Combinations of cabozantinib and atezolizumab to treat cancer
A technology of atezolizumab and a combination, applied in the field of combination of cabozantinib and atezolizumab for the treatment of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
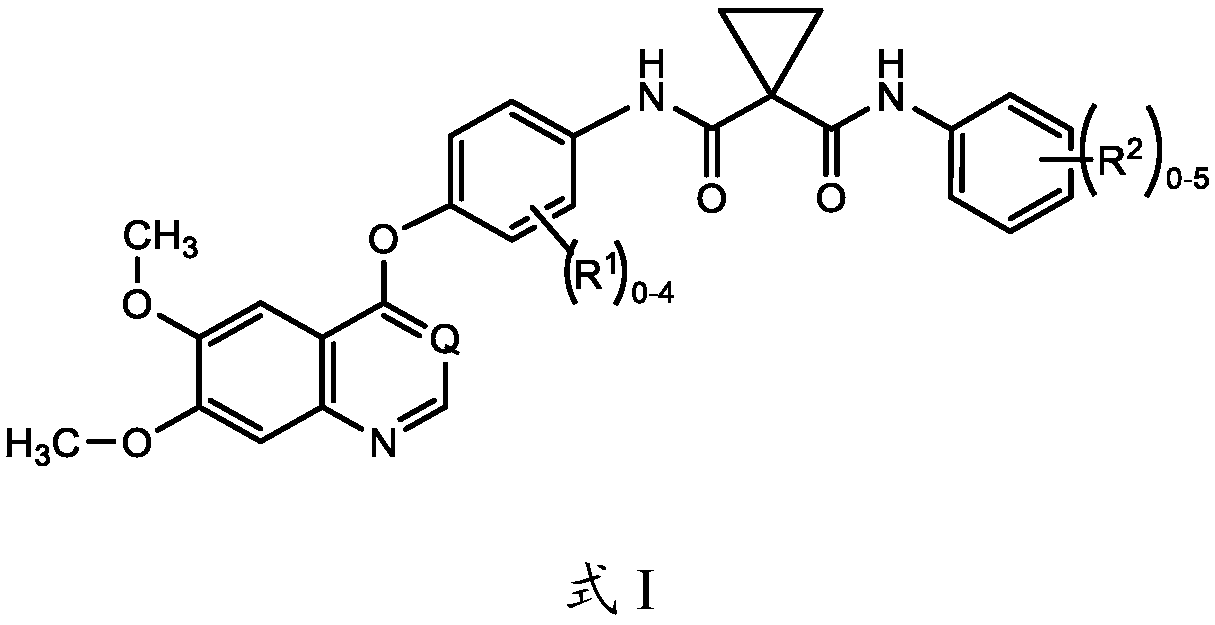
[0194] Embodiment 1. A method of treating locally advanced or metastatic solid tumors comprising administering a compound of formula I to a patient in need of such treatment:
[0195]
[0196] Or a pharmaceutically acceptable salt thereof or a pharmaceutical composition comprising a compound of formula I or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier, wherein:
[0197] R 1 is halo;
[0198] R 2 is halo; and
[0199] Q is CH or N;
[0200] In combination with atezolizumab.
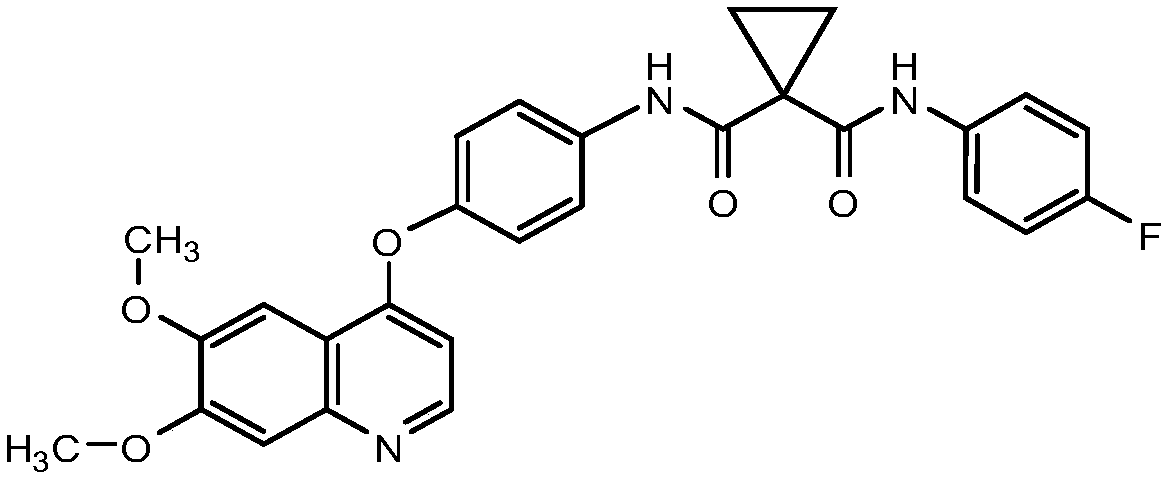
[0201] Embodiment 2. The method of Embodiment 1, wherein the compound of Formula I is Compound 1 or a pharmaceutically acceptable salt thereof.
[0202]
[0203] Embodiment 3. The method of Embodiment 2, wherein Compound 1 is administered as the L-malate salt.
[0204] Embodiment 4. The method of Embodiment 2, wherein Compound 1 is administered as the S-malate salt.
[0205] Embodiment 5. The method of embodiments 2-4, wherein atezolizumab is administere...
Embodiment 1
[0261] Example 1. Summary of an experimental clinical trial investigating the combination of cabozantinib (XL184) and atezolizumab in subjects with locally advanced or metastatic solid tumors such as advanced UC, RCC, CRPC and NSCLC
[0262] Fundamental
[0263] Multitargeted tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) represent two systemic modalities that have made recent advances in anticancer therapy in recent years. Both classes of therapy have shown broad clinical efficacy, resulting in new approved treatment options in multiple tumor types including: Renal Cell Carcinoma (RCC), Urothelial Carcinoma (UC), Melanoma, Non-Small Cell Lung Cancer (NSCLC) and so on. The success of these therapy types as single agents with distinct mechanisms of action has naturally drawn attention to the evaluation of combinations of TKIs and ICIs in search of further, possibly synergistic, anticancer clinical effects.
[0264] Atezolizumab is a humanized immu...
Embodiment 2
[0465] Example 2. Phase Ib Study of Cabozantinib and Atezolizumab for the Treatment of Locally Advanced or Metastatic Solid Tumors
[0466] 1. Background and Rationale
[0467] 1.1 Background
[0468] Multitargeted tyrosine kinase inhibitor (TKI) and immune checkpoint inhibitor (ICI) immunotherapy represent two systemic modalities that have contributed to recent advances in anticancer therapy. Both classes of therapy have shown broad clinical efficacy, resulting in new approved treatment options in multiple tumor types including: Renal Cell Carcinoma (RCC), Urothelial Carcinoma (UC), Melanoma, Non-Small Cell Lung Cancer (NSCLC) and so on. The success of these therapy types as single agents with distinct mechanisms of action has naturally drawn attention to the evaluation of combinations of TKIs and ICIs in search of further, possibly synergistic, anticancer clinical effects.
[0469] 1.1.1 Atezolizumab
[0470] Atezolizumab is a humanized immunoglobulin (Ig) G1 monoclonal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com