Treatment of triple negative breast cancer or colorectal cancer with liver metastases with anti pd-l1 antibody and oncolytic virus
A PD-L1, colorectal cancer technology, applied in the direction of antibody medical components, double-stranded DNA virus, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
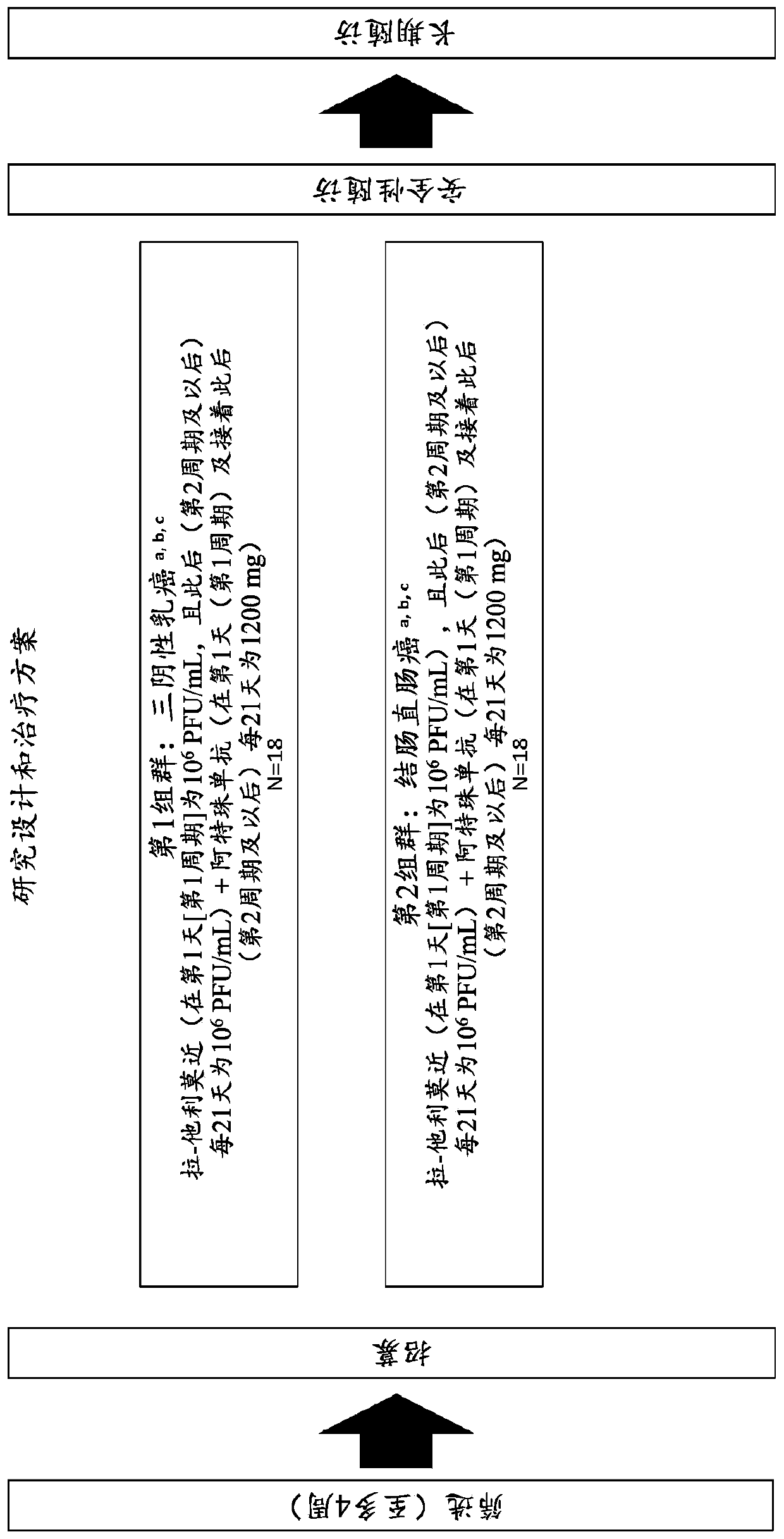
[0206] This example demonstrates an exemplary approach to the treatment of patients with triple negative breast cancer or colorectal cancer with liver metastases.
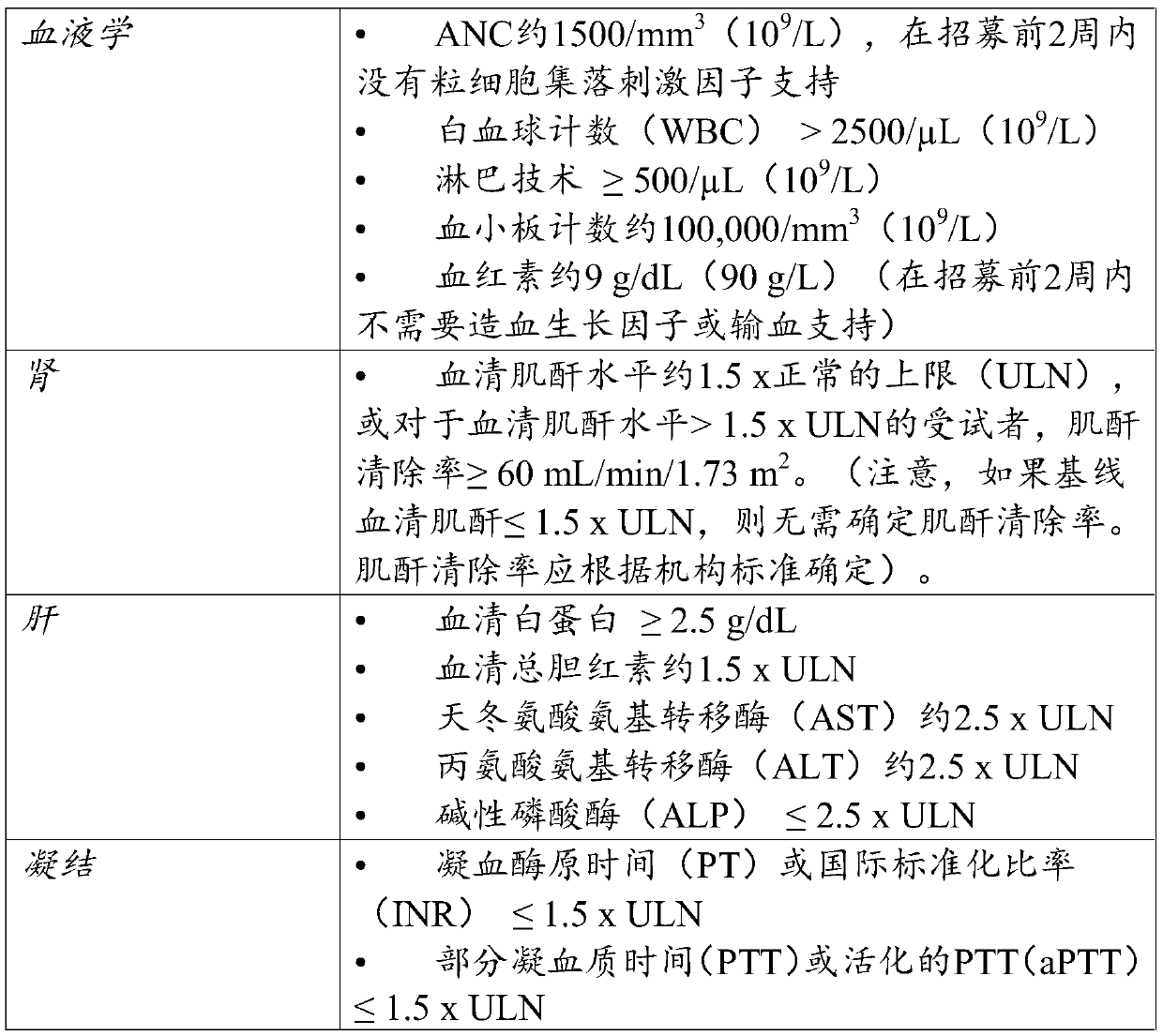
[0207] A phase 1b study was conducted to determine the safety of the combination of intrahepatic injection of latalimoxime to liver metastases with intravenous administration of atezolizumab in patients with triple-negative breast or colorectal cancer, as determined by dose-limiting The incidence of toxicity (DLT) was determined. This study was also conducted to evaluate the efficacy of the combination of latalimogene and atezolizumab, respectively, in subjects with metastatic triple-negative breast cancer or metastatic colorectal cancer with liver metastases, as determined by the following Each item was evaluated: objective response rate (ORR), best overall response (BOR), duration of response (DOR) of cohorts (triple-negative breast cancer and colorectal cancer), injected and uninjected tumor lesions (overall, li...
example 2
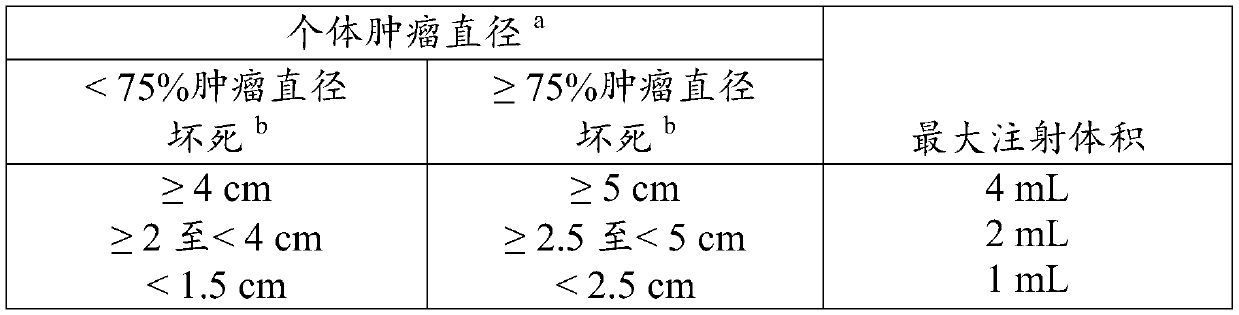
[0223] This example describes an exemplary method of measuring tumor lesions particularly in the context of the study described in Example 1 and additional parameters for assessing the efficacy of treatment.
[0224] Computed tomography scan (or magnetic resonance imaging):
[0225] Computed tomography (CT) scans with contrast enhancement or helical scans (or magnetic resonance imaging [MRI] scans) are performed to assess tumor response in visceral or nodal / soft tissue disease, including lymph nodes. The measurability of lesions on CT scans is based on the assumption that CT slice thicknesses are 5 mm or less. If used throughout the study, MRI may be used to assess the extent of disease.
[0226] Each identified and reported lesion can be characterized at baseline and during follow-up using the same method of assessment and the same technique. Switching from contrast-enhanced CT to non-contrast CT or to MRI (or vice versa) did not preclude response assessment if, in the judg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com