Methods of treating cancer with anti-PD-L1 antibodies
A PD-L1, HVR-L1 technology, applied in the field of cancer treatment with anti-PD-L1 antibodies, can solve problems such as enhancing CD8+ T cell-mediated tumor killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0366] The foregoing written description is considered sufficient to enable any person skilled in the art to practice the invention. The following examples are provided for illustrative purposes only and are not intended to limit the scope of the invention in any way. Indeed, various modifications of the invention in addition to those shown and described herein will become apparent to those skilled in the art from the foregoing description and are within the scope of the appended claims.
[0367] overview
[0368]Immune checkpoint inhibition targeting programmed death-ligand 1 (PD-L1) or programmed death-1 (PD-1) has become an important approach for the treatment of various human cancers because PD-L1 is involved in tumor cells and tumor infiltration Expression on immune cells can suppress anticancer immune responses (Chen et al. (2013) Immunicty doi:10.1016 / j.immuni.2013.07.012). Atezolizumab is a humanized, engineered monoclonal immunoglobulin (Ig) G1 antibody that selecti...
example 1
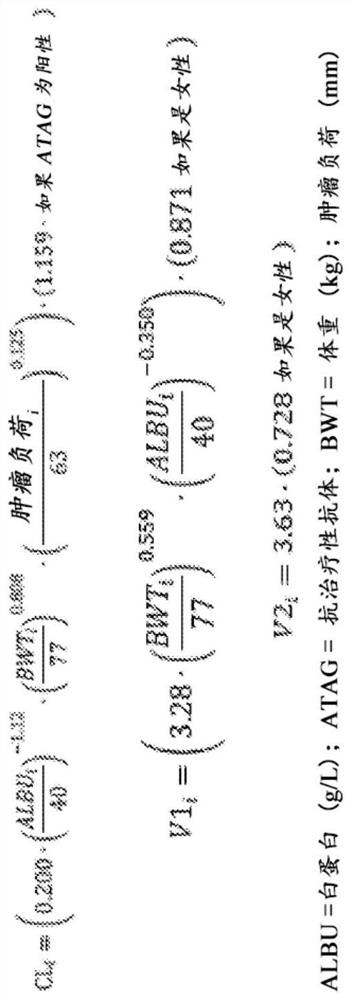
[0383] Pharmacokinetic properties of atezolizumab monotherapy
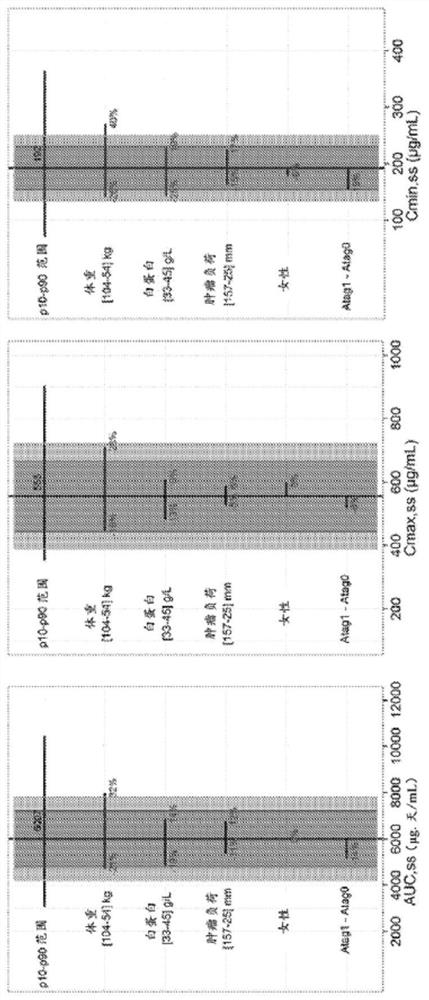
[0384] In this example, the pharmacokinetic (PK) characteristics of atezolizumab were compared in eight atezolizumab studies conducted in the monotherapy setting (see Table 1). Key PK features such as C min 、C max and AUC were calculated based on clinical studies using a fixed 1200mg q3w dose and estimated for fixed 1680mg q4w and 840mg q2w doses. Important patient characteristics were also analyzed as potential covariates.
[0385] Atezolizumab PK was linear over the atezolizumab dose range of 1 to 20 mg / kg, including the fixed 1200 mg atezolizumab dose. Atezolizumab PK appeared to be comparable across studies, as similar cycle 1 C was observed at the same dose levels max and C min Shown (Table 2).
[0386] Table 2. Summary Statistics of Atezolizumab Serum PK Parameters in Cycle 1 of PCD4989g, JO28944, IMvigor210, IMvigor211, BIRCH, POPLAR, FIR, and OAK
[0387]
[0388]
[0389] method
[0390] s...
example 2
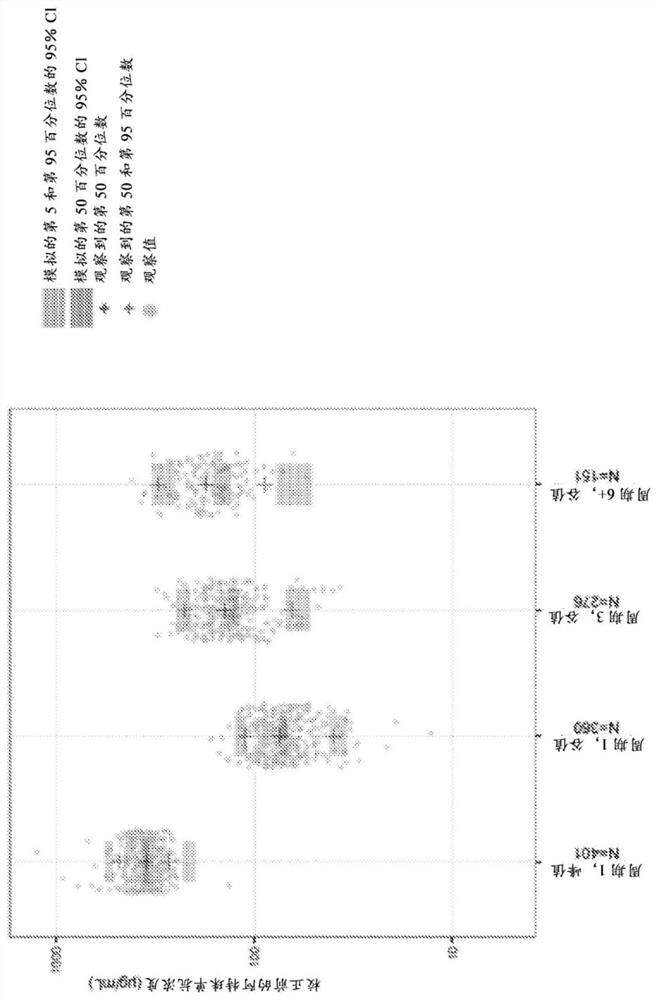
[0450] Exposure-response relationship of atezolizumab in urothelial carcinoma and non-small cell lung cancer
[0451] Exposure-response (ER) analyzes were performed to assess the possible relationship between clinical efficacy and atezolizumab exposure in separate (UC or NSCLC) and pooled (UC and NSCLC) patient populations for each indication.
[0452] method
[0453] Merged ER Analysis Overview
[0454] Objective response rate, overall survival and adverse events were assessed in comparison to pharmacokinetic (PK) measures, as described below.
[0455] Perform ER analysis to inform any relationship between PK measures and ORR, OS, grade 3 to 5 AEs, and AESI endpoints assessed in previous clinical studies based on cycle 1 data to minimize potential confounding from baseline prognostic factors Bias (Yang et al., (2013) J Clin Pharmacol doi:10.1177 / 0091270012445206; Wang et al., (2014) Clin Pharmacol Ther doi:10.1038 / clpt.2014.24) and observations for atezolizumab and other ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com