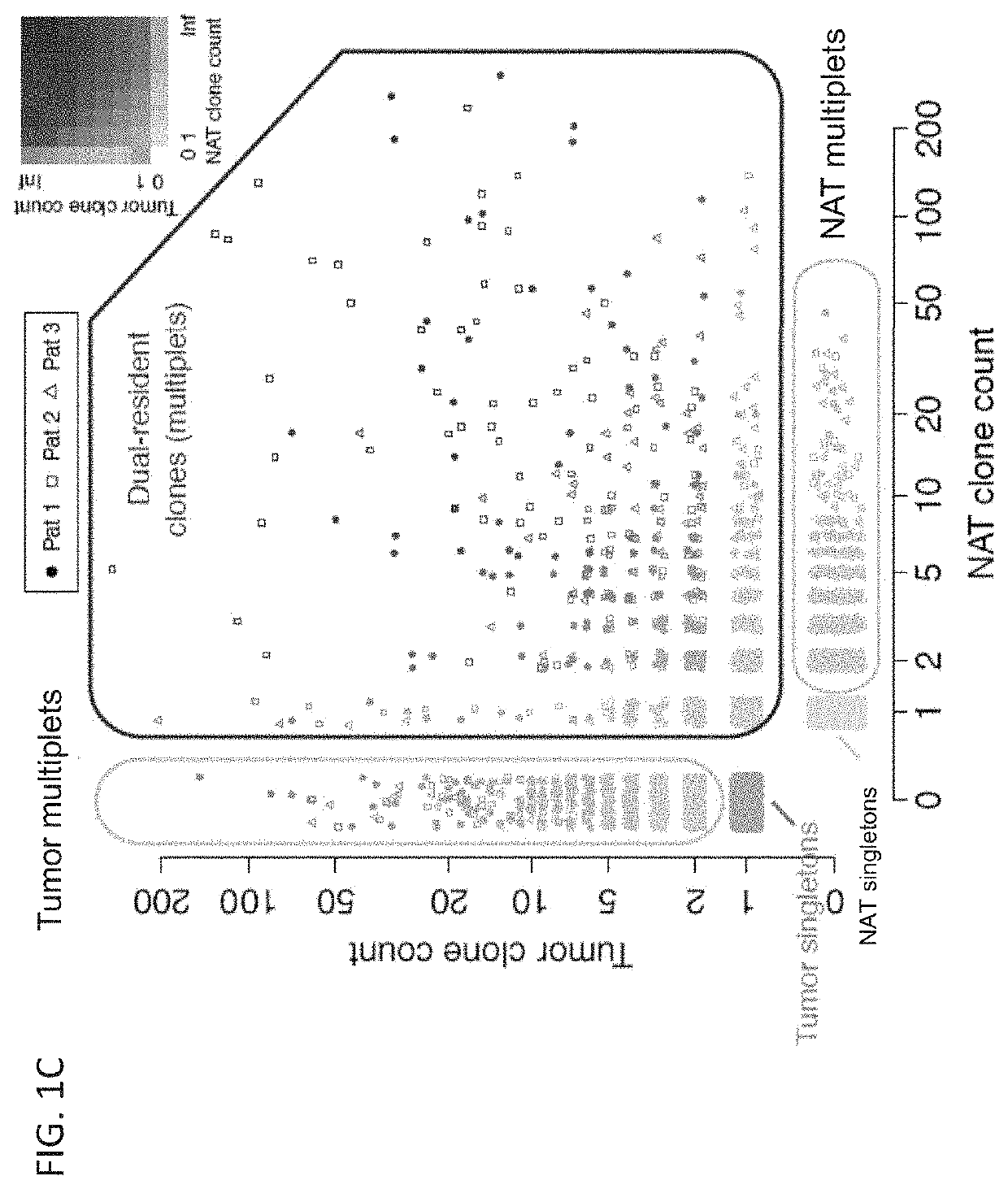
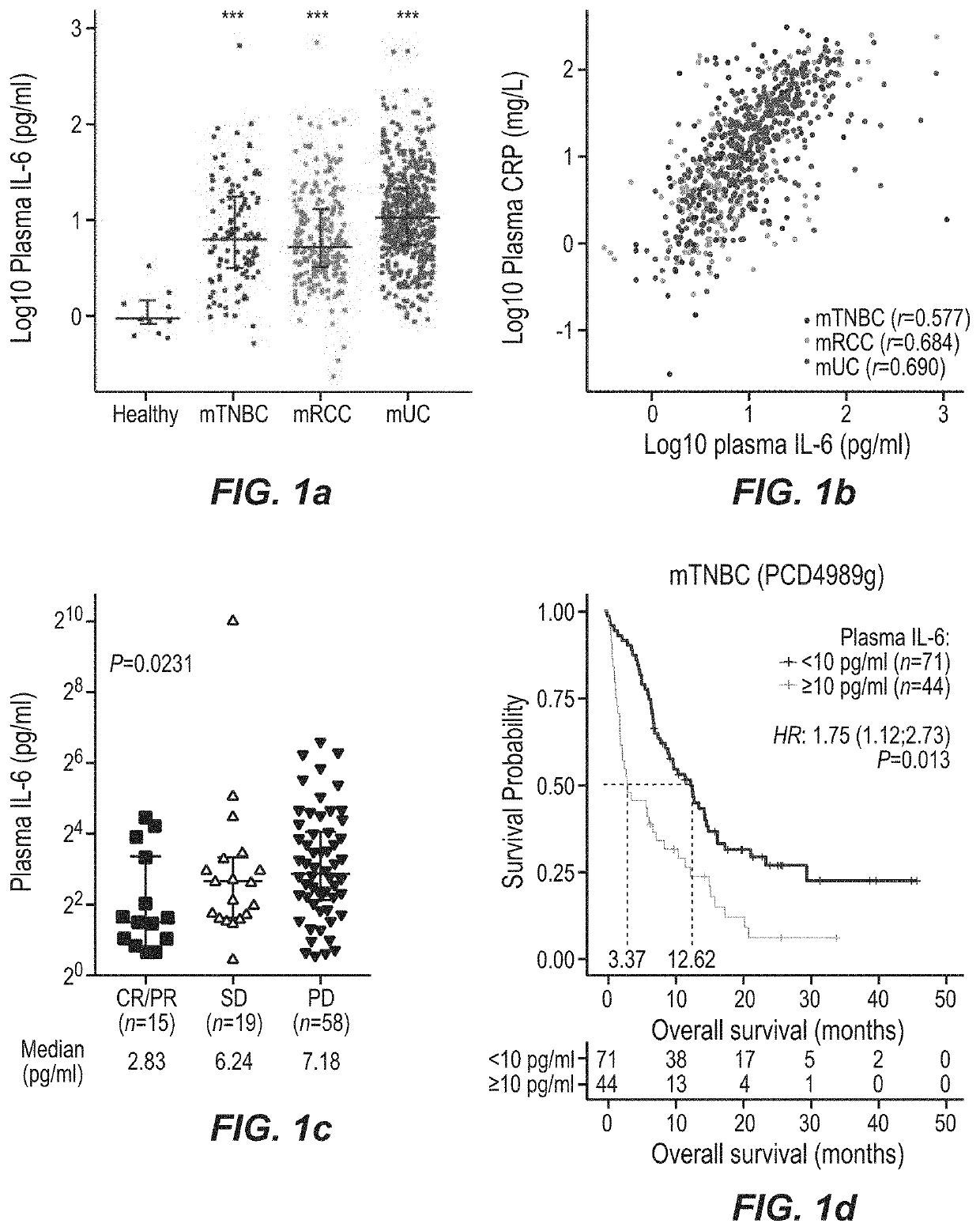
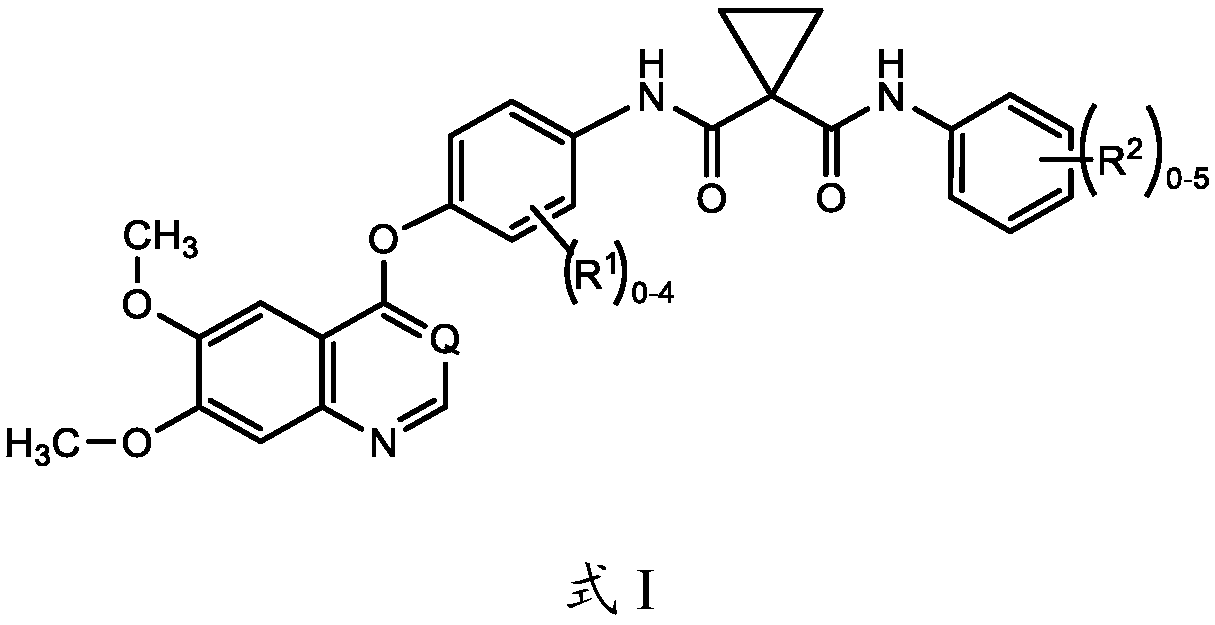
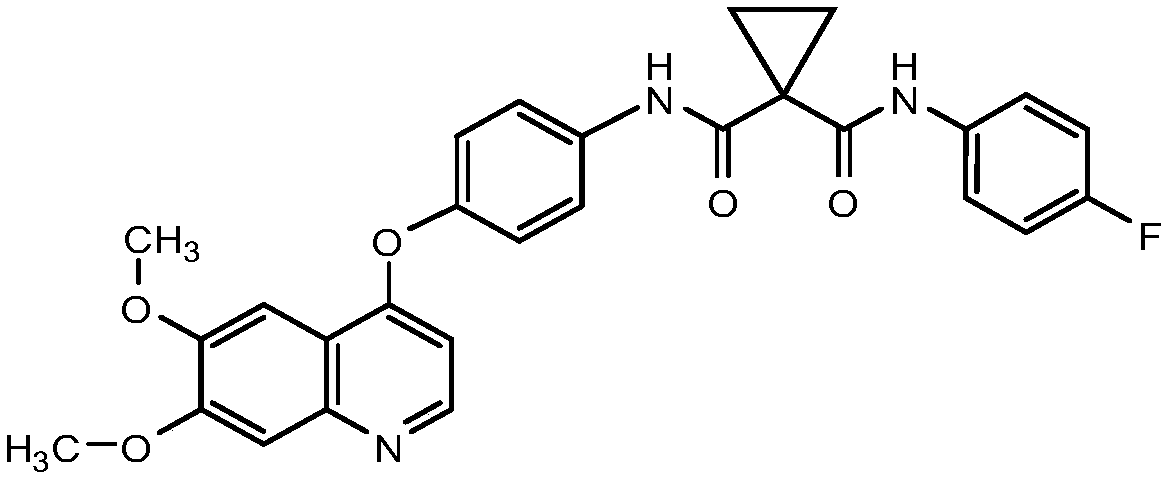
Patents
Literature
30 results about "Atezolizumab" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Atezolizumab (trade name Tecentriq) is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).
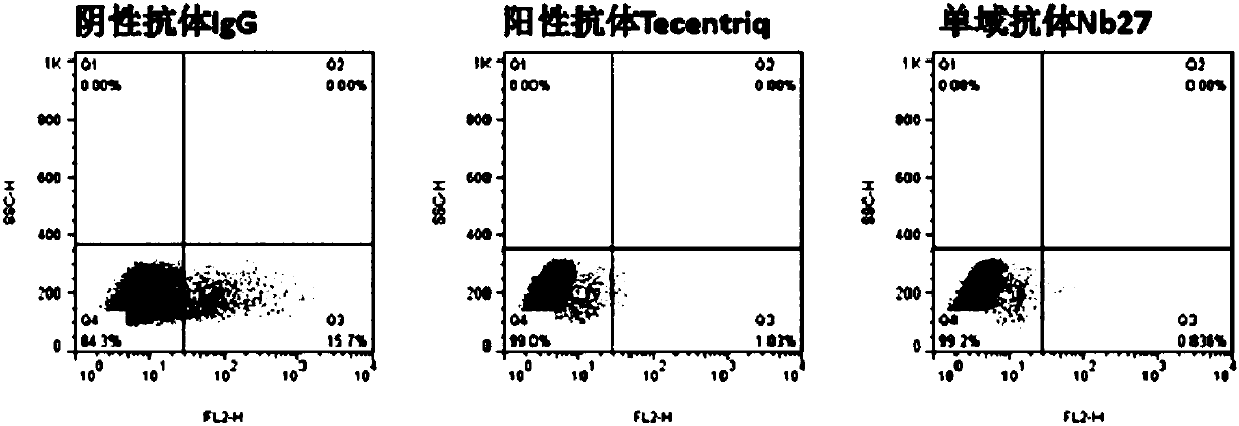
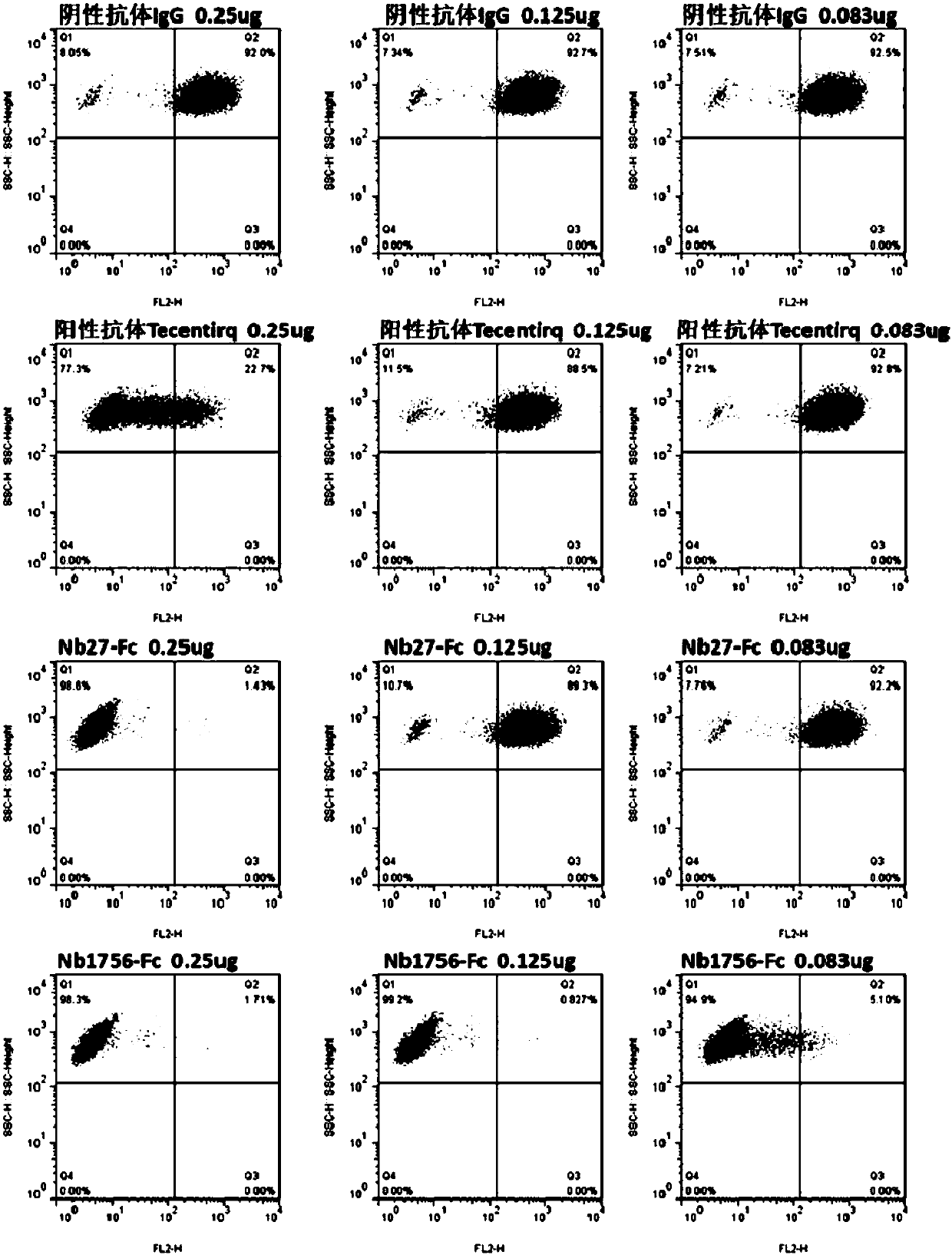
Blocking type PD-L1 camel-source single-domain antibody and use thereof
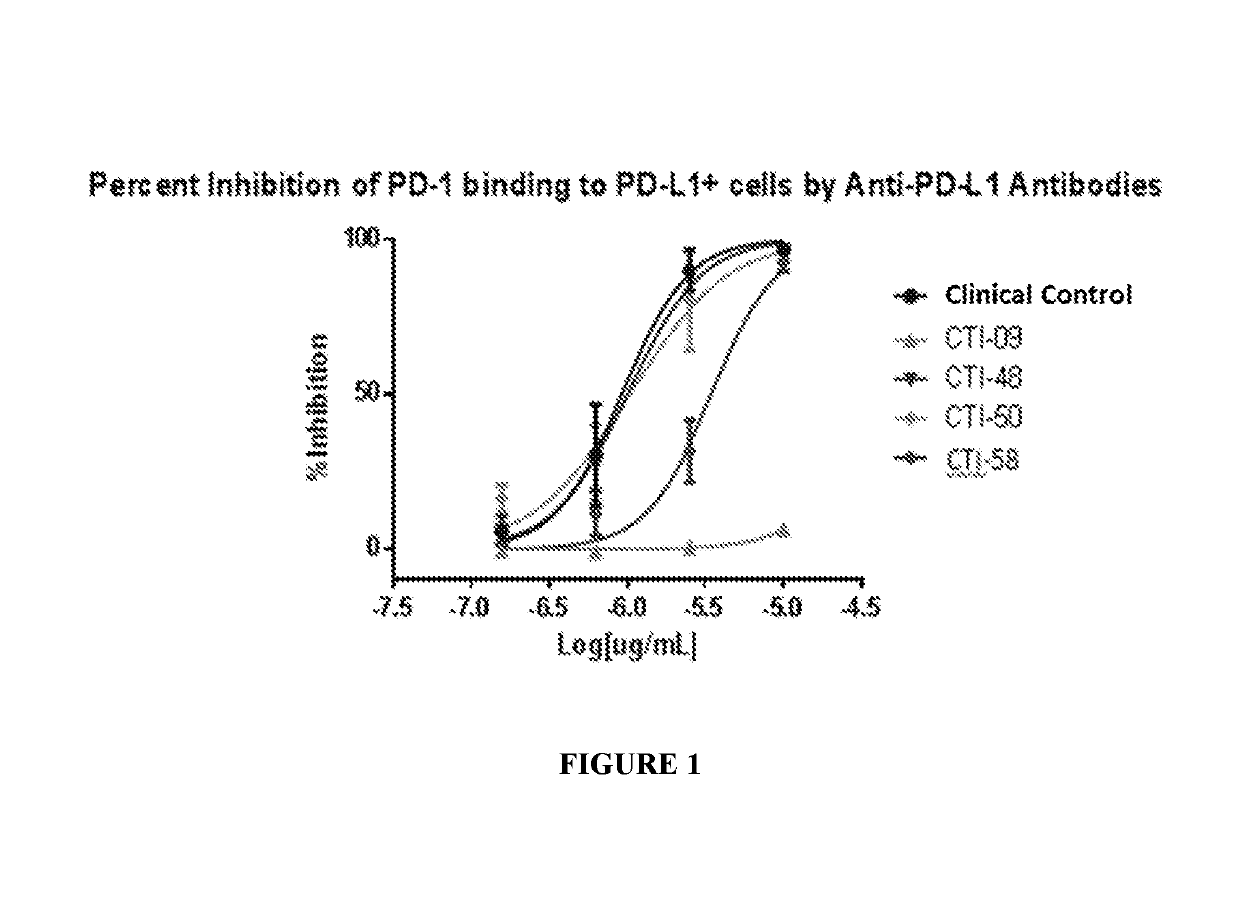
The invention relates to a camel-source single-domain antibody. The antibody specifically recognizes programmed death receptor-ligand 1(PD-L1), the interaction of PD-L1 and the receptor PD-1 thereof can be effectively blocked, and the blocking effect is better than that of atezolizumab which is sold in the market. Specifically speaking, the invention discloses a PD-L1 camel-source single-domain antibody, a coding sequence of derived protein of the PD-L1 camel-source single-domain antibody, and a preparation method and use of the derived protein.
Owner:SHANGHAI NOVAMAB BIOPHARM CO LTD
Diagnostic and therapeutic methods for cancer
PendingCN111213059AMicrobiological testing/measurementDisease diagnosisAntiendomysial antibodiesCancer research
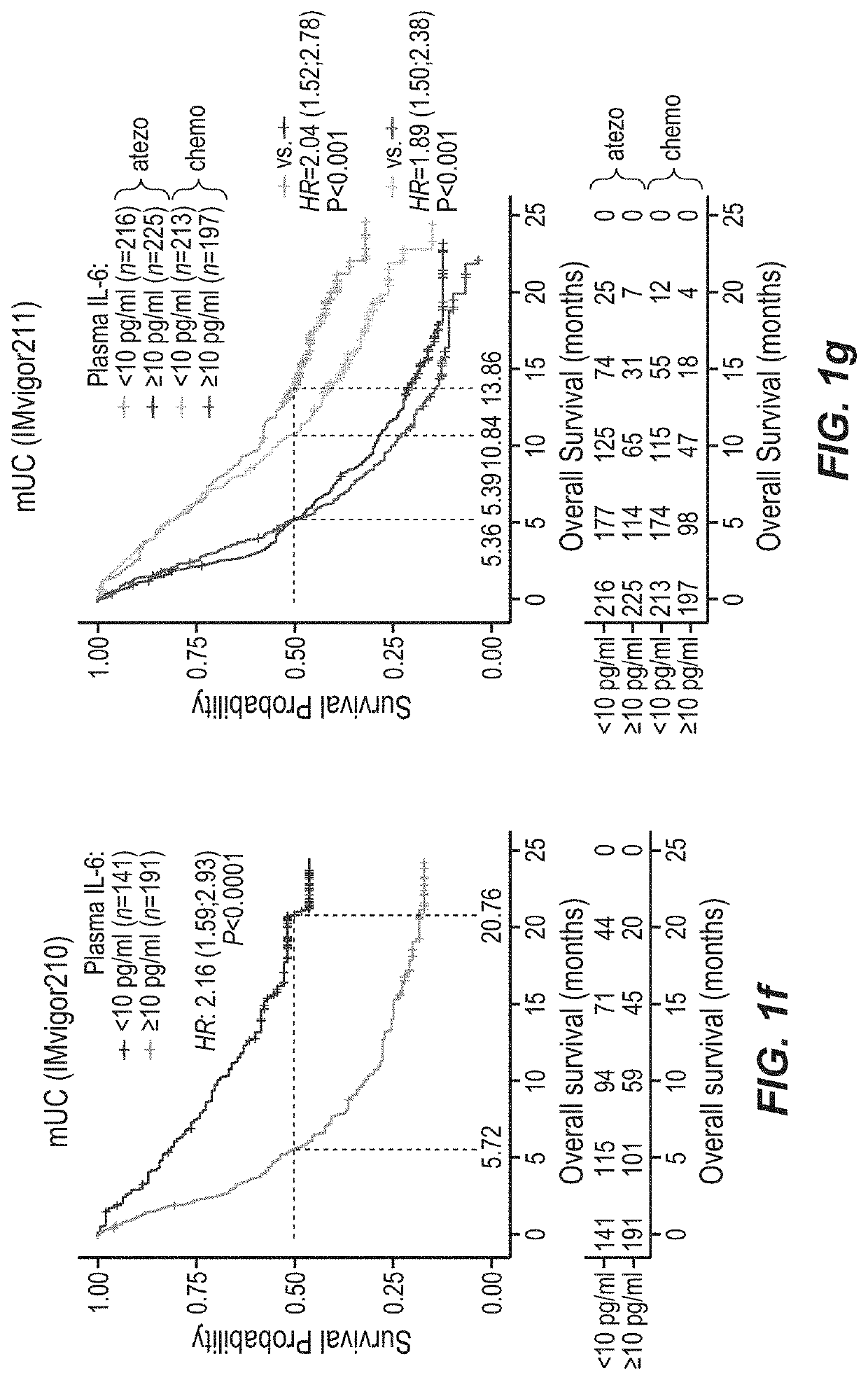
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., a bladder cancer (e.g., UC, e.g., mUC), a kidney cancer, a lung cancer, a liver cancer, an ovarian cancer, a pancreatic cancer, a colorectal cancer, or a breast cancer). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of identifying an individual having a cancer who may benefit with an anti-cancer therapy that includes an immunotherapy (e.g., a PD-L1 axis binding antagonist such as an anti-PD-L1 antibody (e.g., atezolizumab)) and a suppressive stromal antagonist (e.g., a TGF-beta antagonist), methods for selecting a therapy for an individual having cancer, methods of treating an individual having cancer, methods for assessing a response or monitoring the response of an individual to treatment with an anti-cancer therapy that includes an immunotherapy (e.g., a PD-L1 axis binding antagonist such as an anti-PD-L1 antibody (e.g., atezolizumab)) and a suppressive stromal antagonist (e.g., a TGF-beta antagonist), and related kits, anti-cancer therapies, and uses.
Owner:F HOFFMANN LA ROCHE & CO AG
Diagnostic and therapeutic methods for cancer
ActiveUS20190369098A1Organic active ingredientsMicrobiological testing/measurementTyrosine kinaseKidney cancer
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., kidney cancer (e.g., renal cell carcinoma (RCC)), lung cancer (e.g., non-small cell lung cancer (NSCLC)), bladder cancer (e.g., urothelial bladder cancer (UBC)), liver cancer (e.g., hepatocellular carcinoma (HCC)), ovarian cancer, or breast cancer (e.g., triple-negative breast cancer (TNBC))). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a VEGF antagonist (e.g., an anti-VEGF antibody, (e.g., bevacizumab) or a VEGFR inhibitor (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib))) and a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)), or with an angiogenesis inhibitor (e.g., a VEGF antagonist (e.g., a VEGFR inhibitor, (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib)))).
Owner:GENENTECH INC
Diagnostic and therapeutic methods for cancer
ActiveUS20200263261A1Microbiological testing/measurementAntibody ingredientsAntiendomysial antibodiesCancer research
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., a bladder cancer (e.g., UC, e.g., mUC), a kidney cancer, a lung cancer, a liver cancer, an ovarian cancer, a pancreatic cancer, a colorectal cancer, or a breast cancer). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of identifying an individual having a cancer who may benefit with an anti-cancer therapy that includes an immunotherapy (e.g., a PD-L1 axis binding antagonist such as an anti-PD-L1 antibody (e.g., atezolizumab)) and a suppressive stromal antagonist (e.g., a TGF-β antagonist), methods for selecting a therapy for an individual having cancer, methods of treating an individual having cancer, methods for assessing a response or monitoring the response of an individual to treatment with an anti-cancer therapy that includes an immunotherapy (e.g., a PD-L1 axis binding antagonist such as an anti-PD-L1 antibody (e.g., atezolizumab)) and a suppressive stromal antagonist (e.g., a TGF-β antagonist), and related kits, anti-cancer therapies, and uses.
Owner:GENENTECH INC
Diagnostic and therapeutic methods for cancer
PendingCN110621787AOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesEfficacy
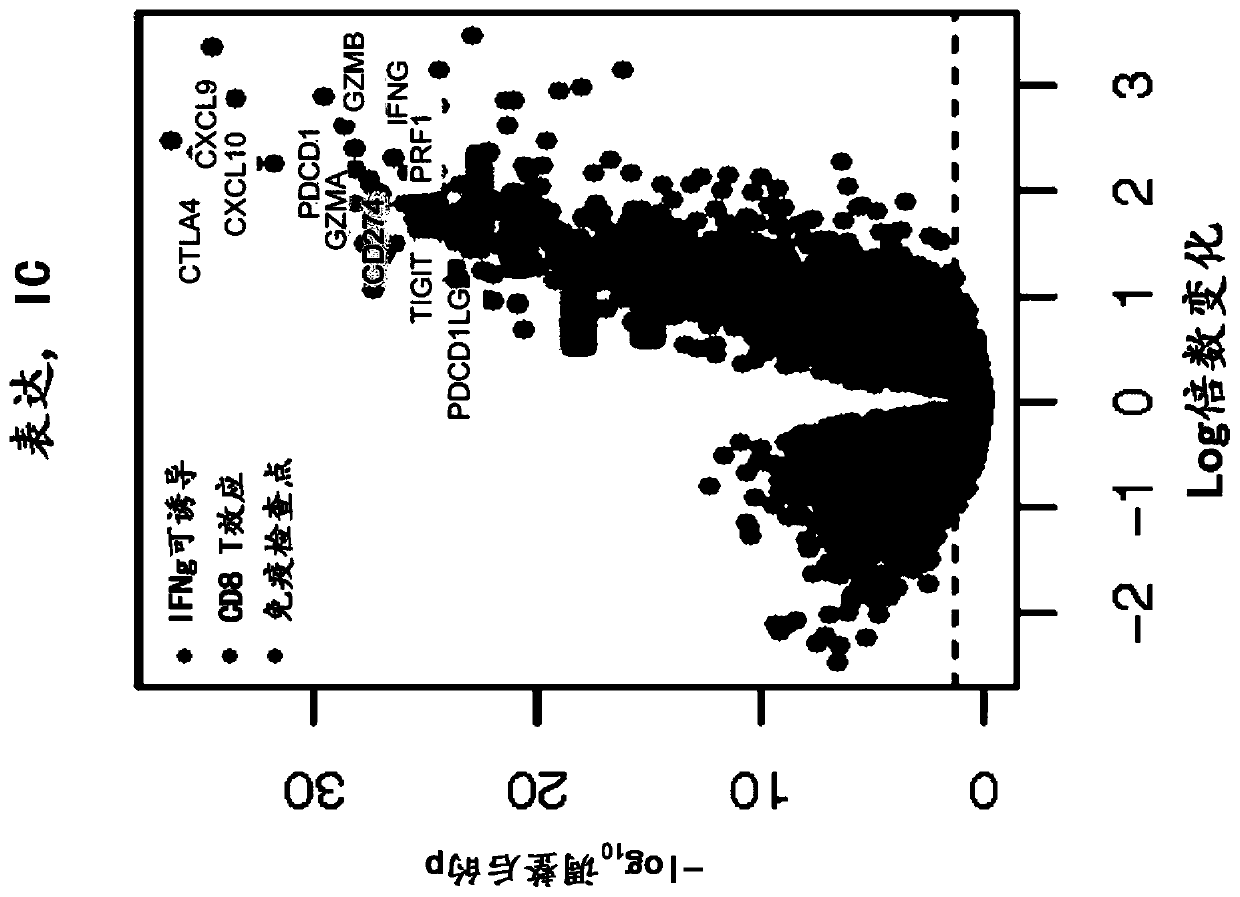
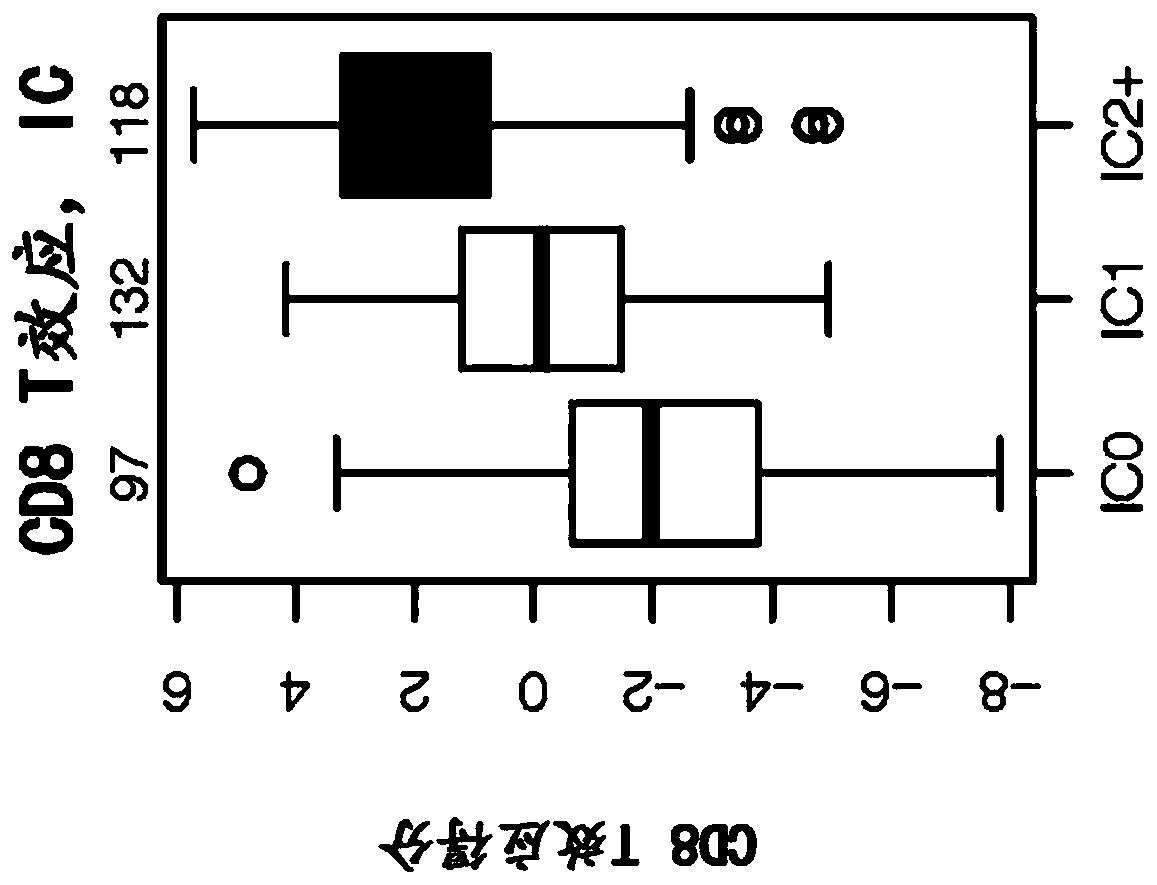
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The invention is based, at least in part, on the discovery that an immune-score expression level based on one or more of PD-L1, CXCL9, IFNG, GZMB, CD8A, and PD-1 in a sample obtained from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)).
Owner:F HOFFMANN LA ROCHE & CO AG
Diagnostic methods and compositions for cancer immunotherapy
PendingCN113260633AOrganic active ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesAntigen Binding Fragment
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The compositions and methods described herein can be used, for example, to determine the propensity of a patient to benefit from treatment with a PD-L1 axis binding antagonist and to treat such patients accordingly. Using the compositions and methods of the disclosure, a patient, such as a human cancer patient, may be determined to be likely to benefit from treatment with a PD-L1 axis binding antagonist if the patient exhibits an elevated pre-treatment expression level of one or more of CST7, NKG7, GZMH, MT-ND4, HLA-H, CCL5, CD8A, CMC1, CD8B, HCST, MT-CYB, MT-ND4L, KLRG1, MT-CO2, MT-ATP6, PLEK, CTSW, HLA-C, LYAR, LITAF, GZMB, KLRD1, FGFBP2, KLRC4-KLRK1, KLRK1, B2M, GZMA, ID2, CX3CR1, PRSS23, GNLY, PRF1, and PATL2. Exemplary PD-L1 axis binding antagonists that may be used in conjunction with the compositions and methods of the disclosure are PD-L1 binding antagonists, such as anti-PD-L1 antibodies and antigen-binding fragments thereof, including atezolizumab, as well as PD-1 binding antagonists, such as anti-PD-1 antibodies and antigen-binding fragments thereof.
Owner:F HOFFMANN LA ROCHE & CO AG
Combination of an Anti-cd20 antibody, pi3 kinase-delta inhibitor, and Anti-pd-1 or Anti-pd-l1 antibody for treating hematological cancers
InactiveCN110191720AOrganic active ingredientsPharmaceutical delivery mechanismAntiendomysial antibodiesOncology
The present disclosure provides methods and kits for treating or slowing the progression of a hematological malignancy, by administering to a subject in need thereof a therapeutically effective amountof: (i) at least one inhibitor of PI3 kinase (PI3K)-delta (e.g., TGR-1202); (ii) at least one anti-CD20 antibody (e.g., ublituximab); and (iii) at least one anti-PD-1 antibody (e.g., pembrolizumab) or anti-PD-L1 antibody (e.g., atezolizumab). Treatment regimens are also provided.
Owner:TG治疗有限公司 +2
Application of anti-PD-1 antibody and/or anti-PD-L1 antibody in preparation of medicine for treating Parkinson's disease
PendingCN111956799AReduce aggregationSymptoms improvedNervous disorderAntibody ingredientsAntiendomysial antibodiesAlpha-synuclein
The invention relates to the technical field of medicines, in particular to an application of an anti-PD-1 antibody and / or an anti-PD-L1 antibody in preparation of a medicine for treating the Parkinson's disease. It is found that the anti-PD-1 antibody and / or the anti-PD-L1 antibody can reduce alpha synuclein aggregation, and the anti-PD-1 / PD-L1 antibodies, namely, pembrolizumab, nivolumab, cemiplimab, toripalimab, sintilimab, camrelizumab, tirelizumab, atezolizumab, durvalumab and avenlimab all can reduce alpha synuclein aggregation. In specific clinical application cases, it is found that through intravenous injection or oral administration of the anti-PD-1 / PD-L1 antibody, the Parkinson's disease can be improved, and development of the disease condition can be effectively prevented.
Owner:生物抗素公司
Methods for treating cancers
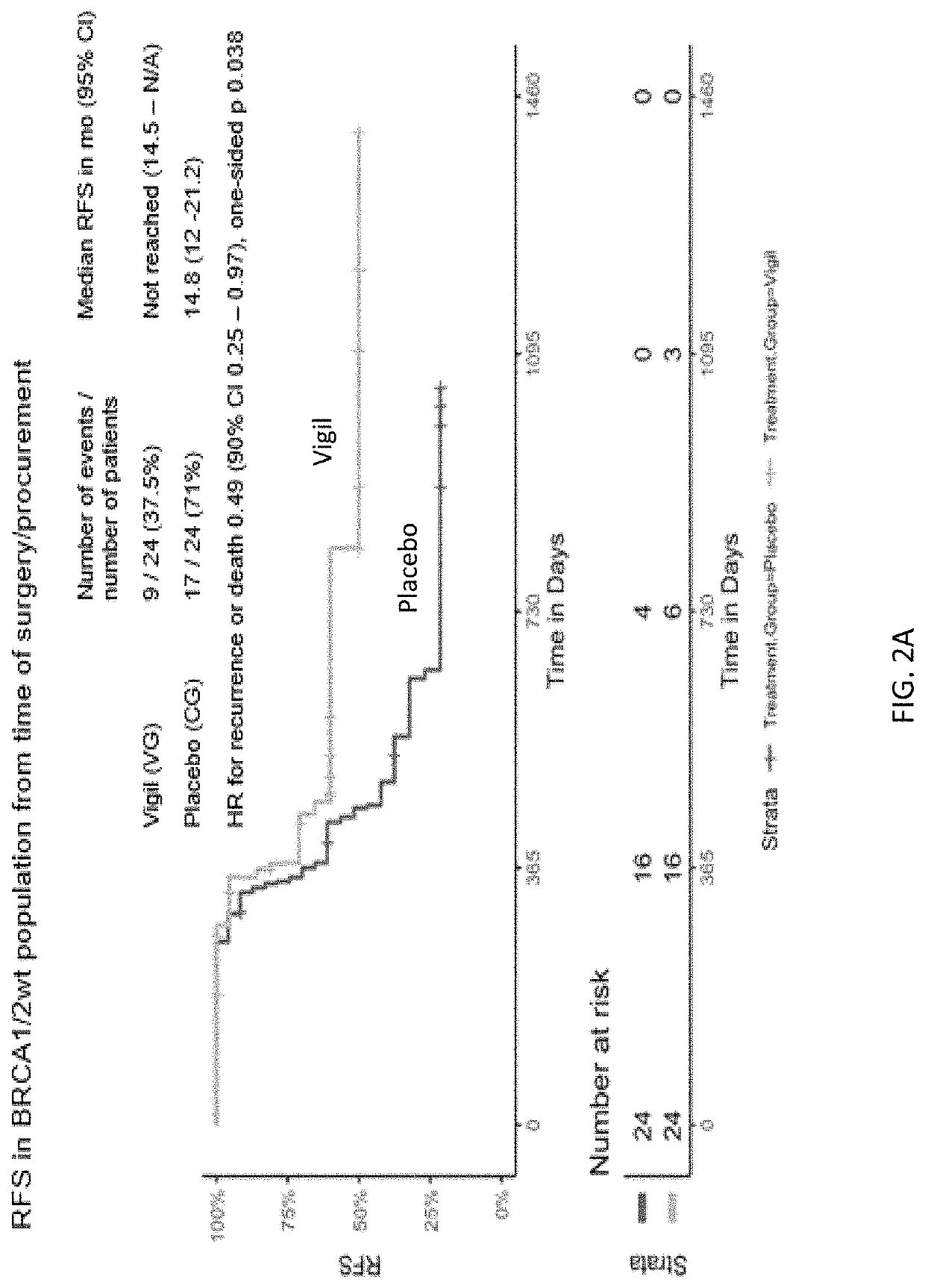
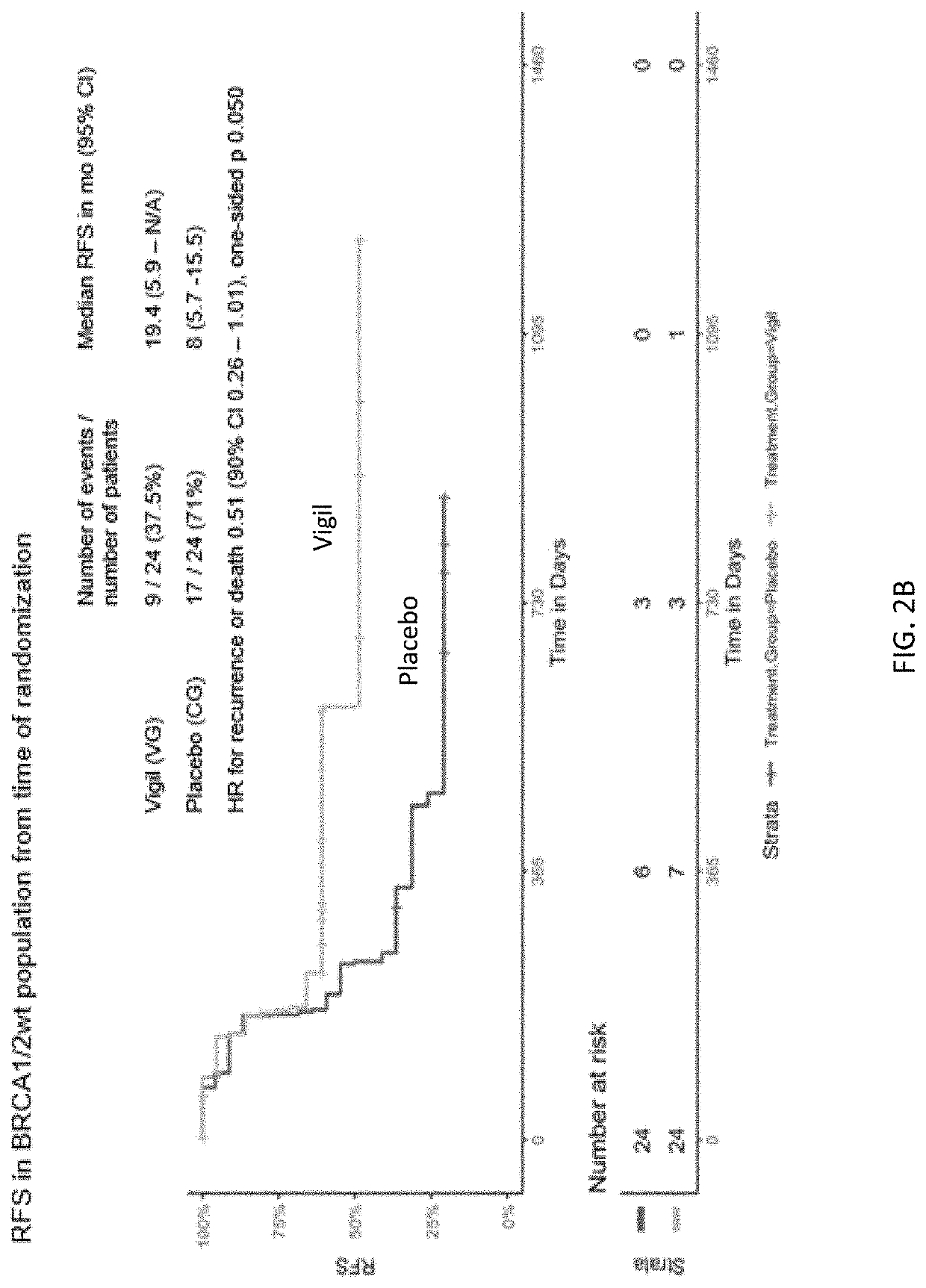
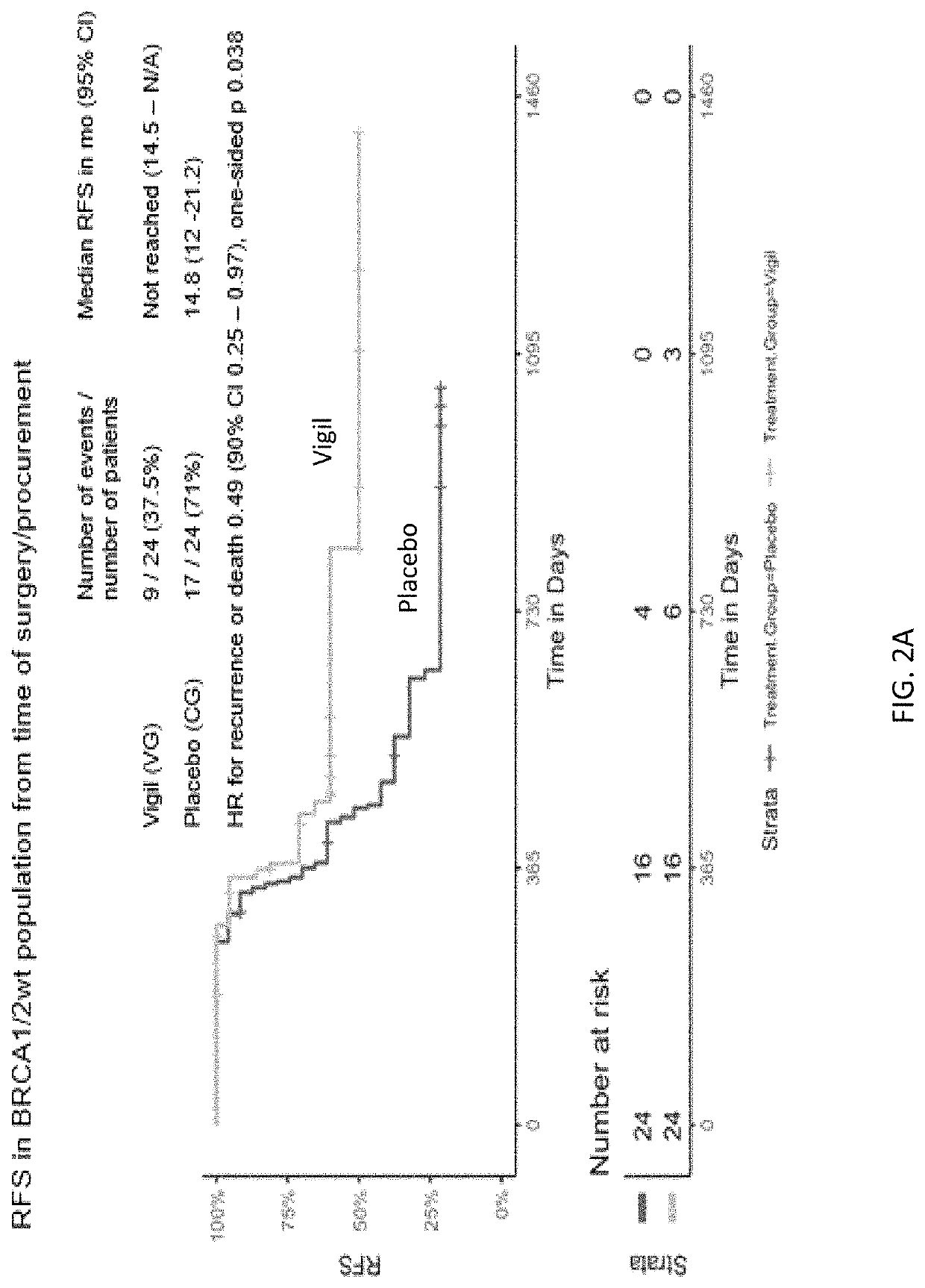
Compositions and methods for prevention of ovarian cancer recurrence and for the treatment of BRCA1 / 2-wild type ovarian cancer are disclosed herein. In some embodiments, the composition comprises an autologous tumor cell vaccine comprising cells genetically modified for furin knockdown and GM-CSF expression. In some embodiments, the method comprises administration of an autologous tumor cell vaccine prior to administration of a combination of the autologous tumor cell vaccine and atezolizumab. Also disclosed herein are methods for treating a cancer in an individual comprising a wild-type BRCA1 gene, a wild-type BRCA2 gene, or a combination thereof, and is identified as homologous recombination deficiency (HRD)-negative.
Owner:GRADALIS
Covalent protein drugs developed via proximity-enabled reactive therapeutics (PERX)
PendingCN113179631AGood effectSmall doseOrganic active ingredientsPeptide preparation methodsAntiendomysial antibodiesIrreversible binding
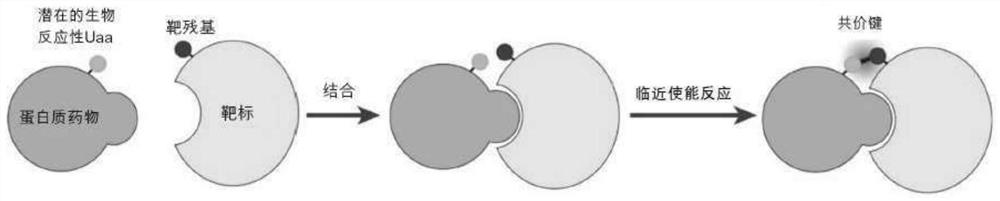
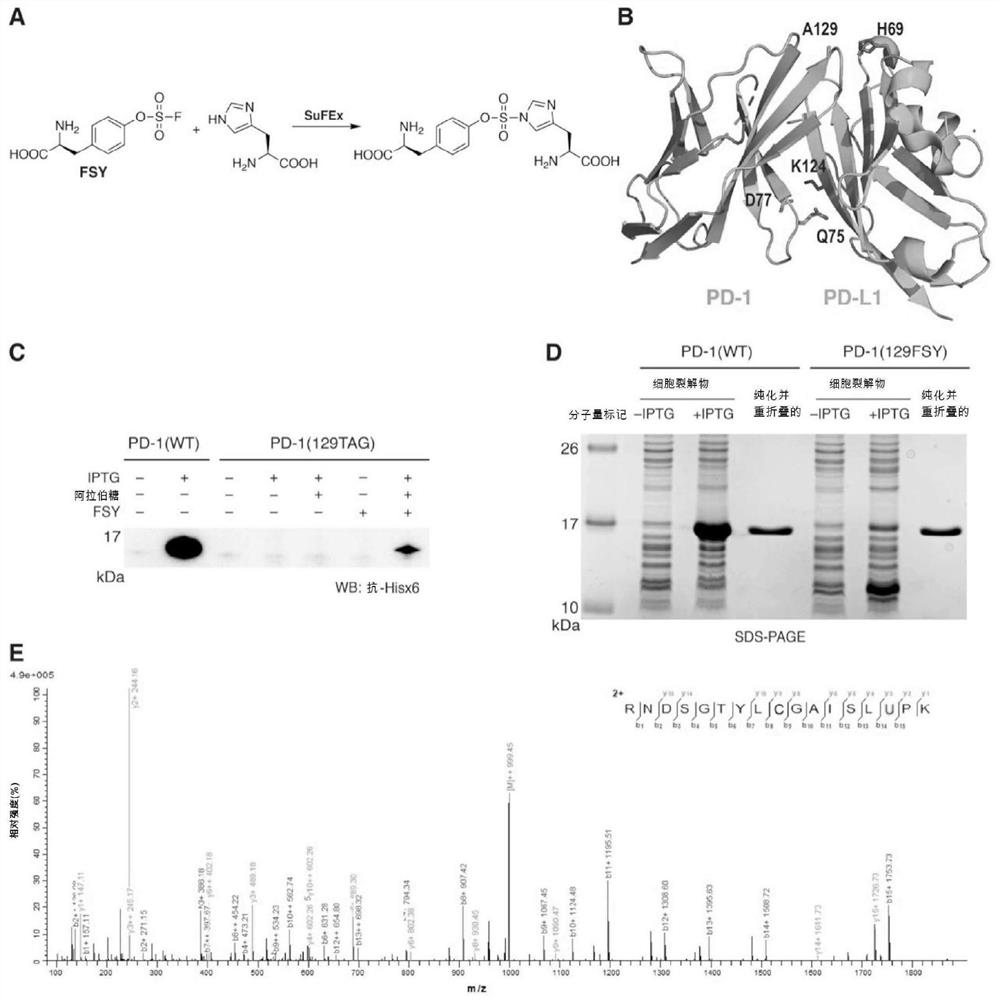
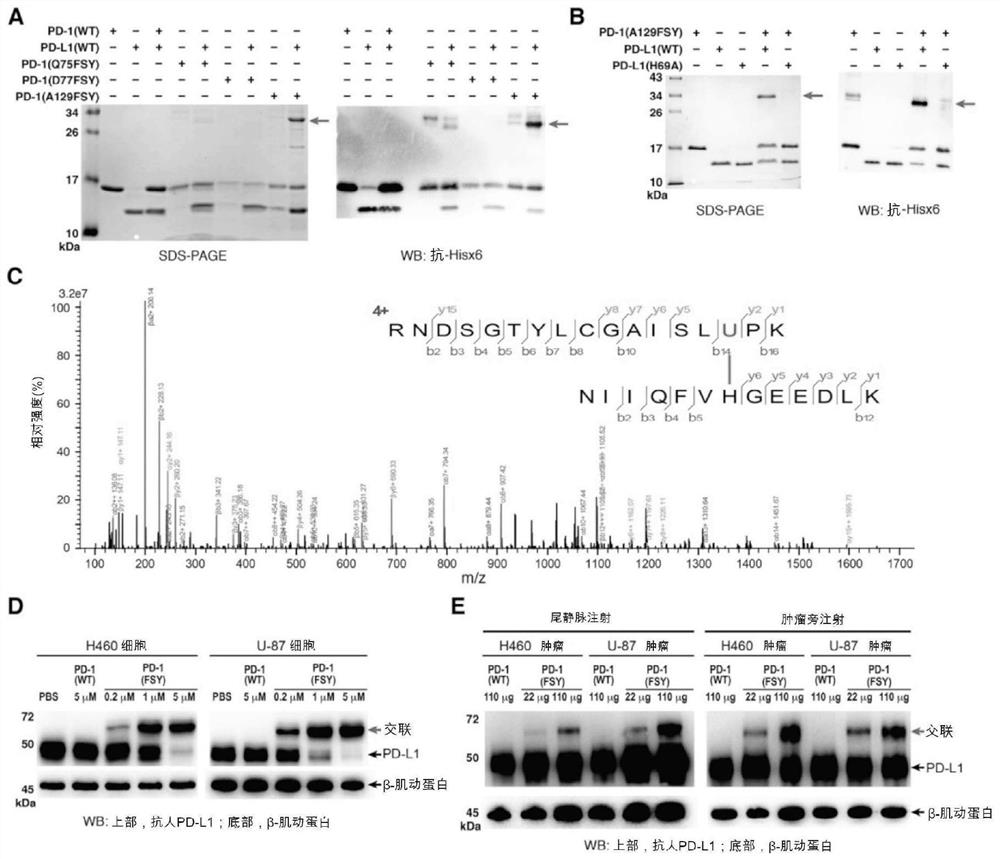
Provided a proximity-enabled reactive therapeutics (PERx) approach to generate covalent protein drugs. A latent bioreactive amino acid FSY was incorporated into human programmed cell death protein 1 (PD-1), which selectively reacted with a proximal histidine of human PD-L1 upon binding, enabling irreversible binding of PD-1 with PD-L1 in vitro, on cancer cells, and in tumor in mice. When administrated in humanized mouse models, the covalent PD-1 (FSY) exhibited robust antitumor effect over wildtype PD-1, achieving therapeutic efficacy equivalent to the FDA approved therapeutic monoclonal antibody atezolizumab. The PERx approach should provide a general method for converting interacting proteins into covalent protein drugs for high therapeutic efficacy.
Owner:HANGZHOU BRANCH OF TECH INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Diagnostic and therapeutic methods for cancer
PendingUS20200157635A1Improve responsivenessResponsiveness to treatmentOrganic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The invention is based, at least in part, on the discovery that an immune-score expression level based on one or more of PD-L1, CXCL9, IFNG, GZMB, CD8A, and PD-1 in a sample obtained from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)).
Owner:GENENTECH INC
Treatment of triple negative breast cancer or colorectal cancer with liver metastases with anti pd-l1 antibody and oncolytic virus
PendingCN111246883APharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAtezolizumab
Provided herein are methods of treating a subject with triple negative breast cancer or colorectal cancer. In exemplary embodiments, the method comprises administering to the subject a combination ofan oncolytic virus, such as talimogene laherparepvec, and an anti-PD-Ll antibody, such as atezolizumab. In exemplary aspects, the oncolytic virus is administered to the subject at an initial dose followed by a second dose, wherein the initial dose is lower than the second dose. In exemplary aspects, the oncolytic virus is intrahepatically administered to the subject.
Owner:AMGEN INC +1
Methods of treating lung cancer with a pd-1 axis binding antagonist, an antimetabolite, and a platinum agent
PendingCN112839644AInorganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesOncology
The present disclosure provides methods for treating lung cancer (such as non-small cell lung cancer, e.g., Stage IV non-squamous non-small cell lung cancer) in an individual. The methods comprise administering to the individual a PD-1 axis binding antagonist (such as an anti-PD-L1 antibody, e.g., atezolizumab), an antimetabolite (e.g., pemetrexed), and a platinum agent (e.g., cisplatin or carboplatin).
Owner:F HOFFMANN LA ROCHE & CO AG
Diagnostic and therapeutic methods for cancer
ActiveUS11402382B2Organic active ingredientsMicrobiological testing/measurementAntiendomysial antibodiesEfficacy
Owner:GENENTECH INC
Biopharmaceutical formulation of Anti-pd-1, Anti-pd-l1, and Anti-vegfr therapeutic monoclonal antibodies and method for treating nsclc by inhalation
InactiveUS20210403568A1Activity loss is largeDispersion deliveryInorganic non-active ingredientsAntiendomysial antibodiesTreatment of lung cancer
This invention relates to pharmaceutical formulations of therapeutic monoclonal antibody drugs and pharmaceutically acceptable excipients and a novel therapeutic strategy for the treatment of lung cancers including metastatic NSCLC by administration of such formulations using a soft mist inhaler and / or nebulizer. The pharmaceutical formulations comprise (a) a therapeutic monoclonal antibody selected from the group consisting of pembrolizumab, atezolizumab, nivolumab, durvalumab, and bevacizumab, (b) water, and (c) a buffer. The pharmaceutical formulations are delivered locally to the lungs by inhalation for treatment of cancer.
Owner:HUANG CAI GU
Methods and compositions comprising a krasg12c inhibitor and a pd-l1 binding antagonist for treating lung cancer
PendingUS20220152029A1Organic active ingredientsPharmaceutical delivery mechanismDepressantPharmaceutical medicine
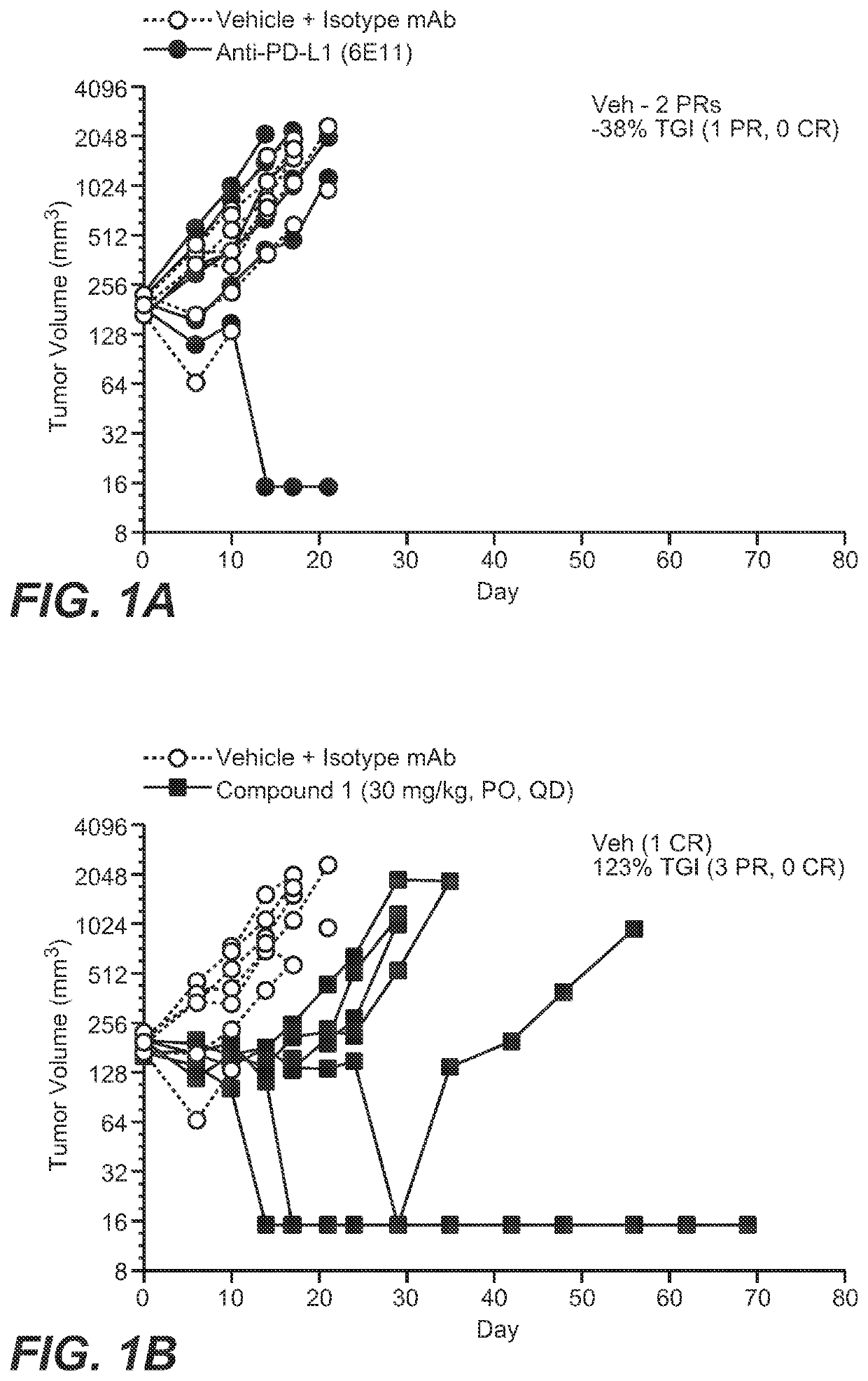
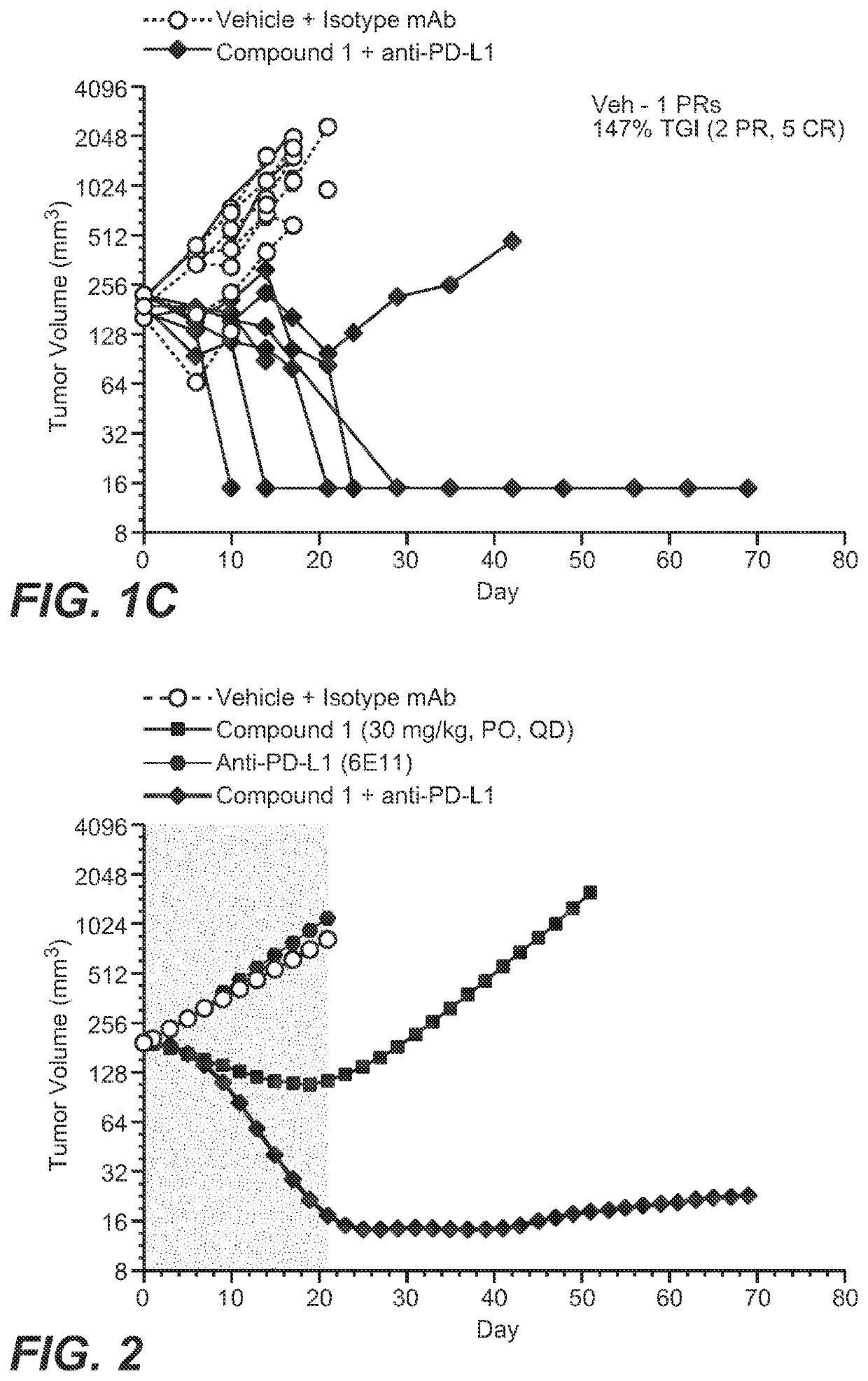
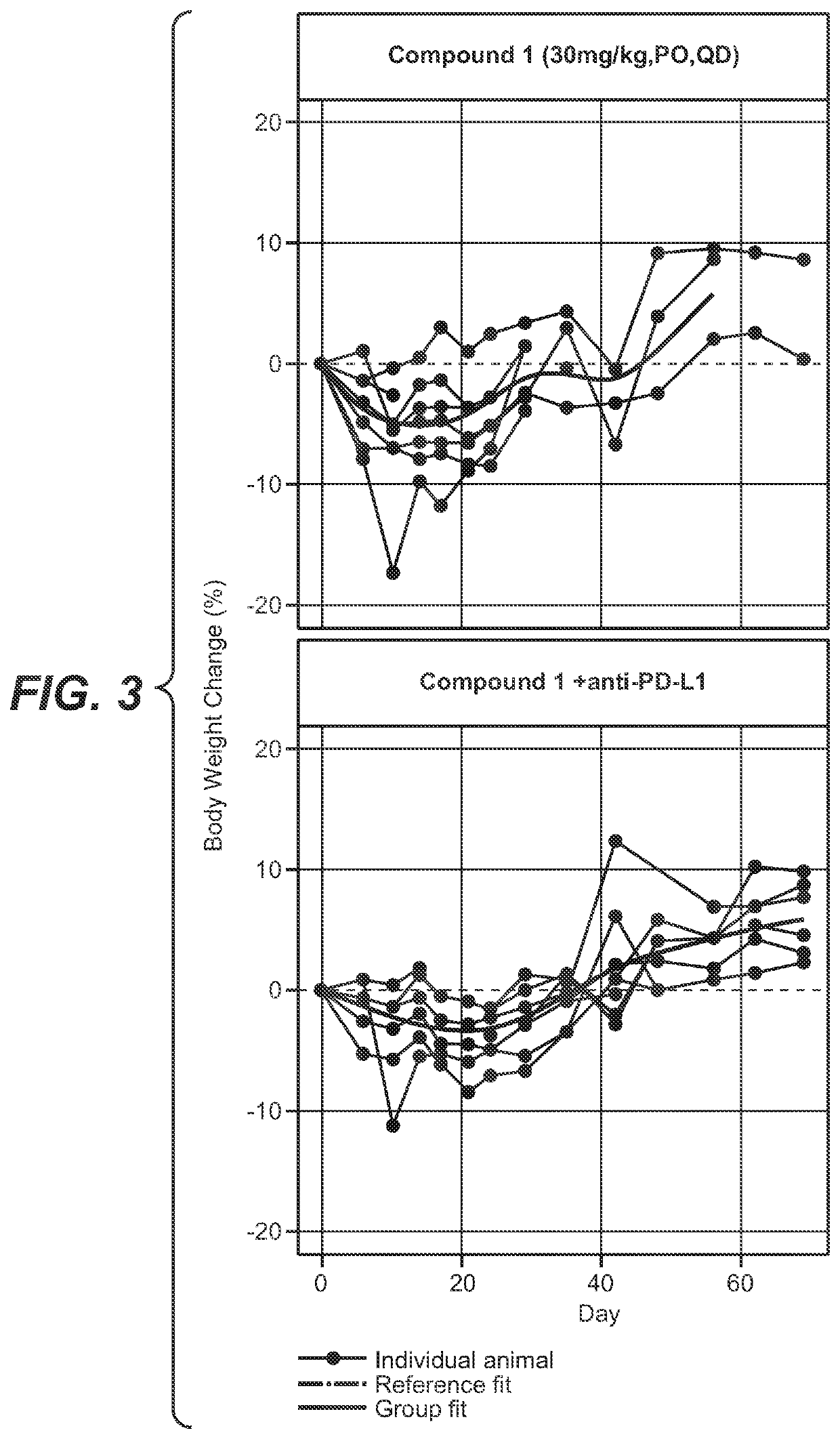
Provided herein are combination therapies (compositions) and methods and uses thereof for the treatment lung cancer, where the combination therapies comprise Compound 1 or a pharmaceutically acceptable salt thereof as described herein and a PD-L1 binding antagonist (e.g., atezolizumab).
Owner:GENENTECH INC
Diagnostic and therapeutic methods for sarcomatoid kidney cancer
PendingCN113196061AKidney cancer vaccineImmunoglobulins against growth factorsAntiendomysial antibodiesEfficacy
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., kidney cancer (e.g., renal cell carcinoma (RCC)). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a VEGF antagonist (e.g., an anti-VEGF antibody, (e.g., bevacizumab) or a VEGFR inhibitor (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib))) and a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)), or with an angiogenesis inhibitor (e.g., a VEGF antagonist (e.g., a VEGFR inhibitor, (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib)))).
Owner:F HOFFMANN LA ROCHE & CO AG
Methods of treating cancer with anti-PD-L1 antibodies
PendingCN114269376AImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAtezolizumab
Owner:F HOFFMANN LA ROCHE & CO AG
Methods of treating lung cancer with a pd-1 axis binding antagonist, a platinum agent, and a topoisomerase ii inhibitor
PendingCN112585166AInorganic active ingredientsPharmaceutical delivery mechanismExtensive Stage Small Cell Lung CarcinomaAntiendomysial antibodies
The present disclosure provides methods for treating lung cancer (such as small cell lung cancer, e.g., extensive stage small cell lung cancer) in an individual. The methods comprise administering tothe individual a PD-1 axis binding antagonist (such as an anti-PD-L1 antibody, e.g., atezolizumab), a platinum agent (e.g., cisplatin or carboplatin), and a topoisomerase II inhibitor (e.g., etoposide).
Owner:F HOFFMANN LA ROCHE & CO AG
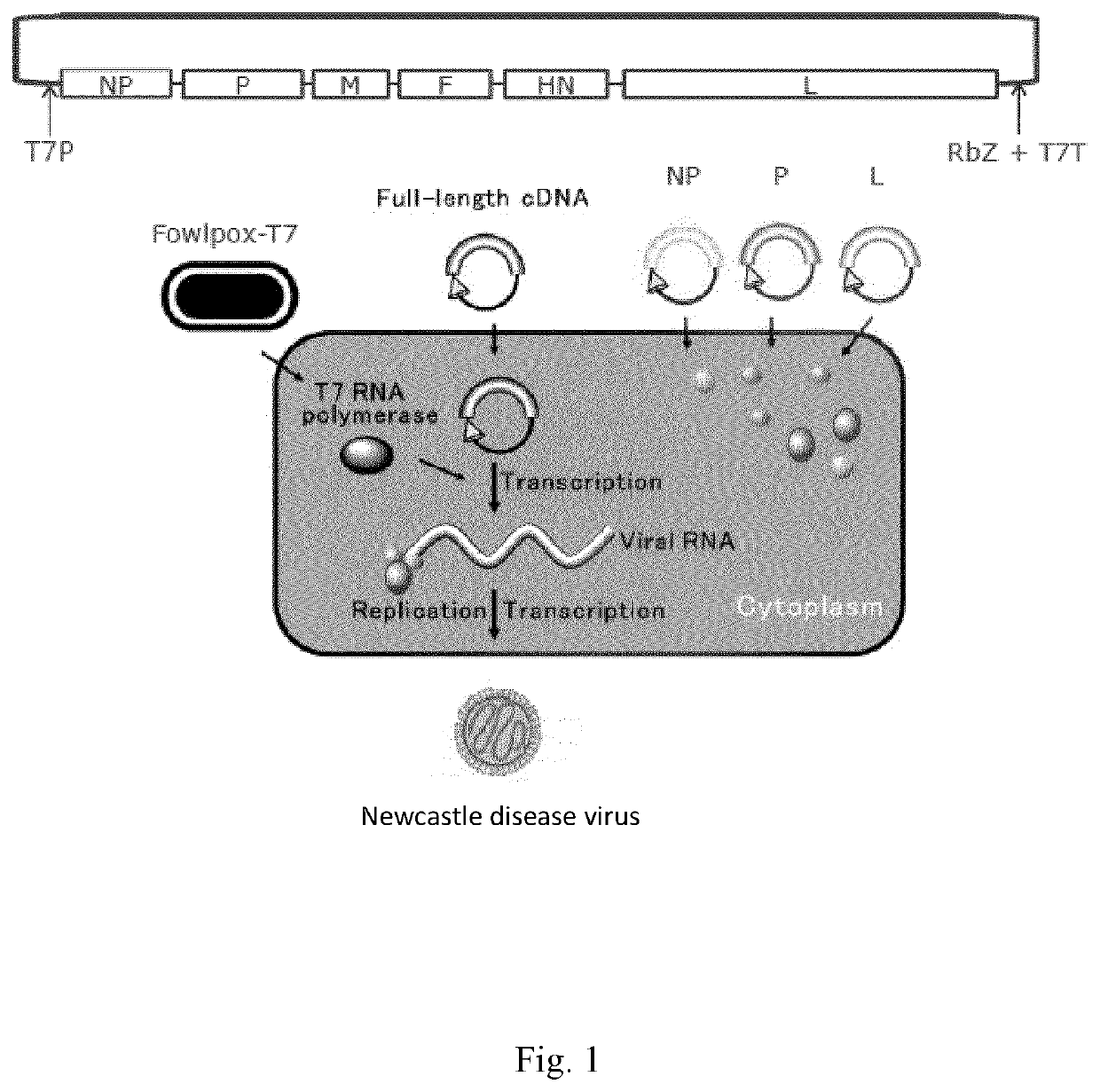
Recombinant oncolytic newcastle disease viruses with increased activity
PendingUS20220325297A1Replication capacity can be improvedImprove securitySsRNA viruses negative-senseVirus peptidesDiseaseOncolytic Newcastle Disease Virus
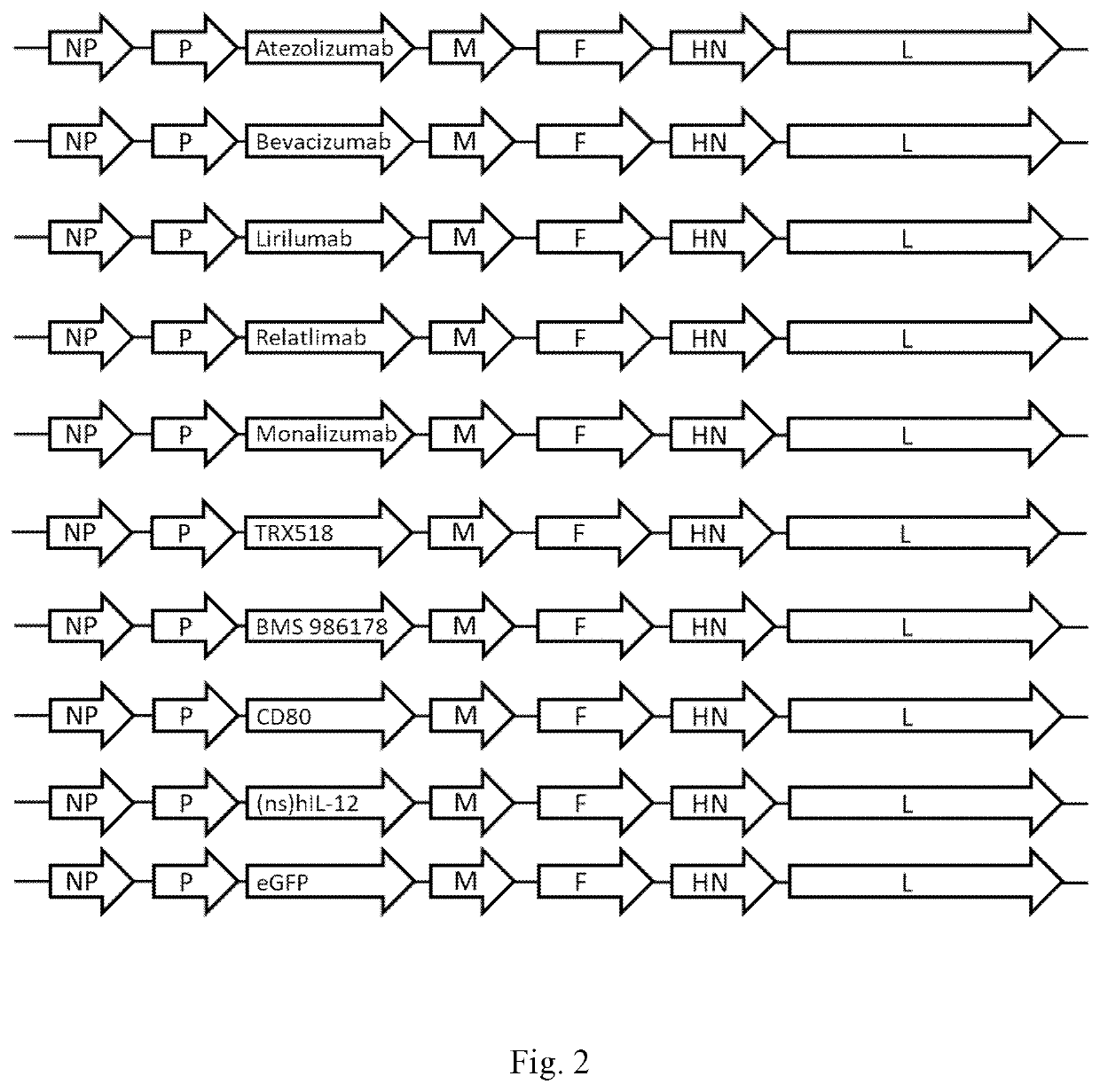
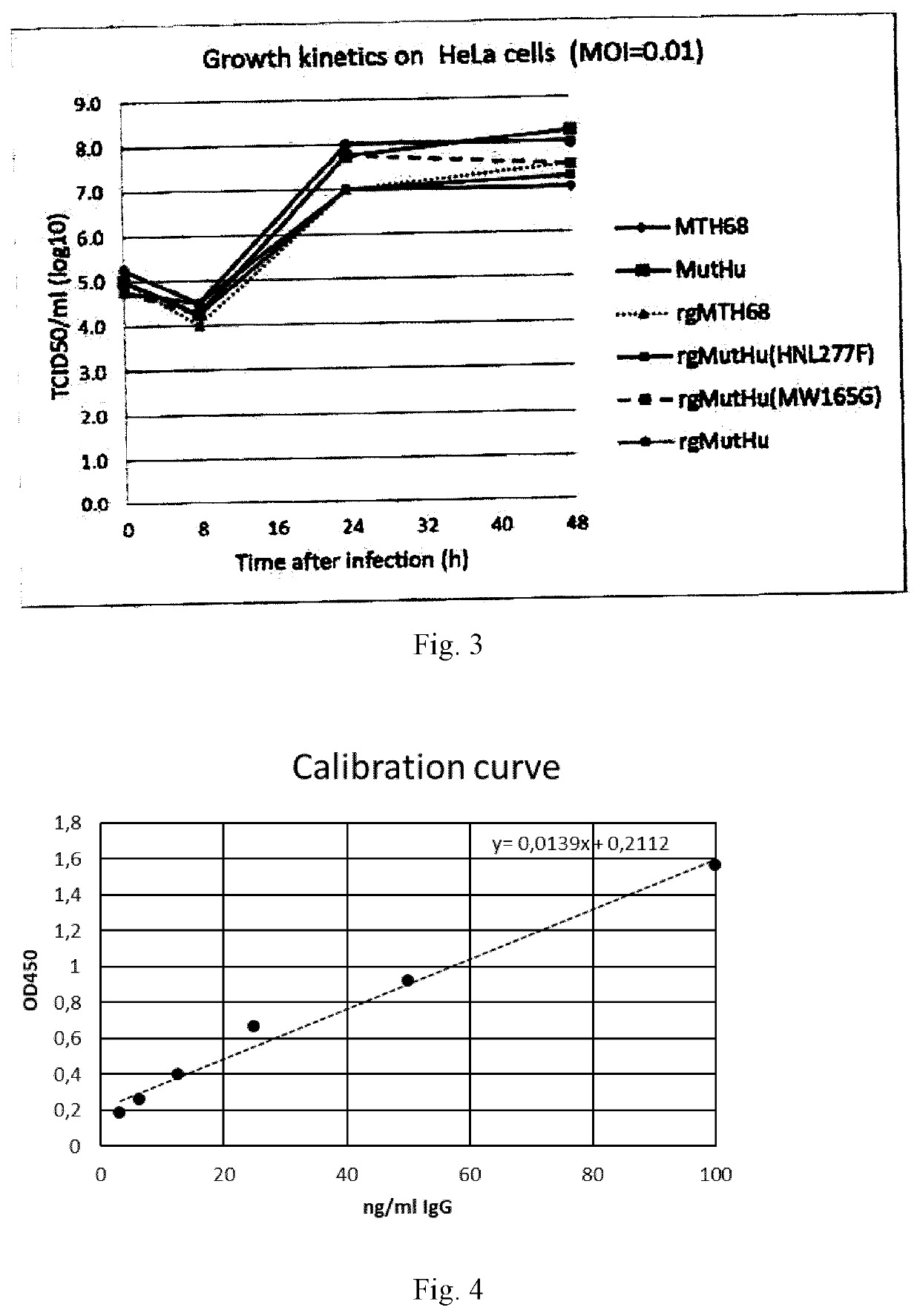
The invention relates to transgene expressing Newcastle Disease Viruses (NDV), which have been demonstrated to possess significant oncolytic activity against mammalian cancers and / or an improved safety profile. The invention provides novel oncolytic viruses through the use of genetic engineering, including the transfer of foreign genes or parts thereof, such as genes encoding Atezolizumab or Bevacizumab. The present invention also provides nucleic acids encoding a reverse genetically engineered (rg-)NDV comprising one or more of these foreign genes and having a mutation in the HN gene, said mutation allowing replication of said rgNDV in a cancer cell to a higher level than replication of an otherwise identical rgNDV not having said mutation in the HN gene.
Owner:THALLER ARNO
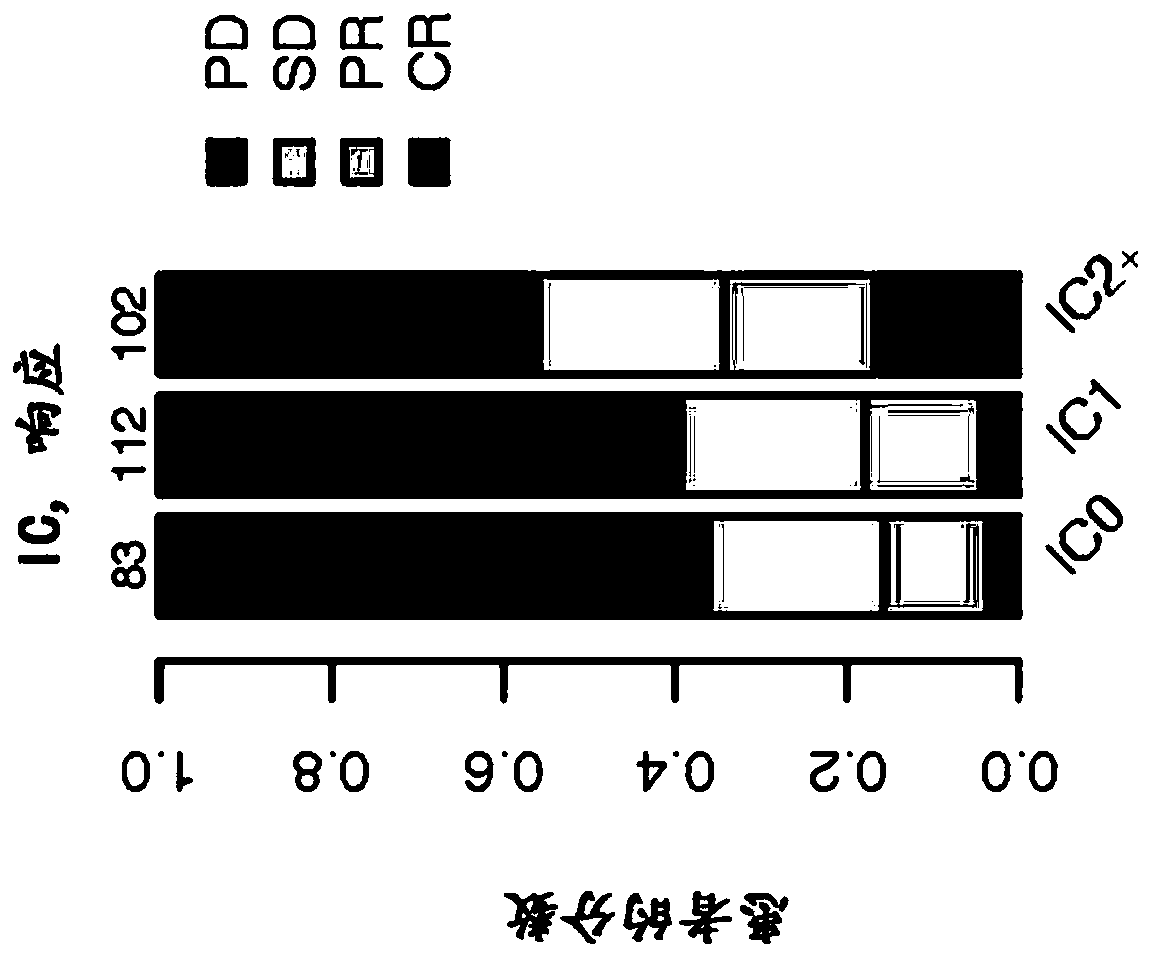
A method for establishing a nomogram model for predicting the curative effect of tumor immunotherapy
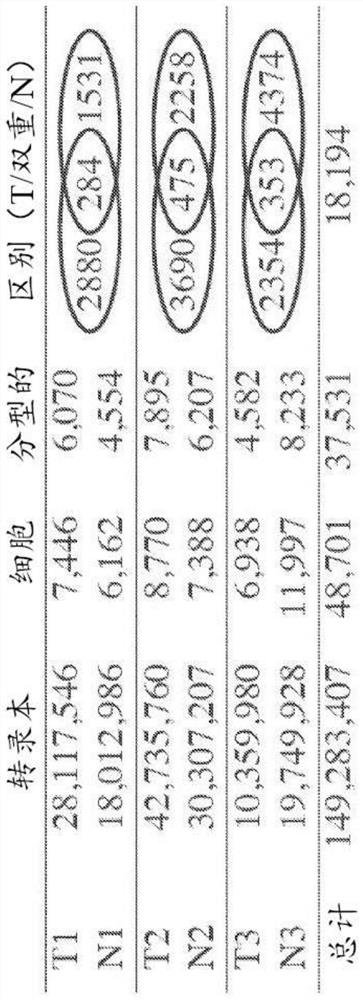
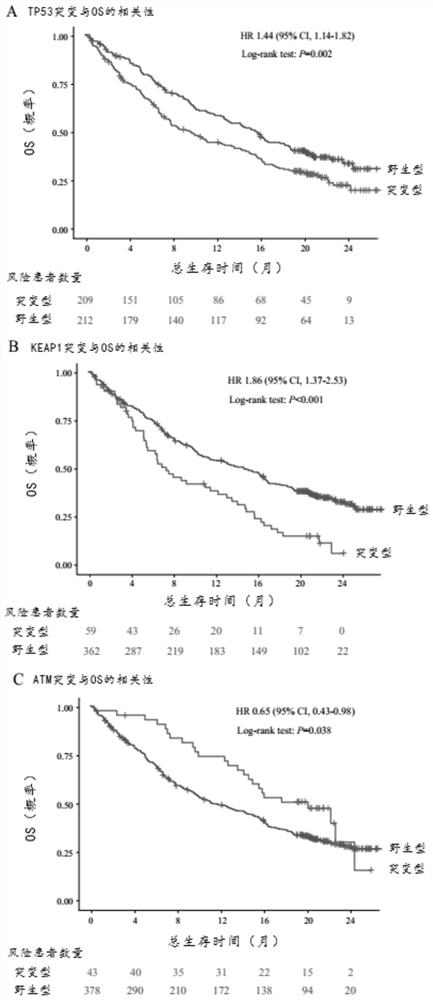
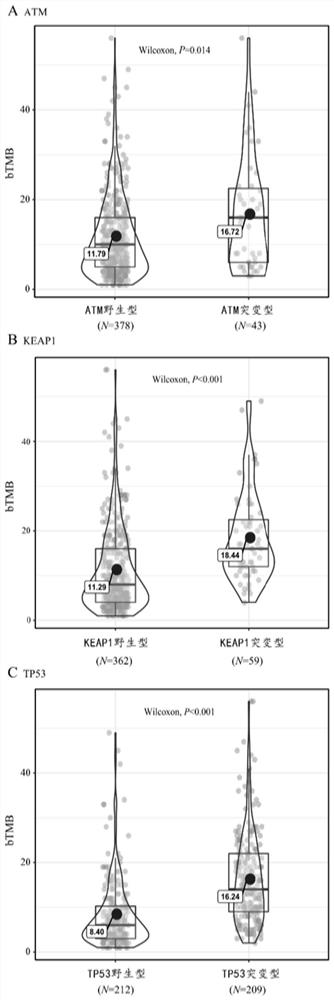
ActiveCN111863159BGood survival benefitAccurate individualized assessmentHealth-index calculationBiostatisticsNomogram ChartAtezolizumab
The invention discloses a nomogram model for predicting the curative effect of tumor immunotherapy and its establishment method. The invention obtains the factors significantly related to immunotherapy by analyzing and screening the mutation gene and clinical characteristic data of patients, and further constructs the corresponding The nomogram model; the present invention provides 3 kinds of nomogram models, which are suitable for different non-small cell lung cancer patients, and assist in judging whether different patients can continue to receive treatment or choose to change the dressing; the model of the present invention is simple, intuitive, and easy to popularize and apply , can effectively help clinicians to accurately and individually evaluate atezolizumab immunotherapy for patients with non-small cell lung cancer when they receive immunotherapy and start treatment, so as to bring better survival benefits to patients. For non-small cell lung cancer patients The effective application of immunotherapy for small cell lung cancer is of great value.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Combination of an Anti-cd20 antibody, pi3 kinase-delta inhibitor, and Anti-pd-1 or Anti-pd-l1 antibody for treating hematological cancers
The present disclosure provides methods and kits for treating or slowing the progression of a hematological malignancy, by administering to a subject in need thereof a therapeutically effective amount of: (i) at least one inhibitor of PI3 kinase (PI3K)-delta (e.g., TGR-1202); (ii) at least one anti-CD20 antibody (e.g., ublituximab); and (iii) at least one anti-PD-1 antibody (e.g., pembrolizumab) or anti-PD-Ll antibody (e.g., atezolizumab). Treatment regimens are also provided.
Owner:RHIZEN PHARM SA +1
Marker for predicting response of esophageal adenocarcinoma patient to combined treatment and application thereof
The invention belongs to the field of biomedicine, and particularly relates to a marker for predicting response of an esophageal adenocarcinoma patient to combined treatment and application thereof. The combined treatment provided by the invention is neoadjuvant radiotherapy and chemotherapy combined with attezumab treatment. In particular, the marker includes RORB, IGKV19, FGD5P1, or a combination thereof.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Diagnostic methods and compositions for cancer immunotherapy
PendingUS20210301023A1Improve responsivenessResponsiveness to treatmentOrganic active ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesAntigen Binding Fragment
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer. The compositions and methods described herein can be used, for example, to determine the propensity of a patient to benefit from treatment with a PD-L1 axis binding antagonist and to treat such patients accordingly. Using the compositions and methods of the disclosure, a patient, such as a human cancer patient, may be determined to be likely to benefit from treatment with a PD-L1 axis binding antagonist if the patient exhibits an elevated pre-treatment expression level of one or more of CST7, NKG7, GZMH, MT-ND4, HLA-H, CCL5, CD8A, CMC1, CD8B, HCST, MT-CYB, MT-ND4L, KLRG1, MT-CO2, MT-ATP6, PLEK, CTSW, HLA-C, LYAR, LITAF, GZMB, KLRD1, FGFBP2, KLRC4-KLRK1, KLRK1, B2M, GZMA, ID2, CX3CR1, PRSS23, GNLY, PRF1, and PATL2. Exemplary PD-L1 axis binding antagonists that may be used in conjunction with the compositions and methods of the disclosure are PD-L1 binding antagonists, such as anti-PD-L1 antibodies and antigen-binding fragments thereof, including atezolizumab, as well as PD-1 binding antagonists, such as anti-PD-1 antibodies and antigen-binding fragments thereof.
Owner:GENENTECH INC
Combination therapy for cancer comprising pd-1 axis binding antagonist and il6 antagonist
PendingUS20210332143A1Increase abundanceReduces and prevents therapeutic resistance to PD-Organic active ingredientsImmunoglobulins against growth factorsAntiendomysial antibodiesAntigen binding
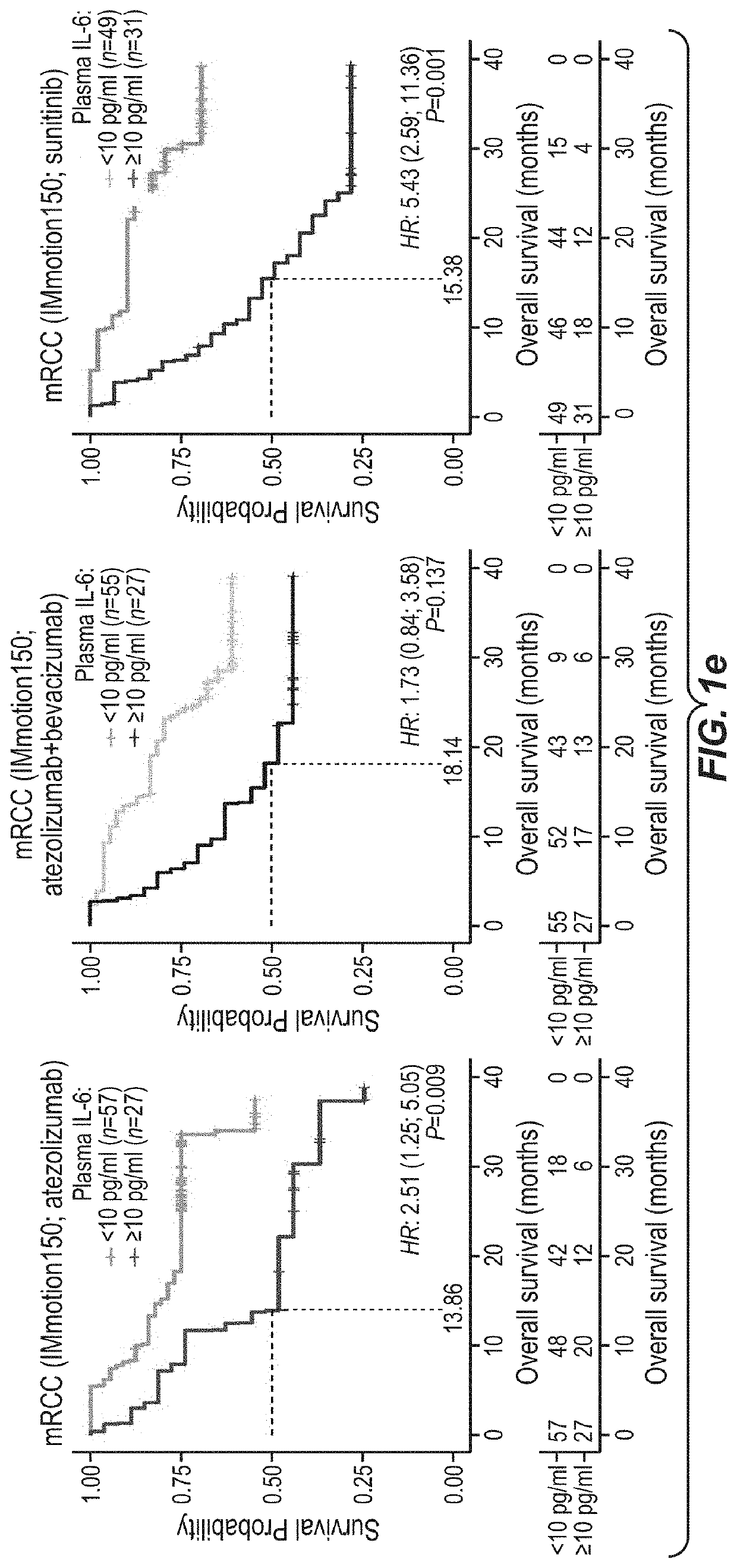
This application discloses methods and compositions for use in treating cancer, including breast cancer (such as metastatic triple negative breast cancer, mTNBC), urothelial carcinoma, renal cell carcinoma, and liver cancer (hepatocellular carcinoma, HCC) with the combination of a PD-1 axis binding antagonist (e.g., a PD-L1 binding antibody such as atezolizumab) and an IL6 antagonist (e.g. an anti-IL6 receptor antibody such as tocilizumab), optionally further comprising a VEGF antagonist (e.g. an anti-VEGF antibody such as bevacizumab). Optionally, the patient has C-reactive protein (CRP) and / or IL-6 level(s) above the upper limit of normal. Optionally, the cancer is PD-L1 positive.
Owner:GENENTECH INC
Blocking pd-l1 camel-derived single-domain antibody and use thereof
ActiveCN110144010B9Hybrid immunoglobulinsAntibody mimetics/scaffoldsAntiendomysial antibodiesReceptor
The invention relates to a camel-derived single-domain antibody, which specifically recognizes programmed death receptor-ligand 1 (PD-L1), and can effectively block the interaction between PD-L1 and its receptor PD-1. The effect is better than the marketed atezolizumab. Specifically, the present invention discloses the coding sequence, preparation method and use of a PD-L1 camel-derived single domain antibody and its derivative protein.
Owner:SHANGHAI NOVAMAB BIOPHARM CO LTD
Application of plant as host in expressing pd-1 antibody and/or pd-l1 antibody
InactiveUS20210087274A1Shorten the production cycleImprove purification effectVaccinesImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesLactuca
Provided is an application of a plant as a host in expressing a PD-1 antibody and / or a PD-L1 antibody, wherein the plant, such as lettuce, is used as an effective expression platform for preparing recombinant proteins, and a simple and effective agrobacterium-mediated vacuum infiltration method is used for expressing the PD-1 monoclonal antibody (Keytruda, pembrolizumab) and the PD-L1 monoclonal antibody (Atezolizumab).
Owner:WANG KEVIN
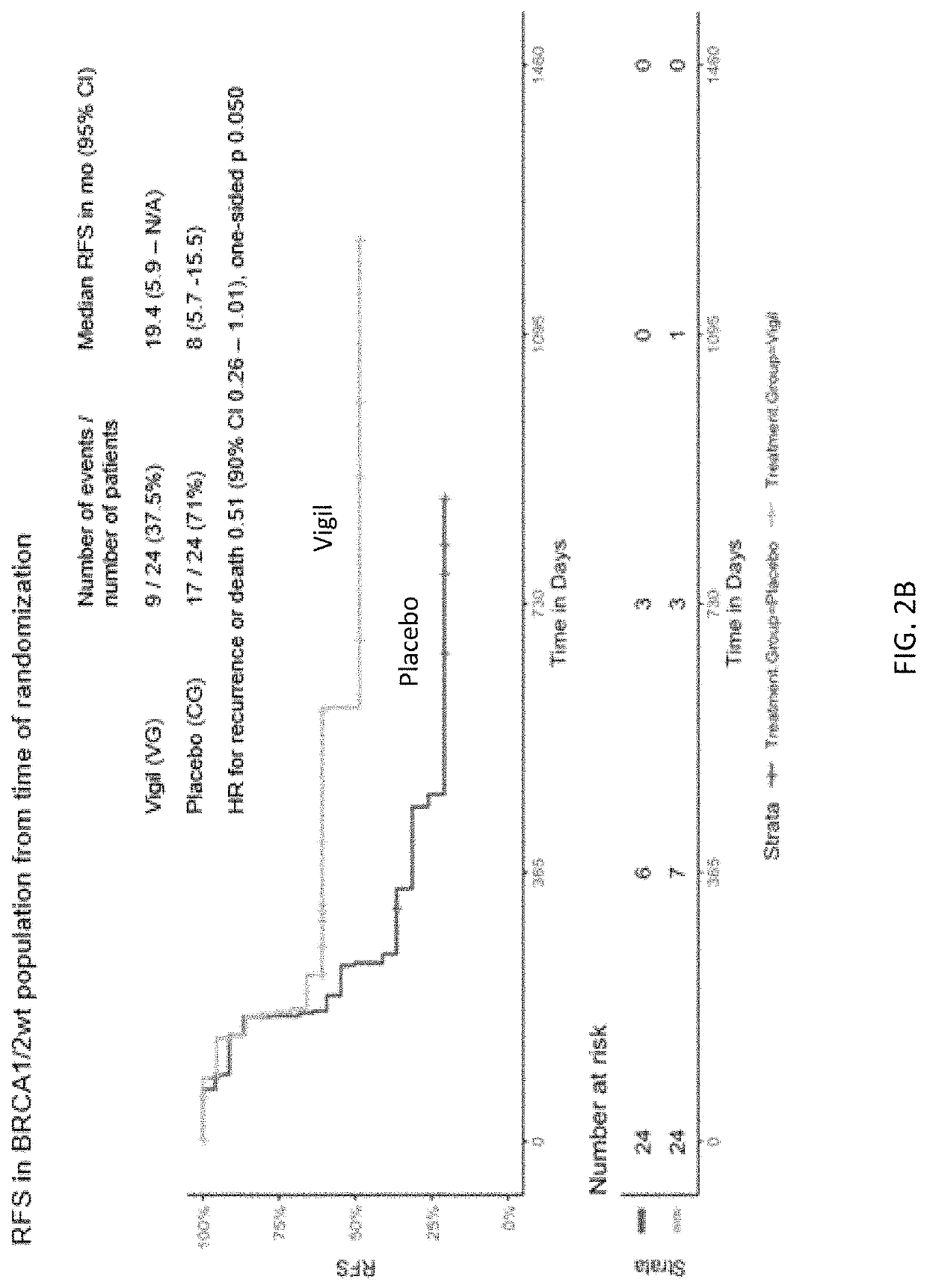
Methods for treating cancers
ActiveUS11400170B2Additional recurrencePeptide/protein ingredientsMammal material medical ingredientsBrca1 geneWild type
Compositions and methods for prevention of ovarian cancer recurrence and for the treatment of BRCA1 / 2-wild type ovarian cancer are disclosed herein. In some embodiments, the composition comprises an autologous tumor cell vaccine comprising cells genetically modified for furin knockdown and GM-CSF expression. In some embodiments, the method comprises administration of an autologous tumor cell vaccine prior to administration of a combination of the autologous tumor cell vaccine and atezolizumab. Also disclosed herein are methods for treating a cancer in an individual comprising a wild-type BRCA1 gene, a wild-type BRCA2 gene, or a combination thereof, and is identified as homologous recombination deficiency (HRD)-negative.
Owner:GRADALIS
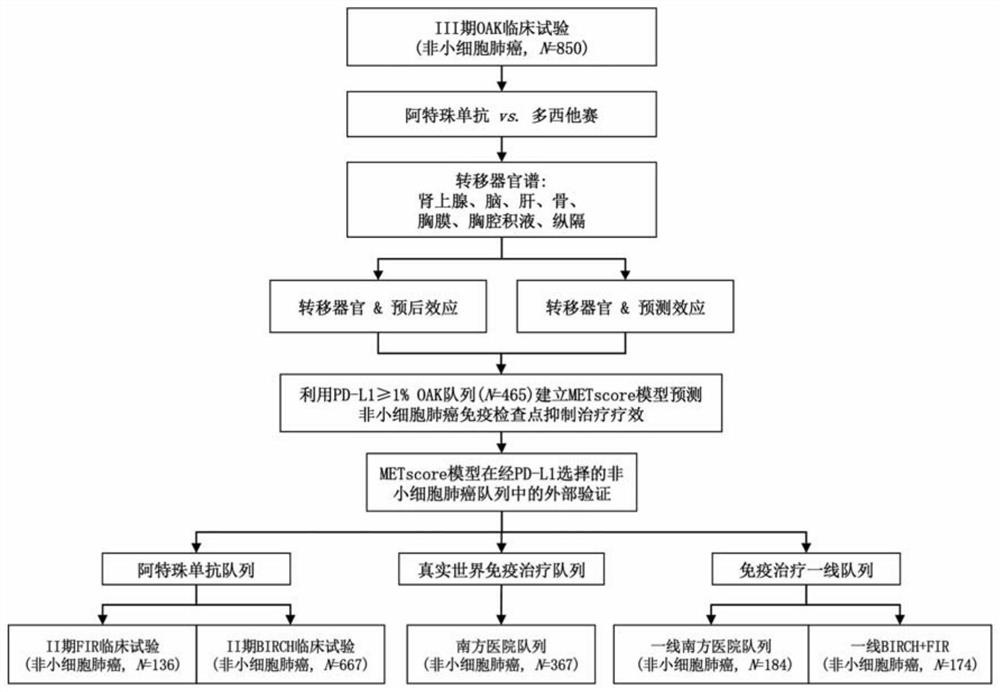
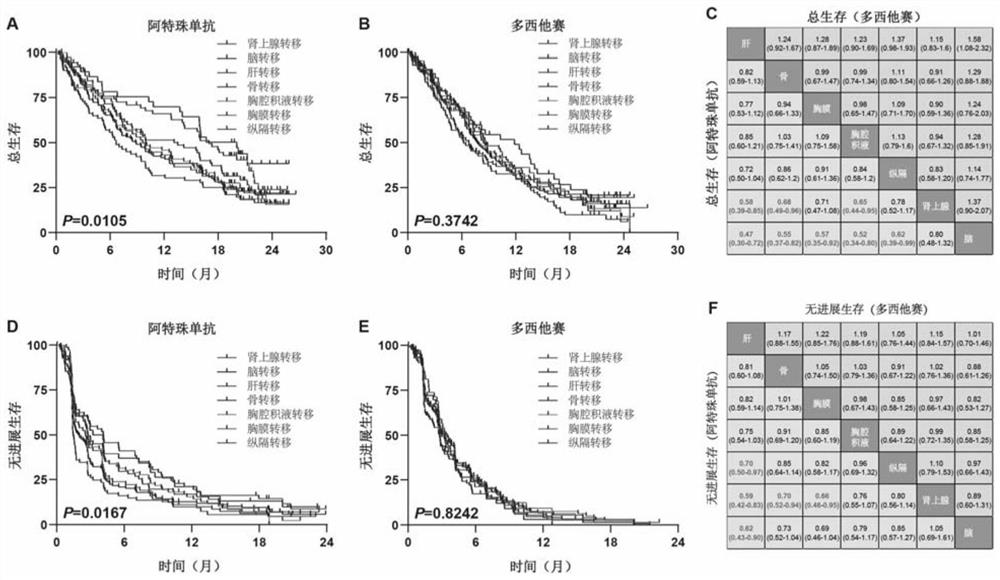
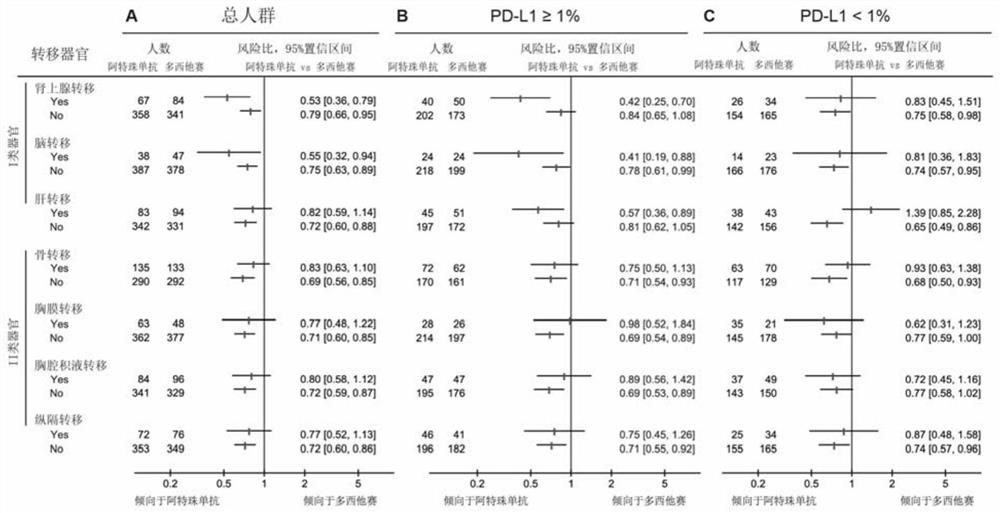
Construction method and application of prediction model for non-small cell lung cancer immune checkpoint inhibition treatment effect based on organ metastasis spectrum
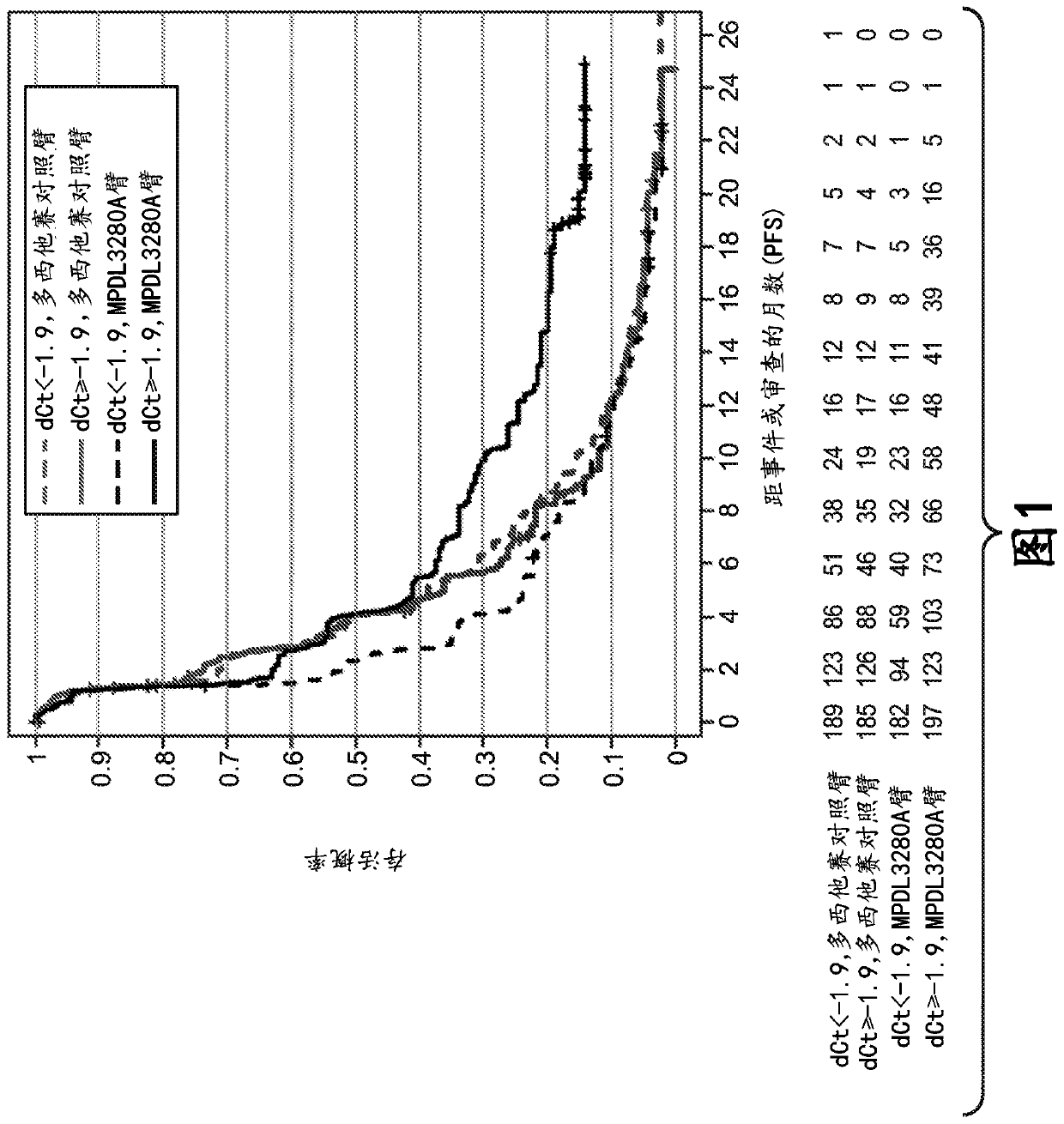
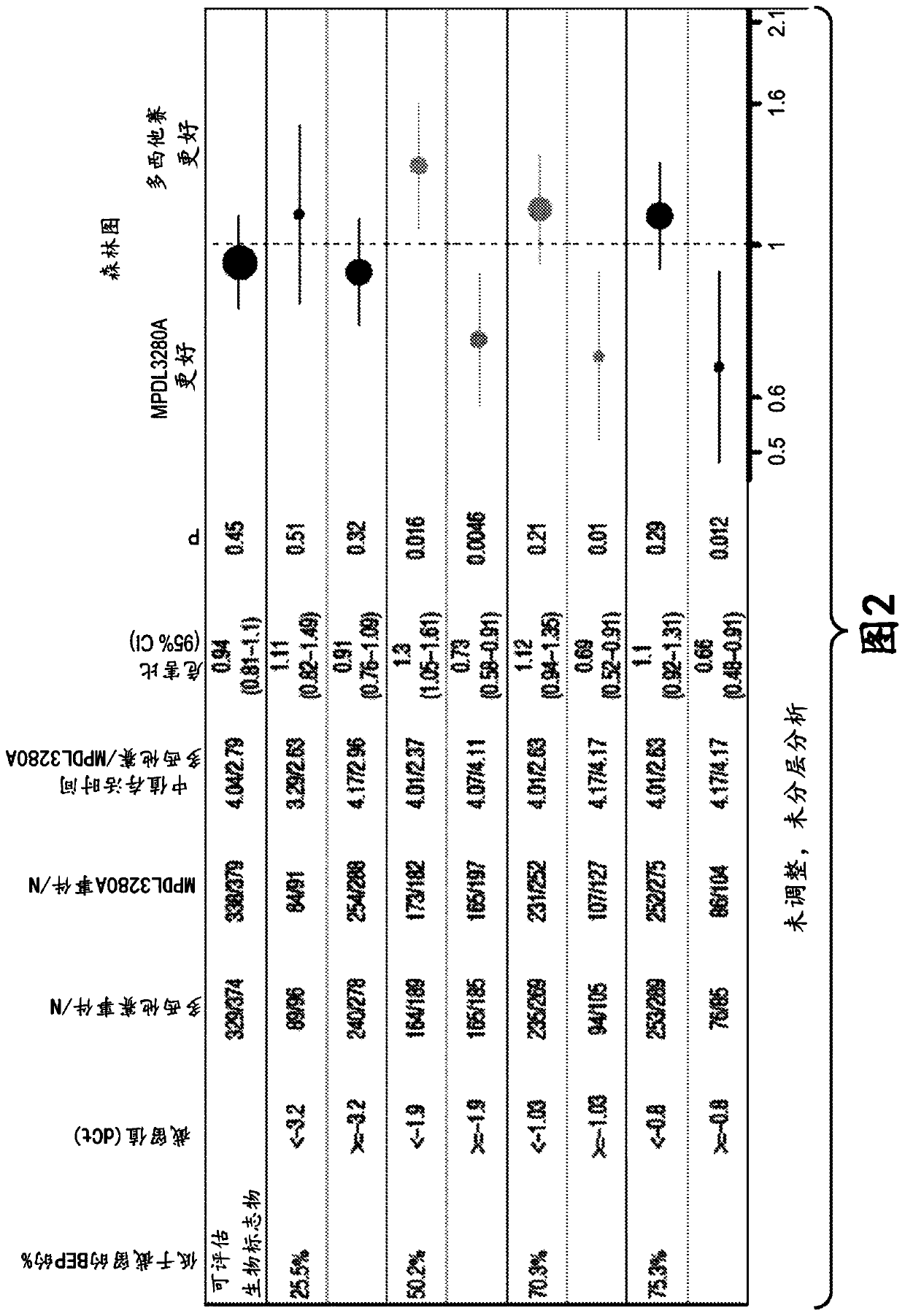
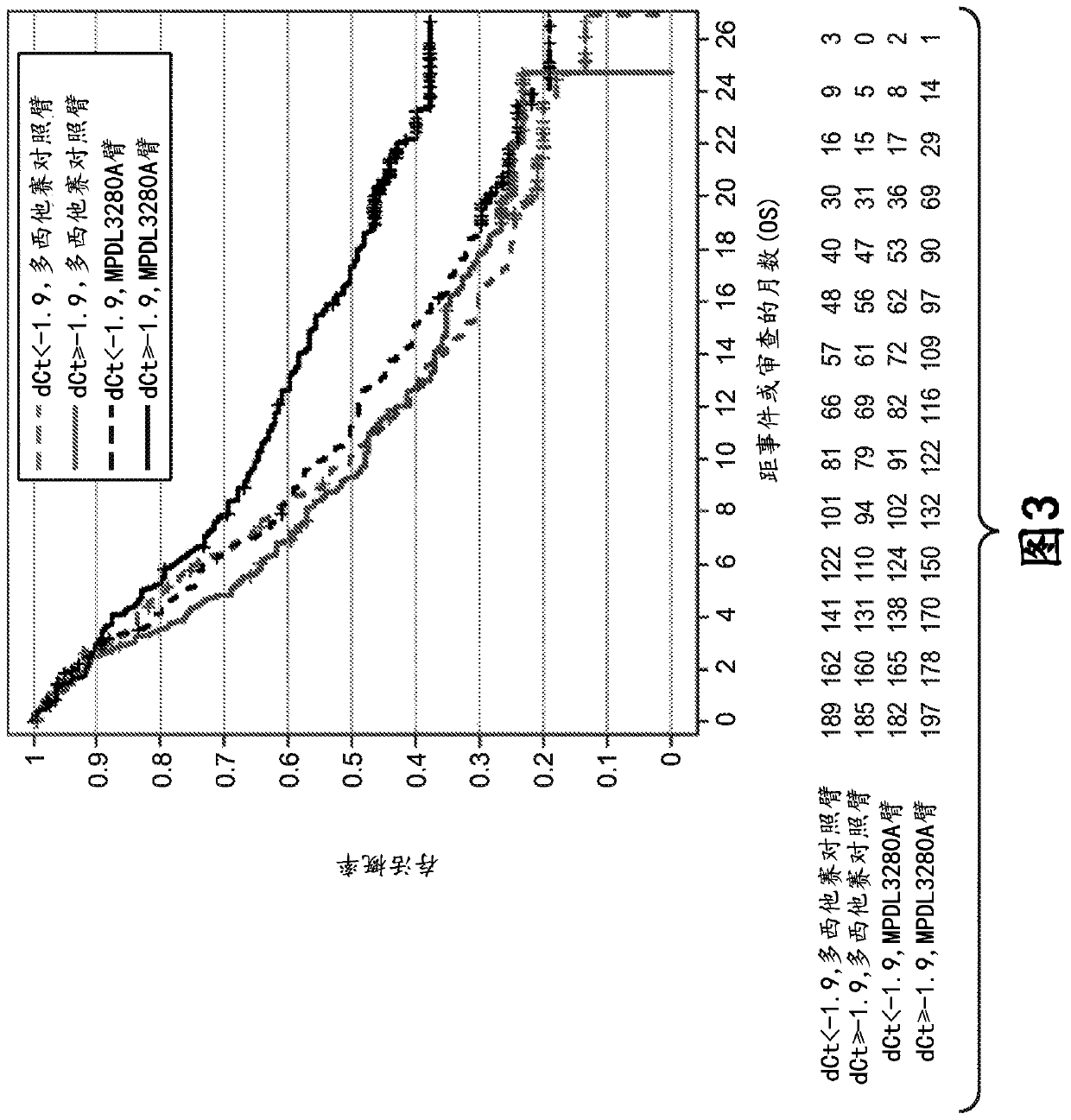
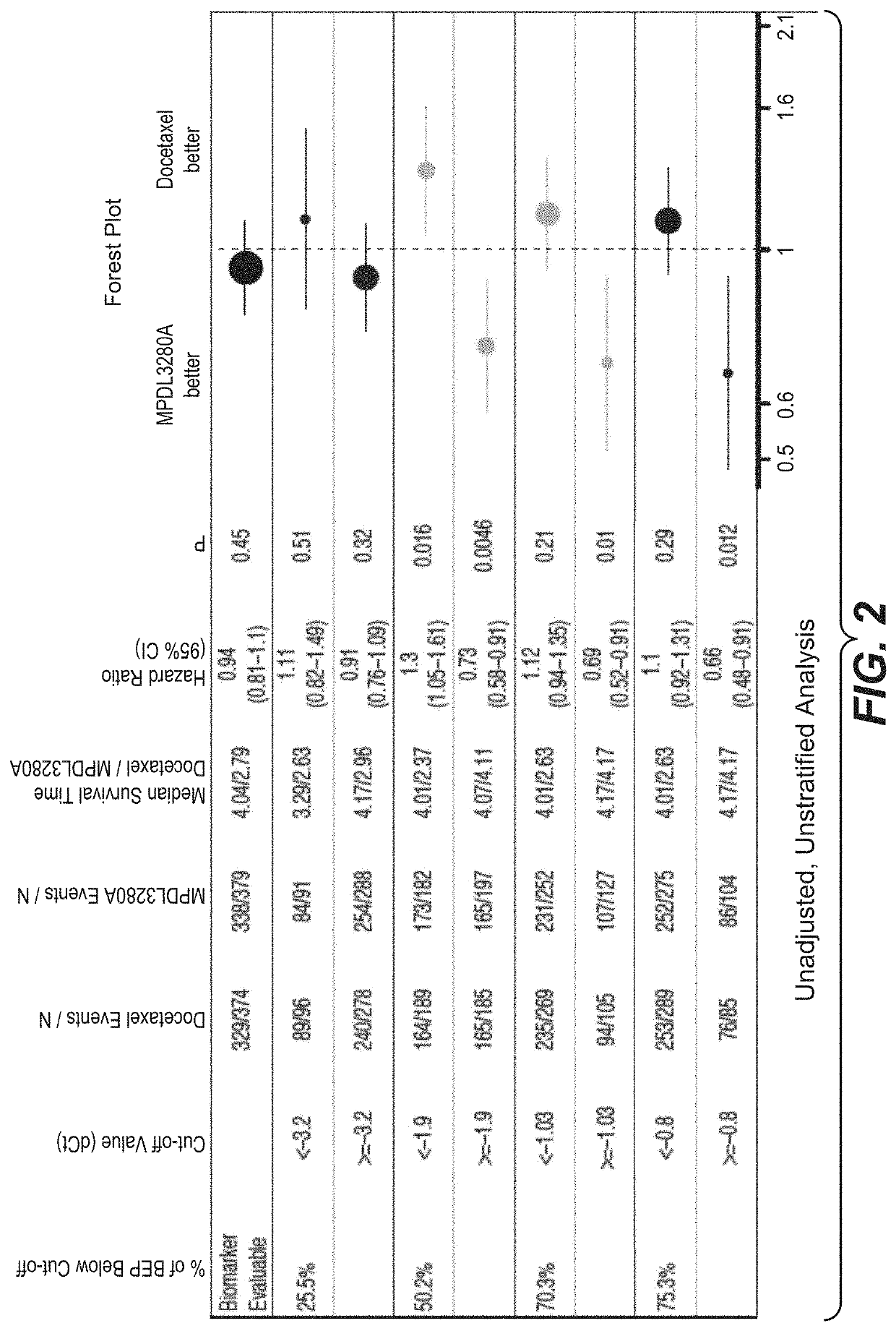
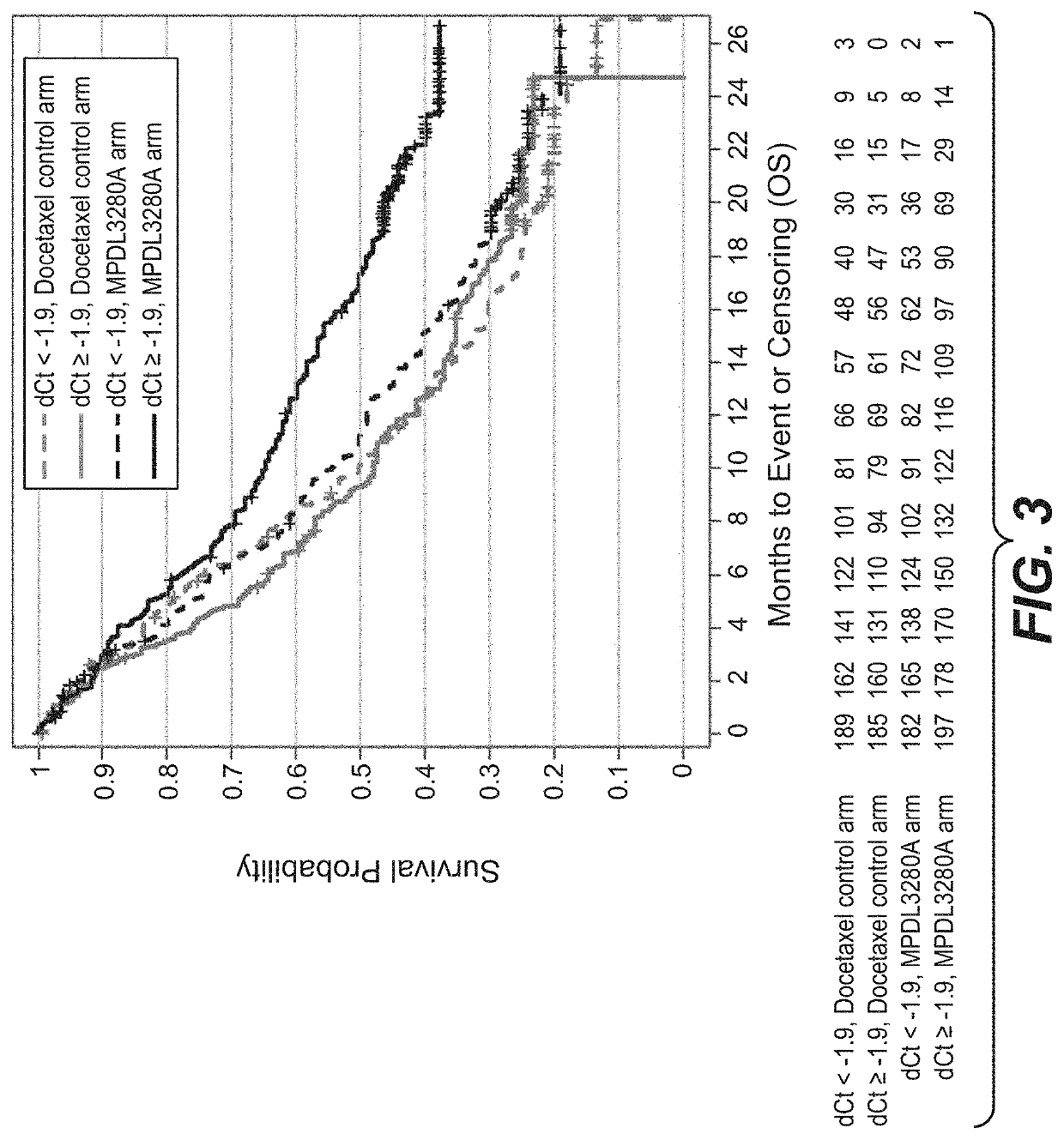
ActiveCN113409949AImprove understandingMedical data miningDrug and medicationsTreatment effectDocetaxel
The invention relates to the field of biomedicine, in particular to a construction method and application of a prediction model for non-small cell lung cancer immune checkpoint inhibition treatment effect based on organ metastasis spectrum. The construction method comprises the following steps: evaluating and comparing the influence of different baseline transfer organs in an OAK queue on OS and PFS of patients in an attezumab group and a docetaxel group, the prediction effect of the transfer organ spectrum of different PD-L1 expression level stratification of the atezumab group and docetaxel group in OAK queue, and OS of different transfer organ types and numbers of the attezumab group and the docetaxel group in a PD-L1 positive OAK queue; establishing a scoring system METscore based on the organ transfer spectrum, performing multivariable Cox proportional risk regression, and predicting a clinical result of ICI treatment by calculating a total score of a prognosis effect and a prediction effect. Immunotherapy prediction and prognosis effects of the metastatic organ spectrum of the advanced NSCLC patient are described, clinical decisions of tumor immunotherapy patients are facilitated, and understanding of metastatic organ specificity anti-tumor immunity is improved.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com