Application of decitabine and oxaliplatin in preparation of combination drug for treating renal cell carcinoma
A technology of oxaliplatin and decitabine, which is applied in the field of medicine and can solve problems such as unsatisfactory curative effect of solid tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The invention provides the application of decitabine and oxaliplatin in the preparation of a combination drug for treating renal cell carcinoma, wherein the preferred molar concentration ratio of decitabine and oxaliplatin is 1:5-1:8.
Embodiment 2
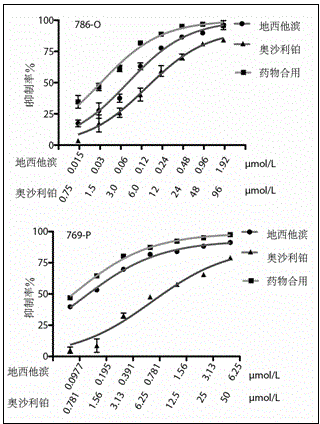
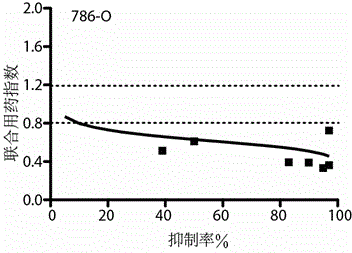
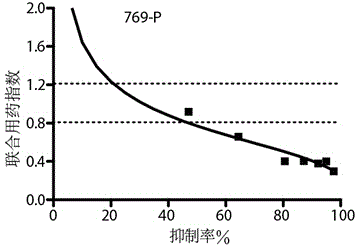
[0022] Example 2 The combination of decitabine and oxaliplatin synergistically enhanced the sensitivity of renal cell carcinoma cell lines to both drugs.
[0023] 1. Reagents and materials:
[0024] Renal cancer cell lines 786-O [CRL-1932?, ATCC] and 769-P [CRL-1933?, ATCC] were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. Both cell lines were cultured in RPMI-1640 medium (GIBCO, product number 31800022, added NaHCO 3 1.5 g / L, glucose 2.5 g / L, Sodium Pyruvate 0.11 g / L), and contains 10% high-quality fetal bovine serum. Culture conditions are 37°C, 5% CO 2 .
[0025] Decitabine was purchased from Sigma-Aldrich (5-Aza-2'-deoxycytidine, A3656). Oxaliplatin was purchased from China National Institutes for Food and Drug Control.
[0026] 2. Experimental method:
[0027] Decitabine, oxaliplatin stock solution and working solution preparation:
[0028] Using DMSO as a solvent, dilute decitabine and oxaliplatin to 1000 x stock solution and 250 x stock so...
Embodiment 3
[0048] The present invention finds that the combination of decitabine and oxaliplatin enhances the accumulation of oxaliplatin in the tumor cells of the renal cell carcinoma mouse model and enhances the sensitivity of the renal cell carcinoma mouse model to oxaliplatin.
[0049] 1. Reagents and Materials
[0050] Female Balb / C nude mice were purchased from Shanghai Experimental Animal Research Center. They were housed in the Experimental Animal Center of Zhejiang University.
[0051] Renal carcinoma cell line 786-O [CRL-1932?, ATCC] was purchased from Shanghai Cell Bank, Chinese Academy of Sciences. Both cell lines were cultured in RPMI-1640 medium (GIBCO, product number 31800022, added NaHCO 3 1.5 g / L, glucose 2.5 g / L, Sodium Pyruvate 0.11 g / L), and contains 10% high-quality fetal bovine serum. Culture conditions are 37°C, 5% CO 2 .
[0052] Decitabine was purchased from Sigma-Aldrich (5-Aza-2'-deoxycytidine, A3656). Oxaliplatin was purchased from China National Instit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com