Diagnostic and therapeutic methods for cancer
A kind of therapy and cancer technology, applied in the field of cancer diagnosis and treatment, can solve the problems of timely detection and treatment, downregulation of T cell activation, suppression of anti-tumor immune activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
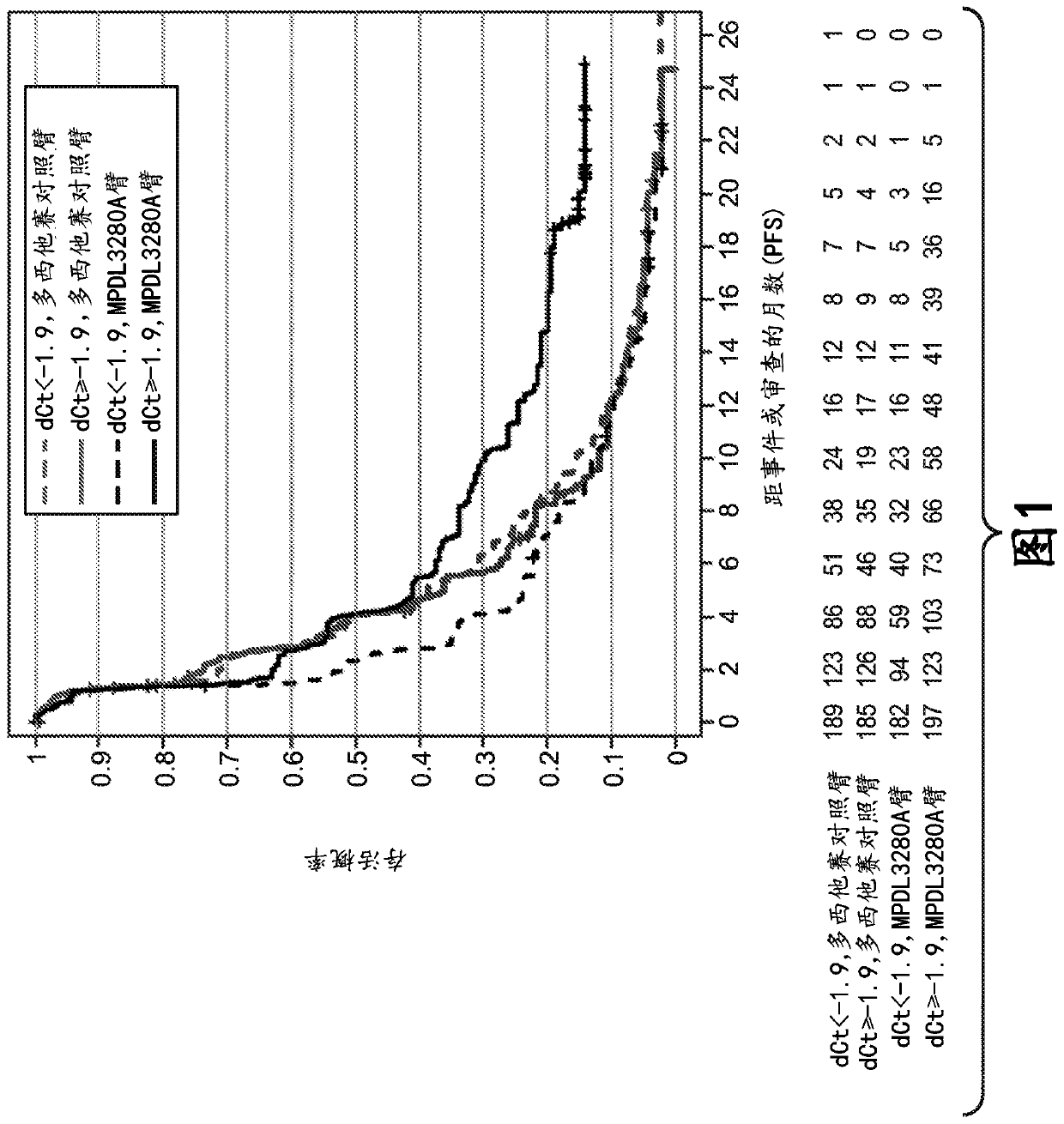
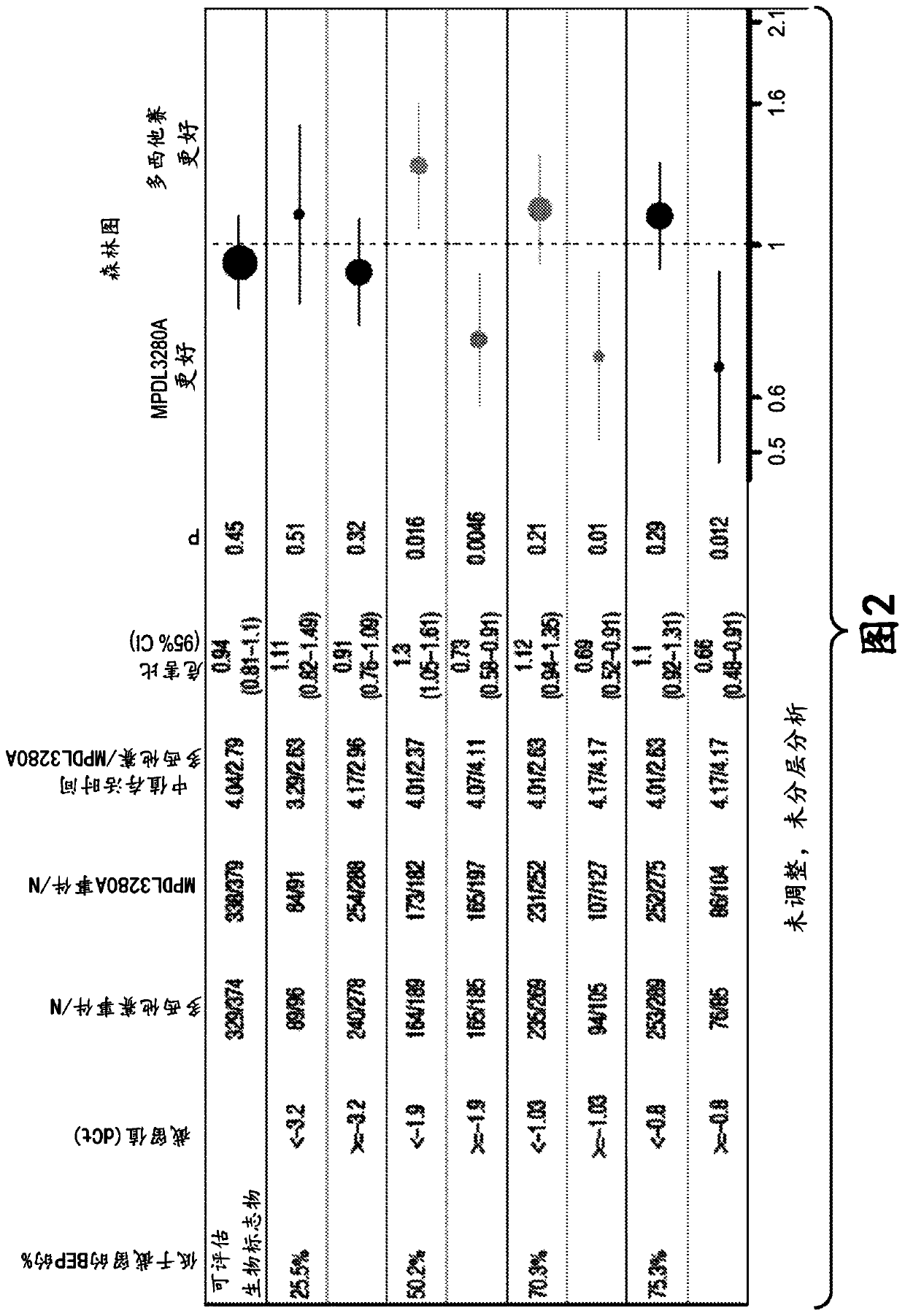
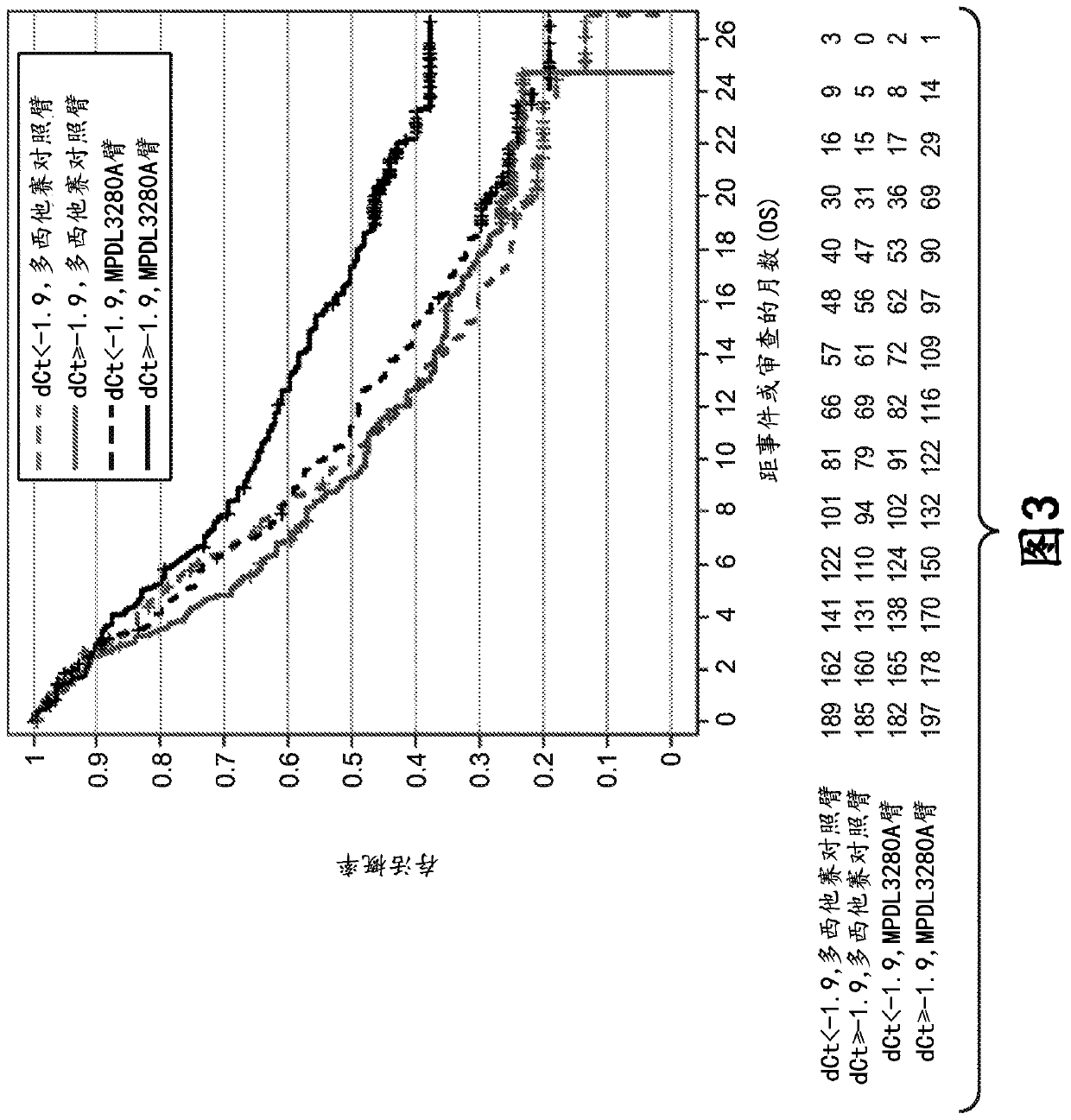
[0815] Example 1: Immune Score Expression Levels of (i) PD-L1, CXCL9, and IFNG or (ii) PD-L1, IFNG, GZMB, and CD8A in Patients with Non-Small Cell Lung Cancer (NSCLC) Association between clinical responses to zizumab (MPDL3280A) treatment
[0816] An RNA-based molecular assay was used to assess response to the anti-PD-L1 antibody atezolizumab in patients with non-small cell lung cancer (NSCLC) enrolled in a phase III clinical trial in which atezolizumab was administered as monotherapy (MPDL3280A) Association between clinical response to treatment and immune score expression levels of (i) PD-L1, CXCL9, and IFNG or (ii) PD-L1, IFNG, GZMB, and CD8A.
[0817] Research design
[0818] The OAK (Clinical Trial ID No.: NCT02008227) patient population consisted of 753 patients to evaluate the expression levels of (i) PD-L1, CXCL9, and IFNG and (ii) PD-L1, IFNG, GZMB, and CD8A. If the patient has locally advanced or metastatic (e.g., stage IIIB, stage IV, or recurrent) NSCLC; during o...
Embodiment 2
[0841] Example 2: Association Between Expression Levels of PD-L1, CXCL9, and IFNG and Clinical Response to Treatment with Atezolizumab (MPDL3280A) in Patients with NSCLC
[0842] An RNA-based molecular assay was used to assess the clinical response to treatment with the anti-PD-L1 antibody atezolizumab (MPDL3280A) in individuals with NSCLC enrolled in a phase II clinical trial in which atezolizumab was administered as monotherapy Correlation between response and expression levels of PD-L1, CXCL9, and IFNG.
[0843] Research design
[0844] The POPLAR (Clinical Trial ID No.: NCT01903993) patient population evaluating PD-L1, CXCL9, and IFNG expression levels consisted of 215 patients. If the patient has locally advanced or metastatic (e.g., stage IIIB, stage IV, or recurrent) NSCLC; during or after treatment with a prior platinum-containing regimen for locally advanced, unresectable / inoperable, or metastatic NSCLC Disease progression, or disease recurrence within 6 months of t...
Embodiment 3
[0855] Example 3: Association Between Expression Levels of PD-L1, CXCL9, and IFNG and Clinical Response to Treatment with Atezolizumab (MPDL3280A) in Patients with UBC
[0856] Use of an RNA-based molecular assay to assess response to atezolizumab in individuals with advanced urothelial bladder cancer (UBC) enrolled in a phase II clinical trial (IMvigor210 trial) in which atezolizumab was administered as monotherapy (MPDL3280A), Association between clinical response to anti-PD-L1 antibody therapy and expression levels of PD-L1, CXCL9, and IFNG.
[0857] Research design
[0858] The expression levels of PD-L1, CXCL9, and IFNG were assessed on pre-treatment tumor specimens from patients with advanced UBC in cohort 2 of the phase II IMvigor210 trial (Clinical Trial ID No.: NCT02108652). If patient has histologically or cytologically proven locally advanced or metastatic transitional cell carcinoma or urothelium (eg, renal pelvis, ureter, bladder, or urethra); disease progression...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com