Patents
Literature
40 results about "Esophageal adenocarcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adenocarcinoma of the esophagus is the most common type of cancerous esophageal tumour. It usually develops in the lower third of the esophagus, often in an area containing Barrett’s esophagus.
Methods and probes for detecting esophageal cancer
ActiveUS20060211019A1Promote resultsSugar derivativesMicrobiological testing/measurementEsophageal cancerOmodysplasia
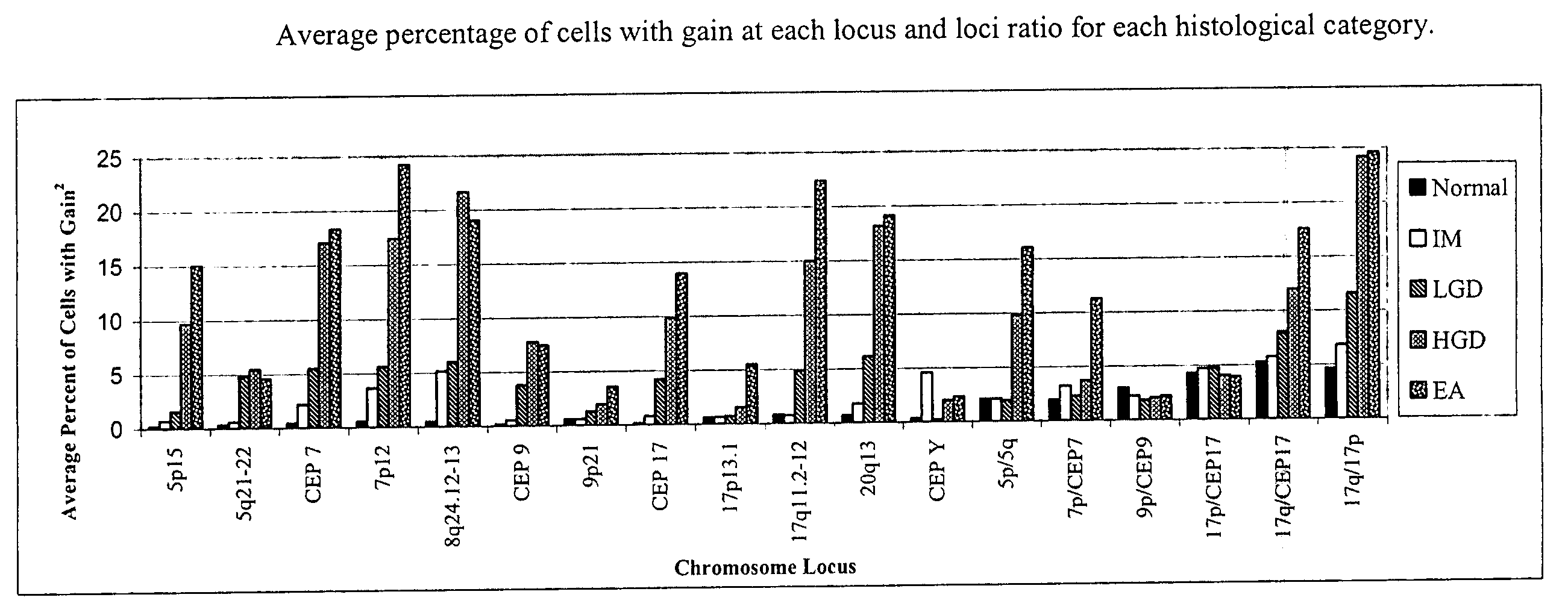
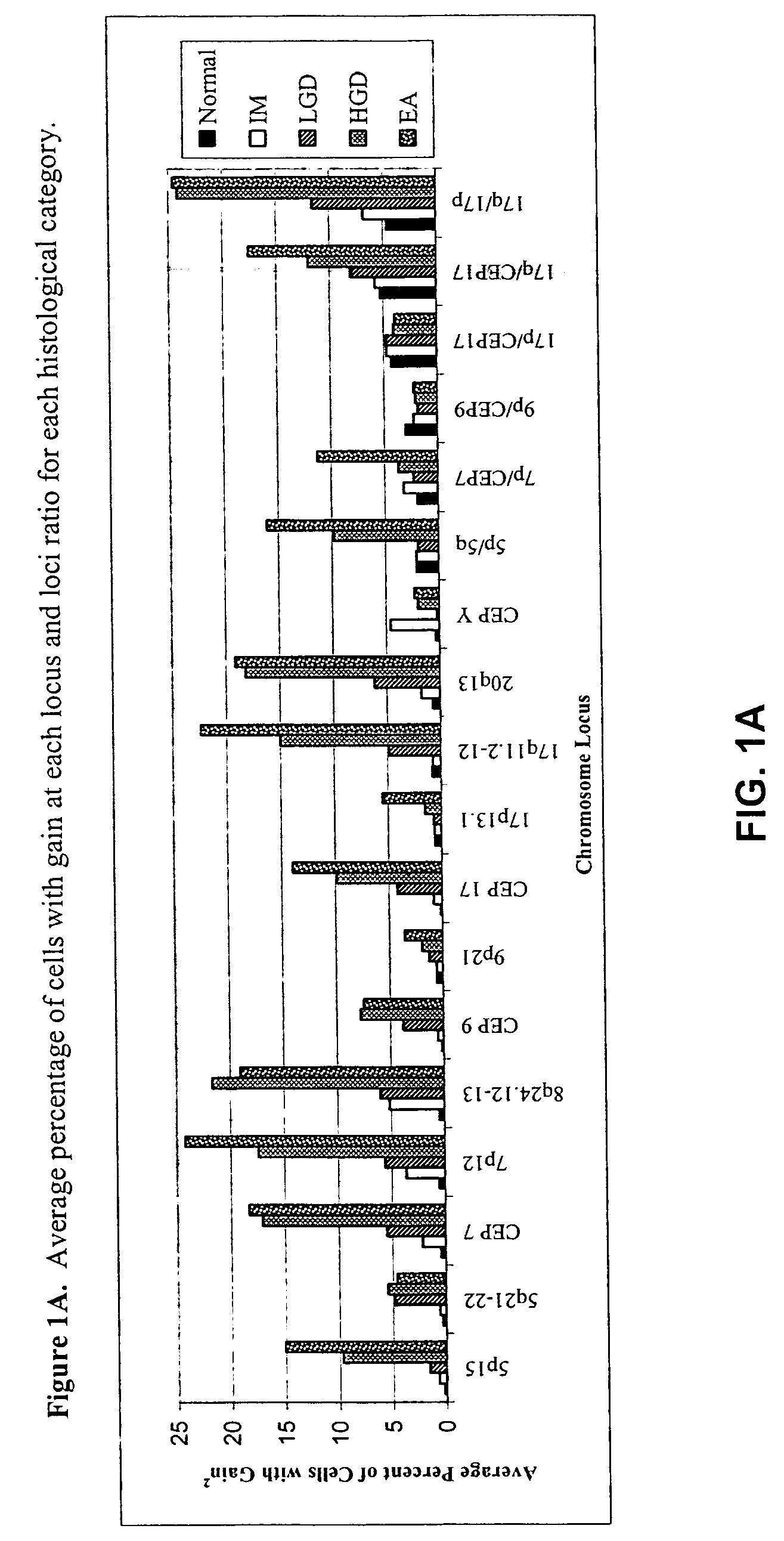
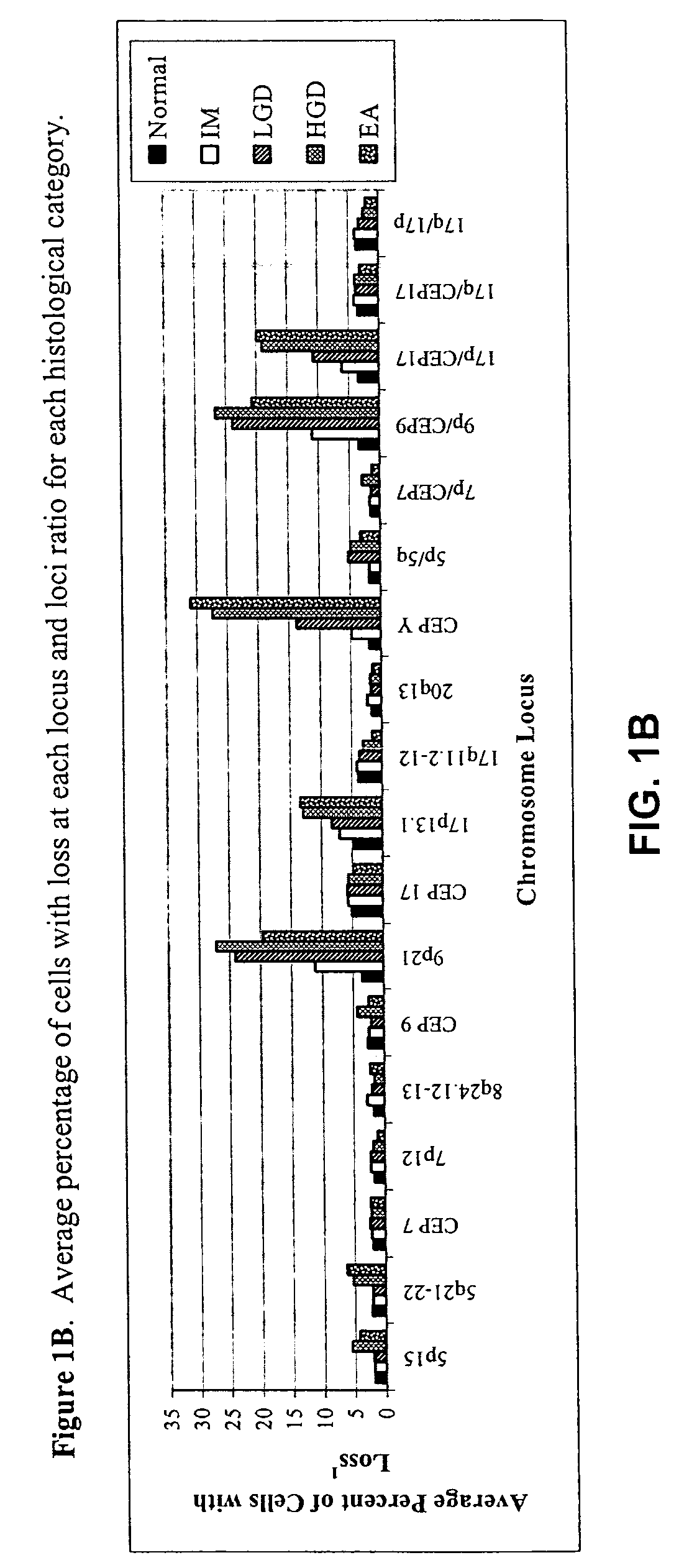
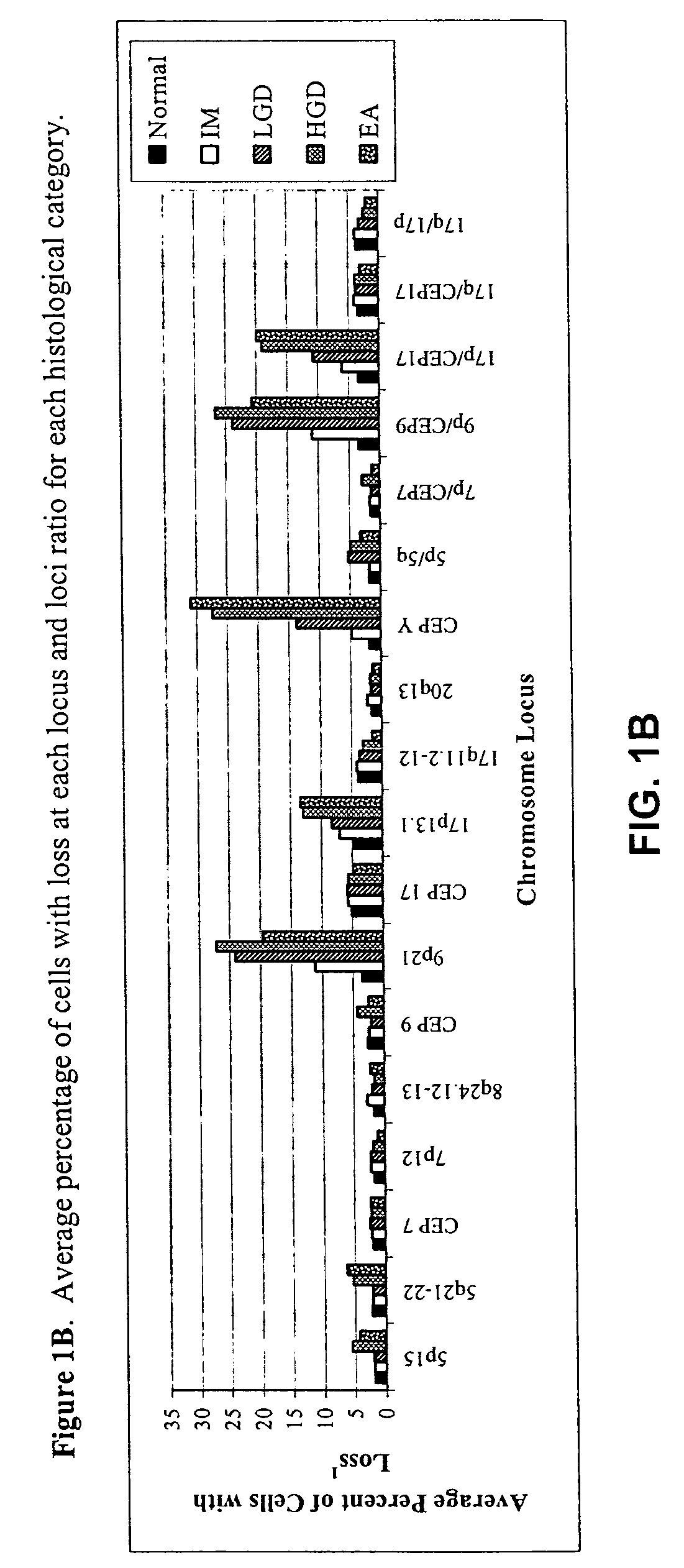
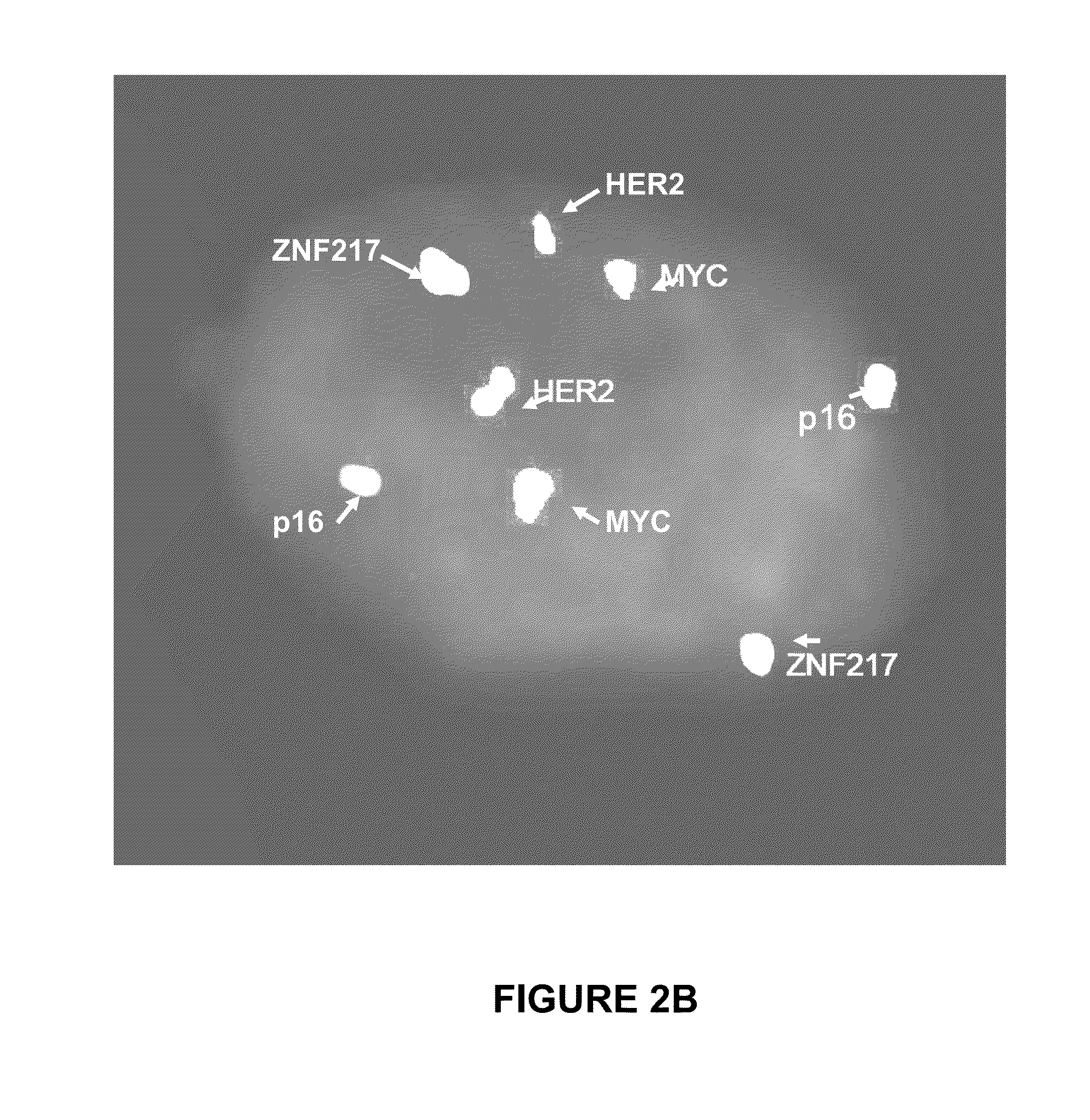
Probe sets and methods of using probes and probe sets for selectively detecting high grade dysplasia and esophageal adenocarcinoma or low grade dysplasia from biologic samples are described. Methods of the invention include contacting a biological sample obtained from a subject with a set of chromosomal probes to selectively detect an esophageal carcinoma or precursor lesion in the sample, if any, under conditions for specifically hybridizing the probes to their nucleic targets present in the sample. The presence or absence of high grade dysplasia and esophageal adenocarcinoma or low grade dysplasia is thereafter specifically determined from the hybridization pattern detected for the set of chromosomal probes to the biological sample.
Owner:ABBOTT MOLECULAR INC +1
Epigenetic sequences for esophageal adenocarcinoma
InactiveUS20080096768A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBarrett's esophagusBiology
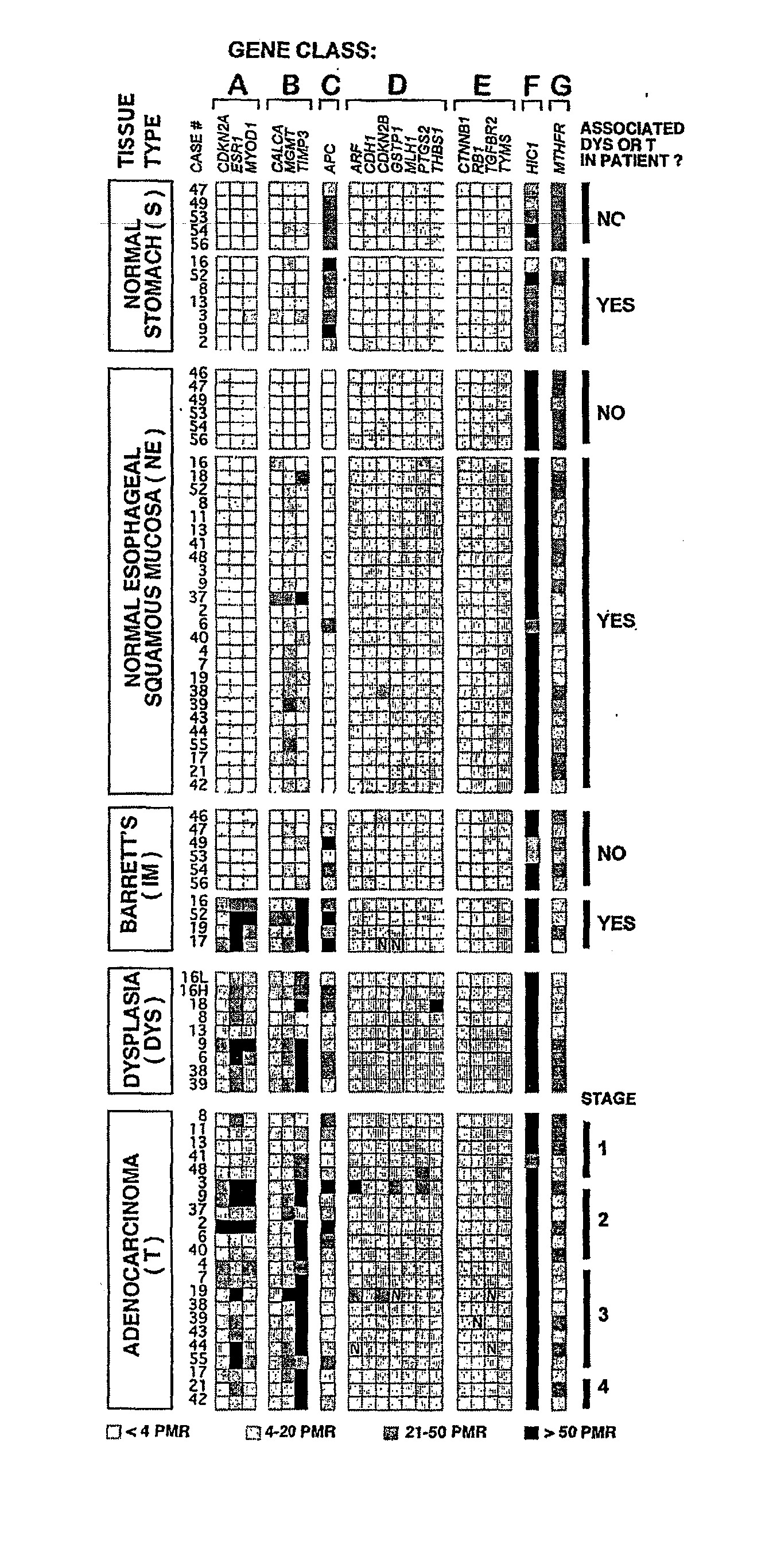
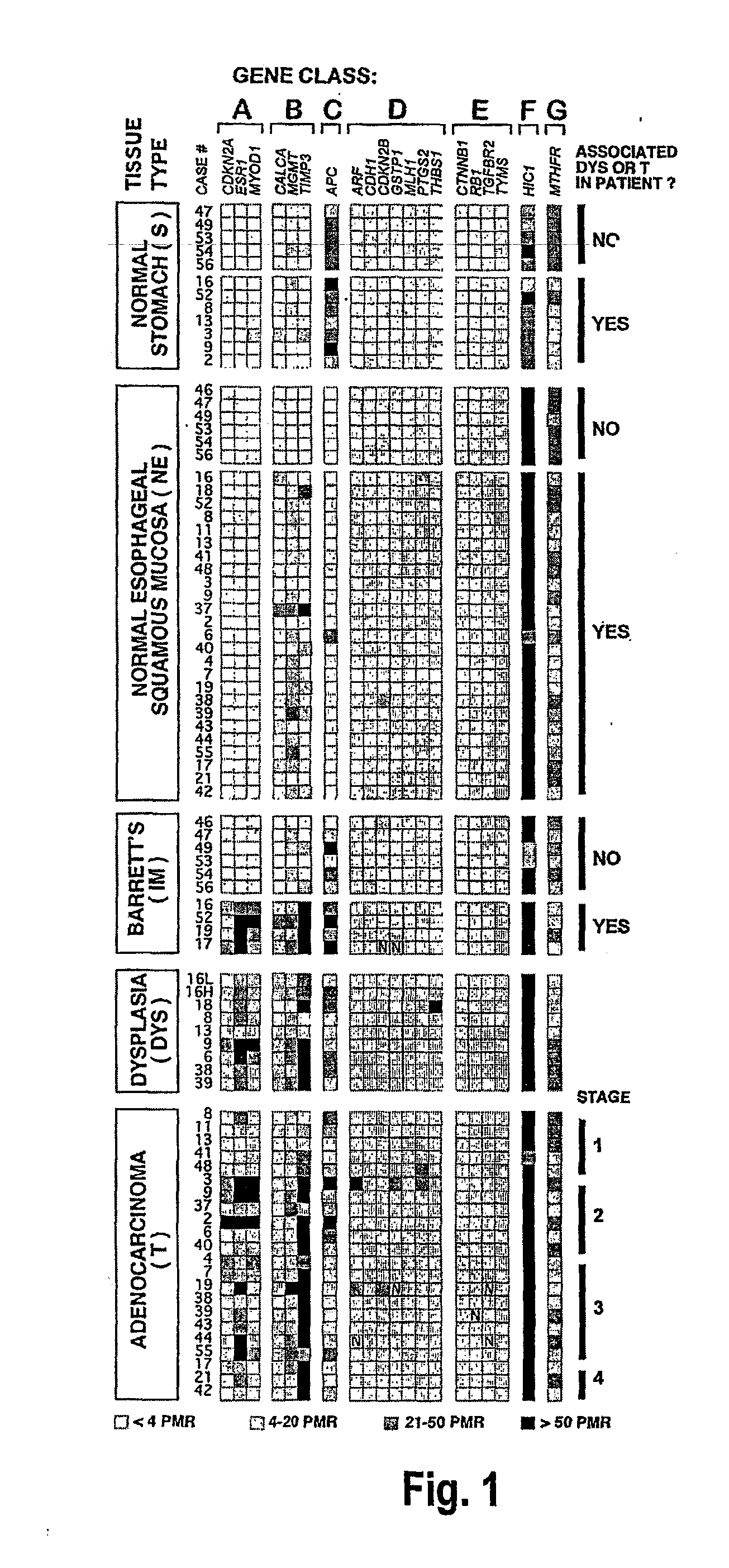
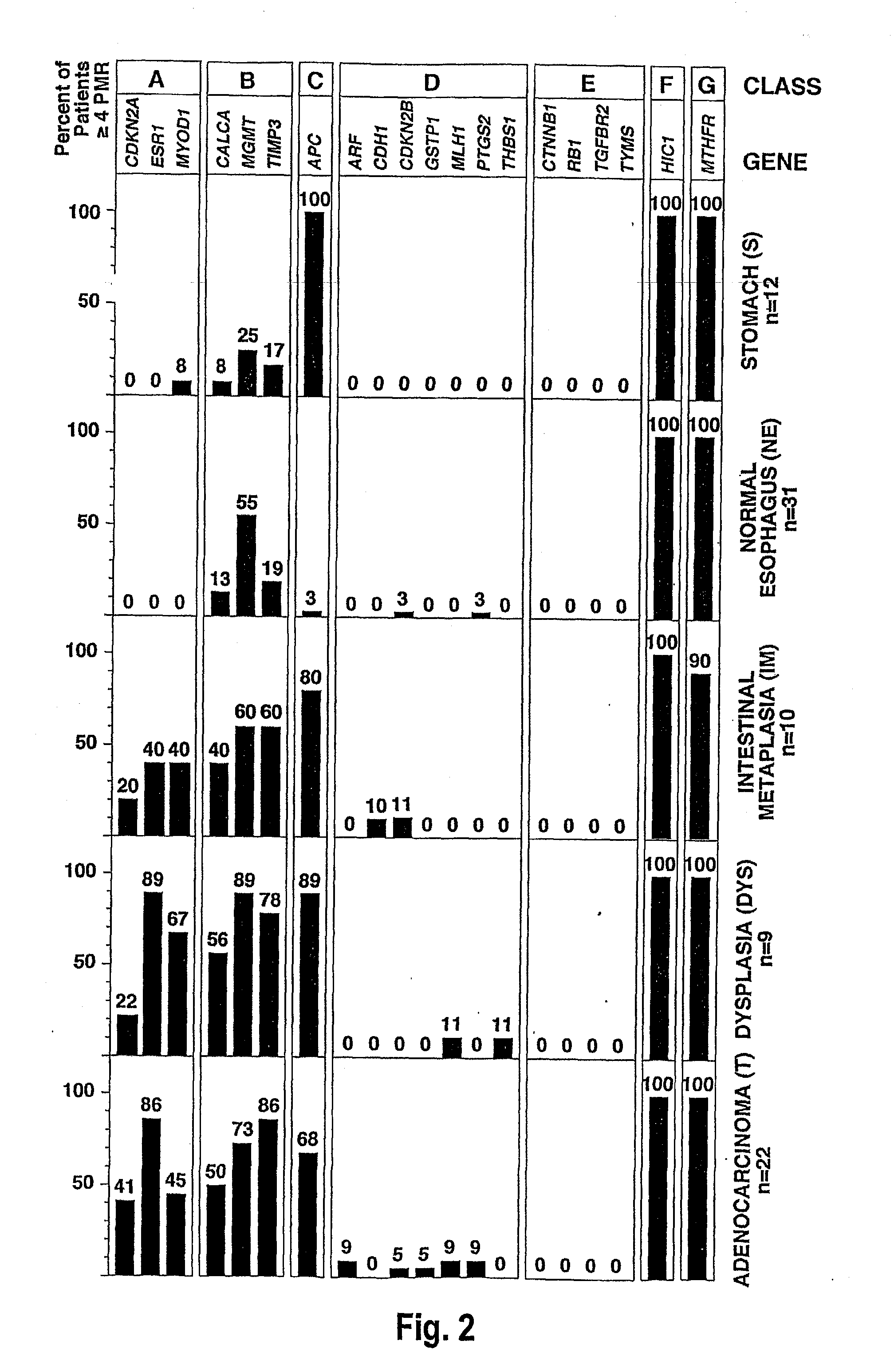
There is disclosed a diagnostic or prognostic assay for cancer, particularly gastrointestinal and esophageal adenocarcinoma. Specifically, the present invention provides a methylation pattern that can be assayed by standard methylation assays of CpG islands, including which genes are hypermethylated and which genes are unmethylated in gastrointestinal and esophageal adenocarcinomas, Barrett's esophagus, and normal squamous mucosa.
Owner:UNIV OF SOUTHERN CALIFORNIA
Methods and compositions for the diagnosis and treatment of esphageal adenocarcinomas
InactiveCN101861401ASugar derivativesMicrobiological testing/measurementSquamous CarcinomasSquamous Cell Cancers
Methods and compositions for the diagnosis, prognosis and / or treatment of esophageal adenocarcinoma and Barrett's esophagus associated adenocarcinoma are disclosed, along with more markers where a difference is indicative of esophageal adenocarcinoma and squamous cell carcinoma, and / or Barrett's esophagus associated adenocarcinomas or a predisposition thereto. The invention also provides methods and compositions of identifying anti-cancer agents therefor.
Owner:THE OHIO STATE UNIV RES FOUND +1
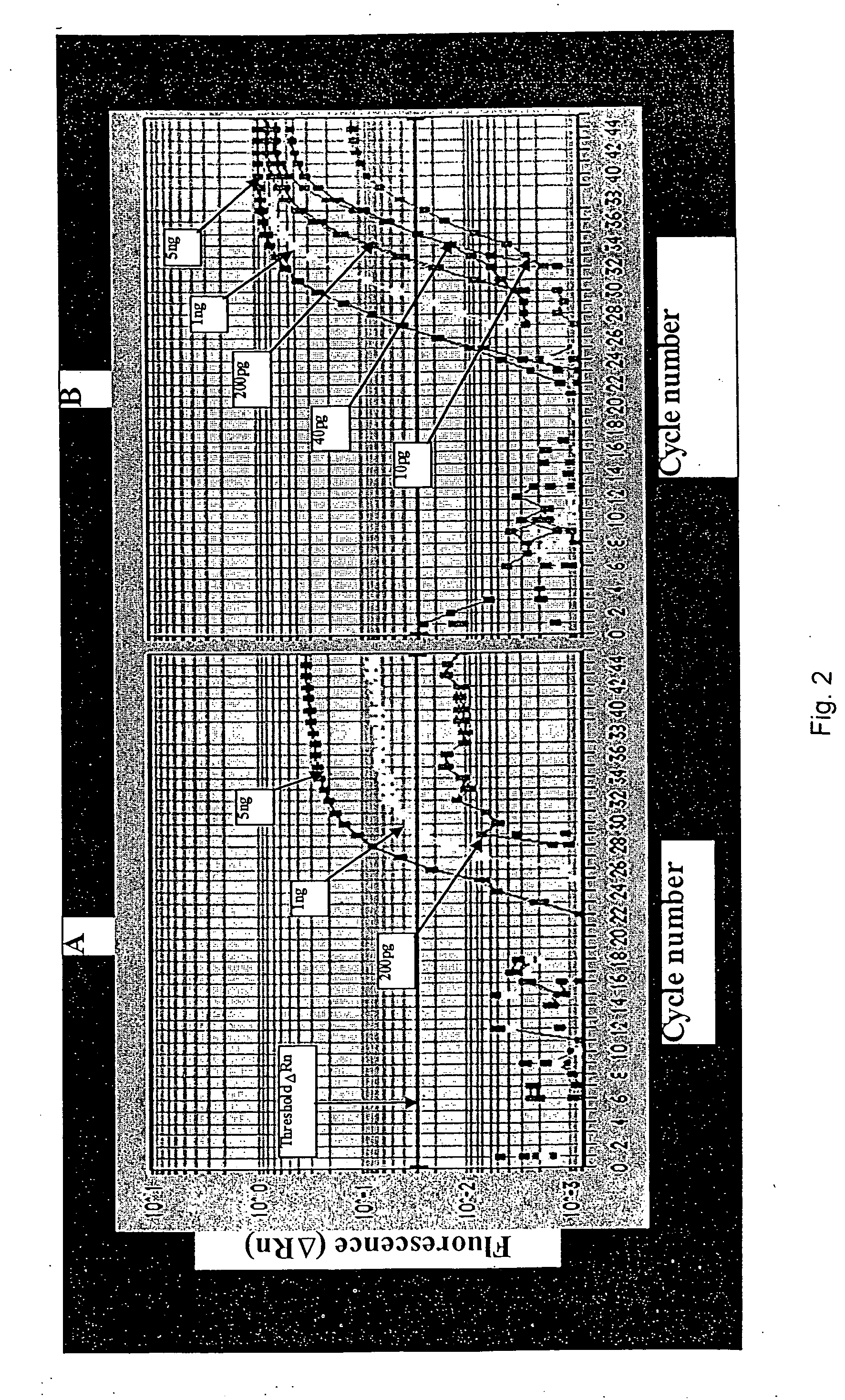
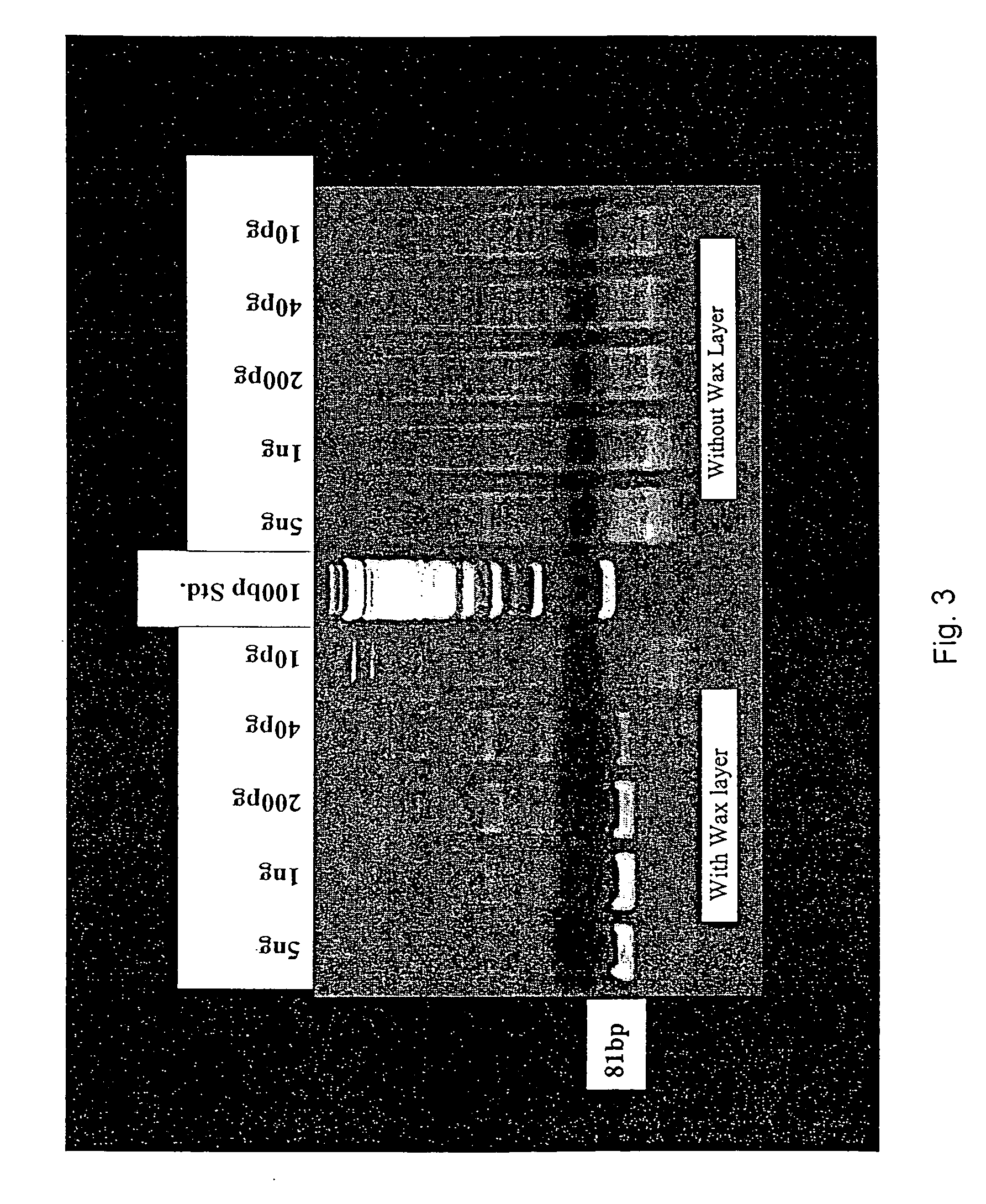
PCR method and related apparatus
InactiveUS20060205006A1Permit useReduce errorsMicrobiological testing/measurementRecombinant DNA-technologySentinel lymph nodeCarcinoembryonic antigen
A method for balancing multiplexed PCR methods is provided. In the method, two or more sequential temporal PCR stages are used to effectively separate two or more PCR reactions in a single tube as an alternative to primer limiting to modulate the relative rate of production of a first amplicon by a first primer set and a second amplicon by a second primer set during the first and second amplification stages. Also provided are rapid RT-PCR methods that find particular use in intraoperative diagnoses and prognoses, for instance in diagnosing malignant esophageal adenocarcenoma by determining expression levels of carcinoembryonic antigen (CEA) in sentinel lymph nodes.
Owner:UNIVERSITY OF PITTSBURGH
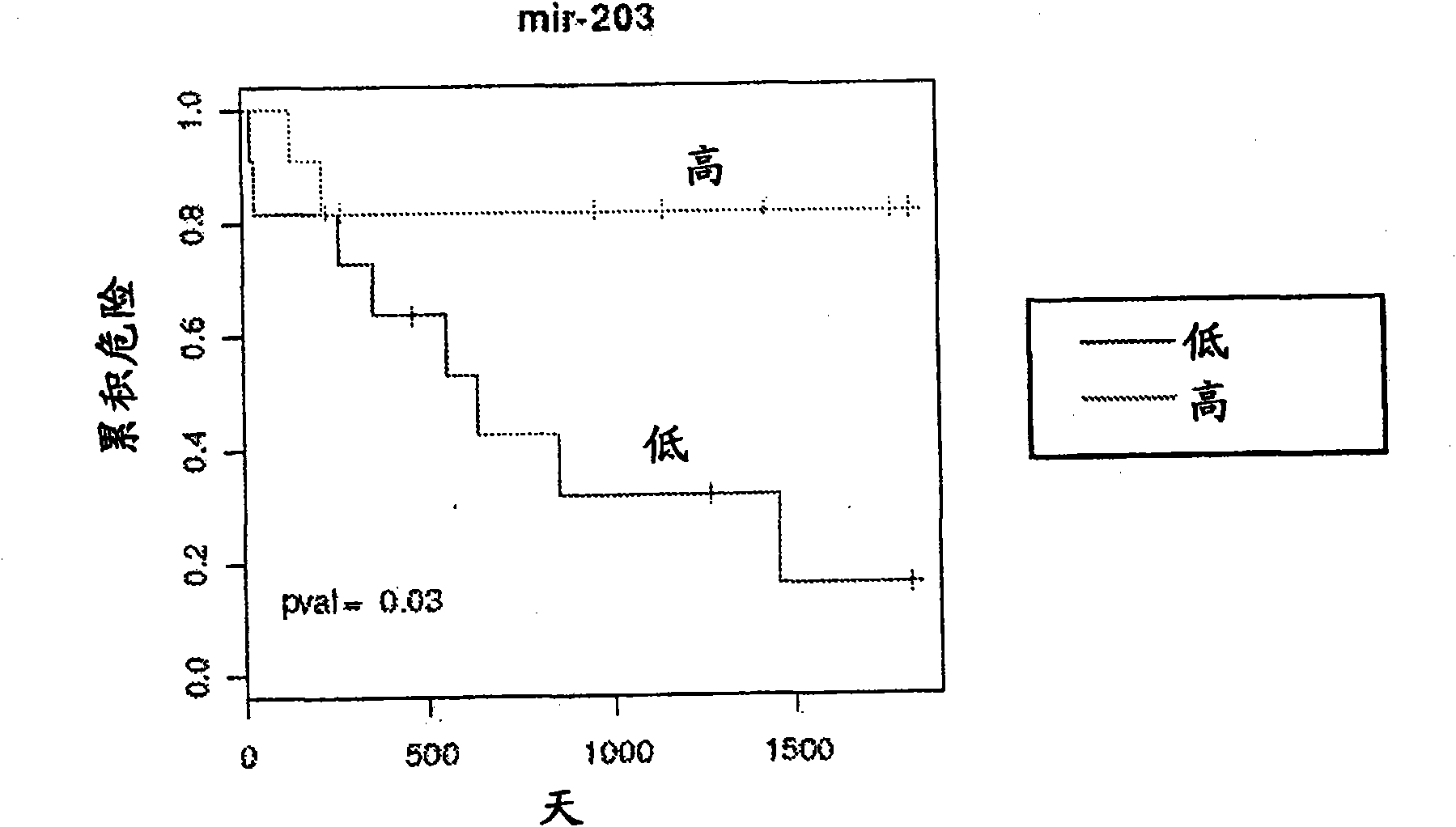
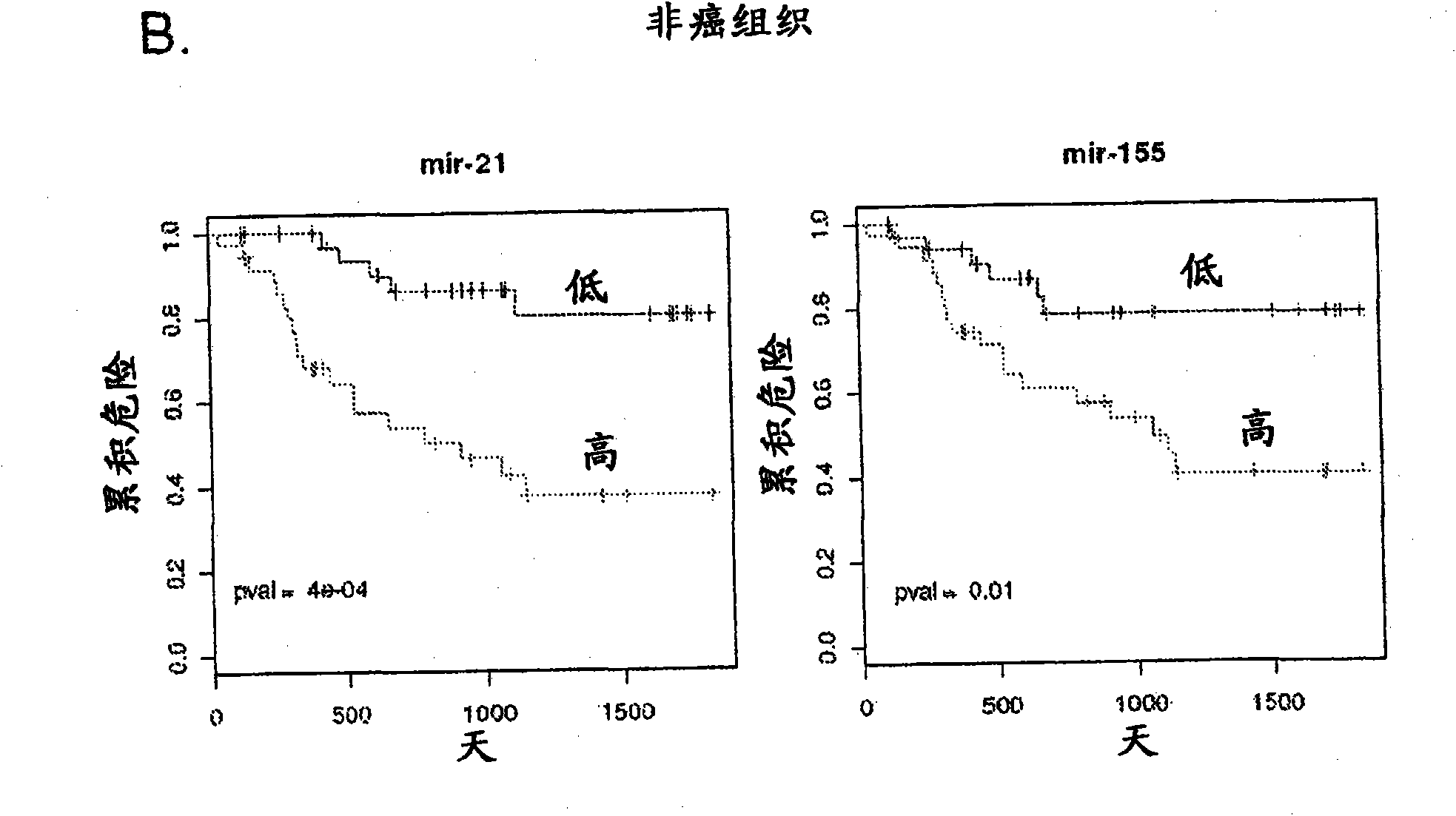
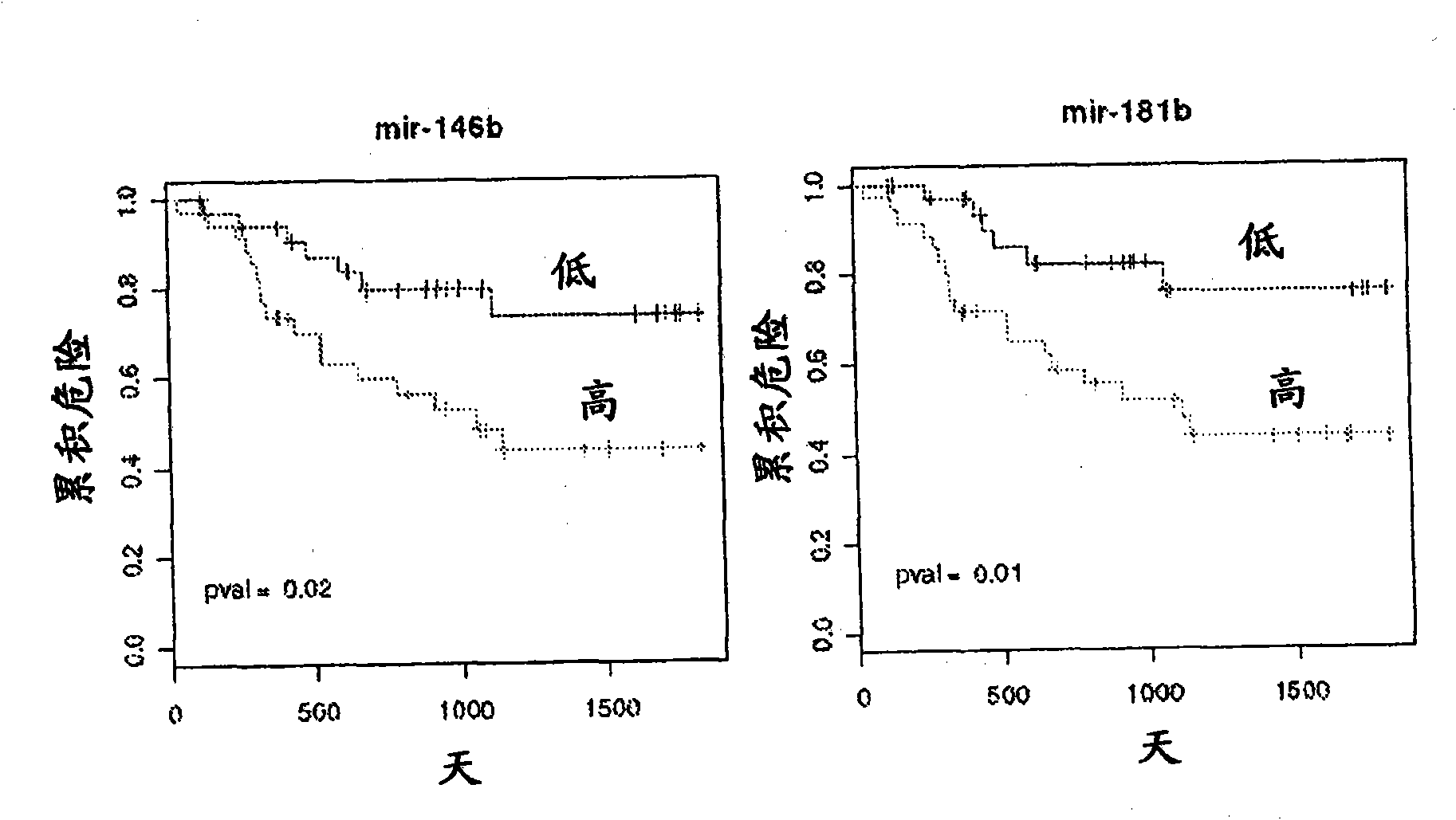
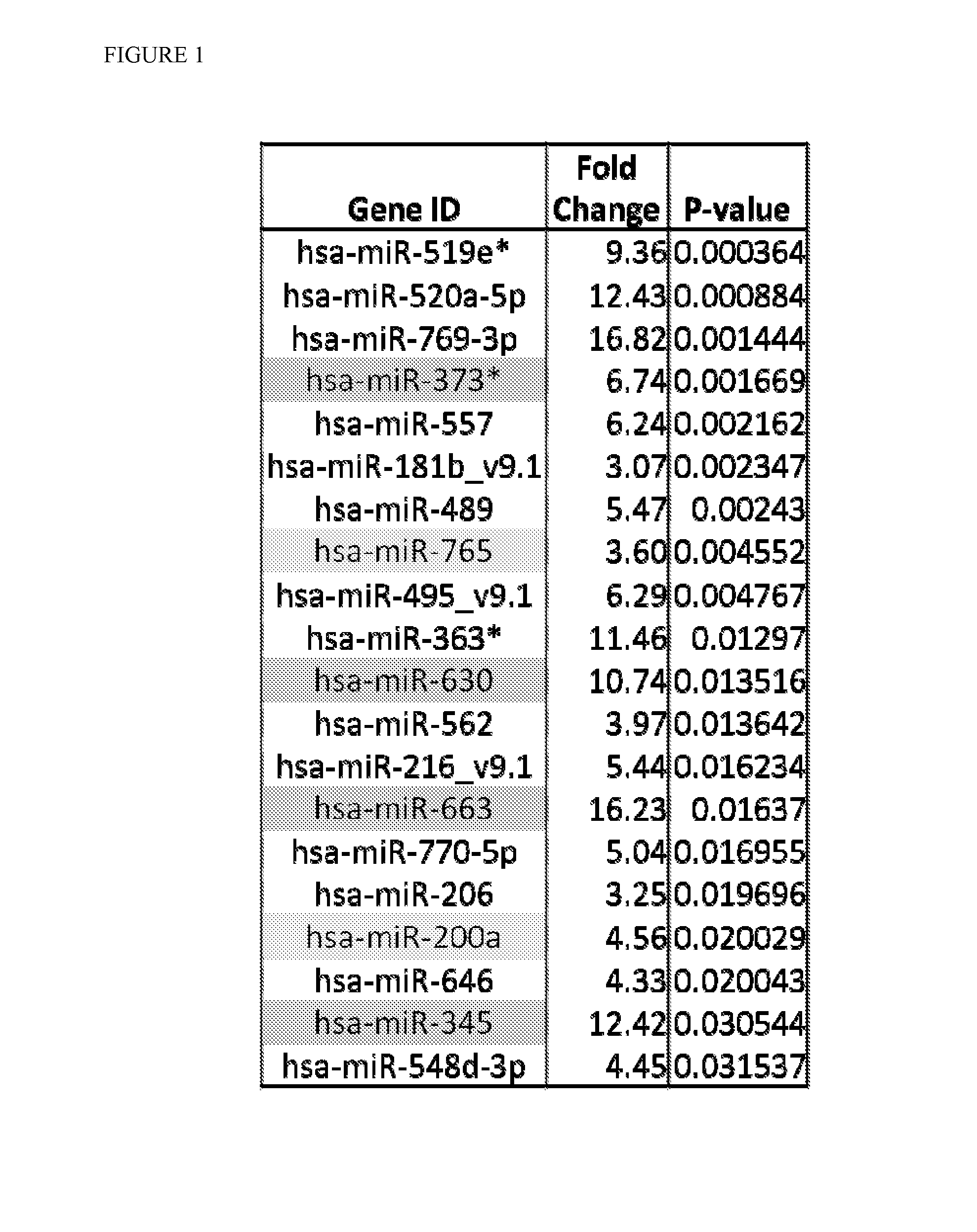
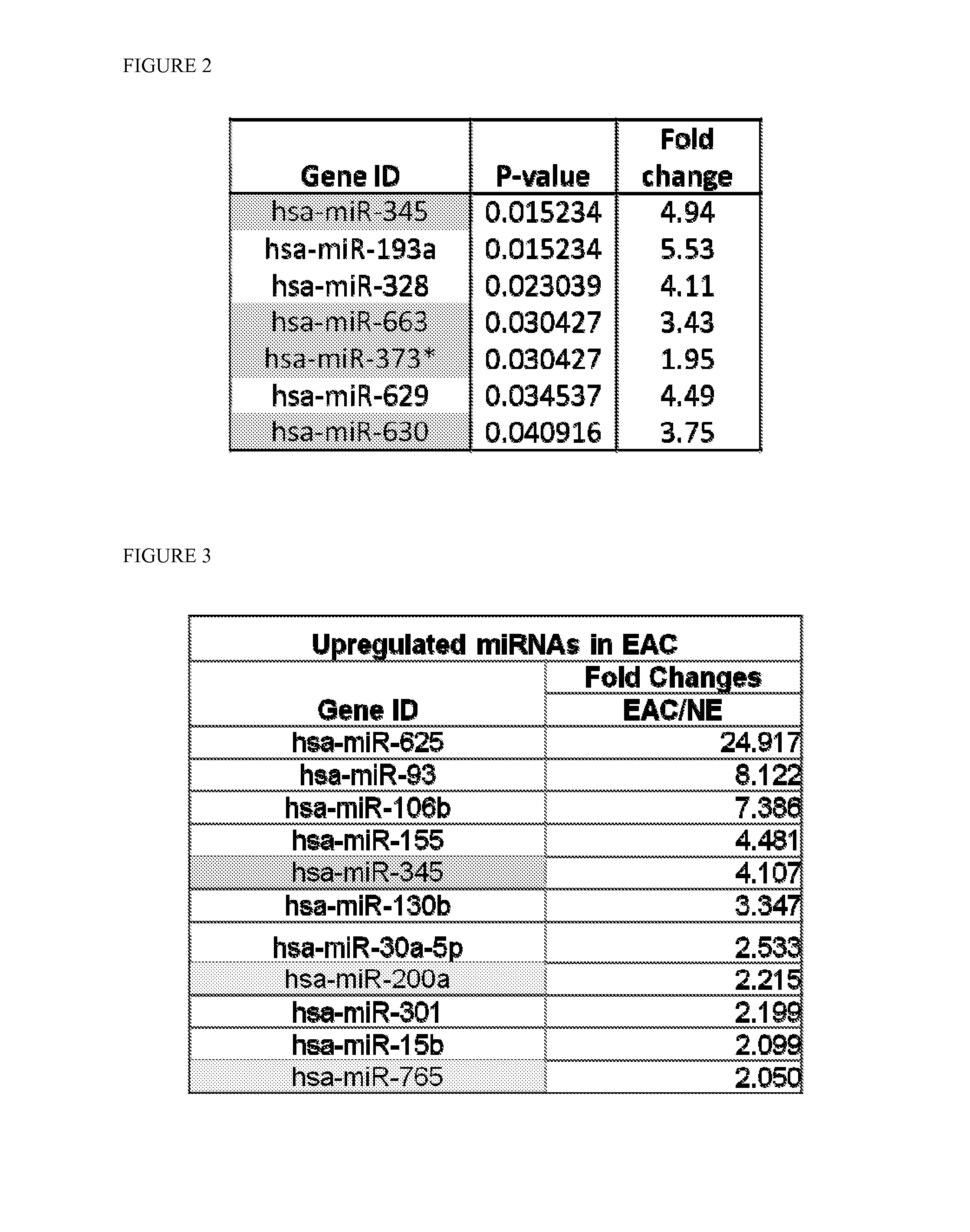
Serum-based mirna microarray and its use in diagnosis and treatment of barrett's esophagus (BE) and esophageal adenocarcinoma (EAC)
InactiveUS20140031258A1Nucleotide librariesMicrobiological testing/measurementSerum samplesMirna microarray
Robust and reliable molecular diagnostic screening tools for early detection of esophageal and gastrointestinal tract cancers and pre-cancerous lesions, such as Barrett's Esophagus, and esophageal adenocarcinoma are provided. Included in the invention is an array of miRNA probes specific for identifying, diagnosing and prognosticating esophageal and gastrointestinal tract cancers and pre-cancerous lesions in subjects from blood or serum samples. A biochip comprising the array as well as methods for its use are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods to assess the likelihood of dysplasia or esophageal adenocarcinoma
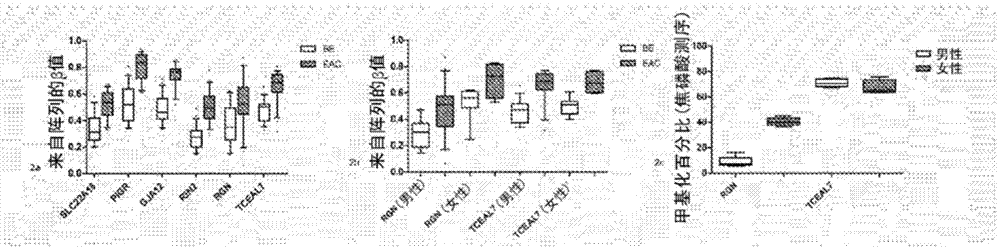
ActiveCN104508147AProblems with "lost" dysplasia/EAC are reduced or eliminatedMicrobiological testing/measurementBiologyMethylation
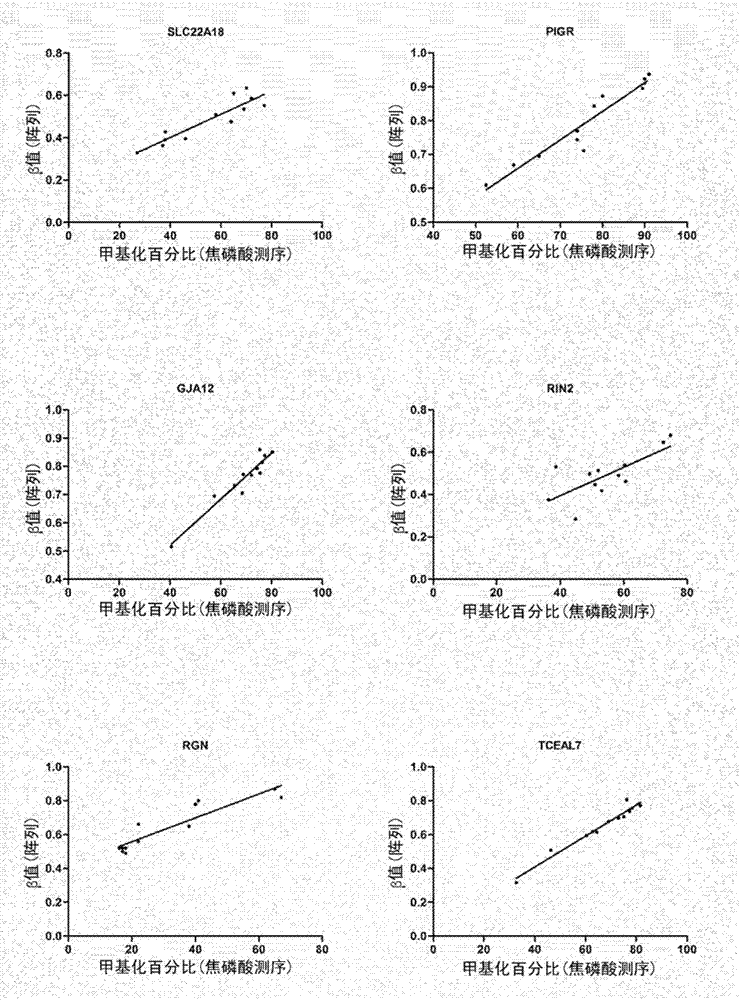
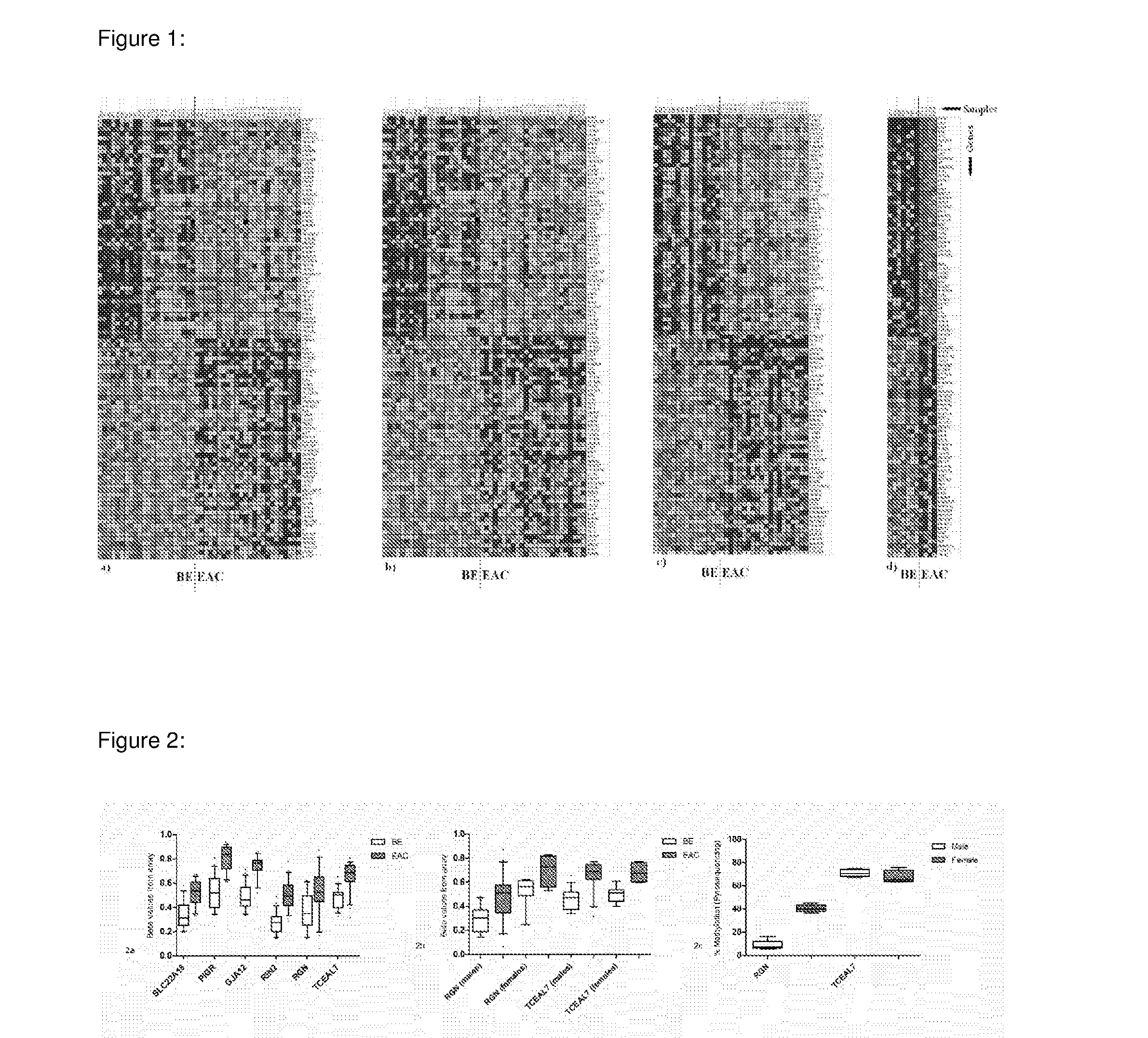
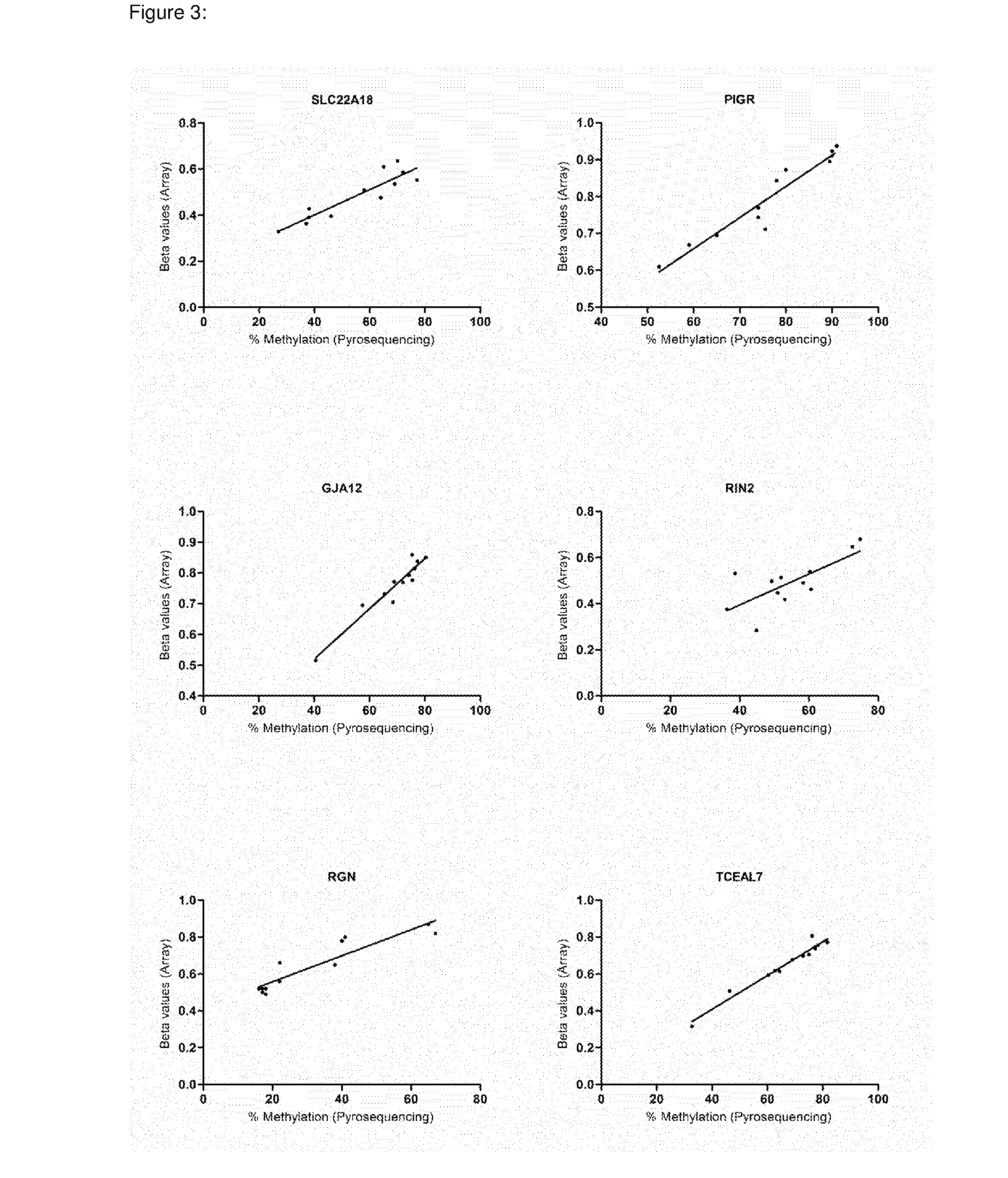
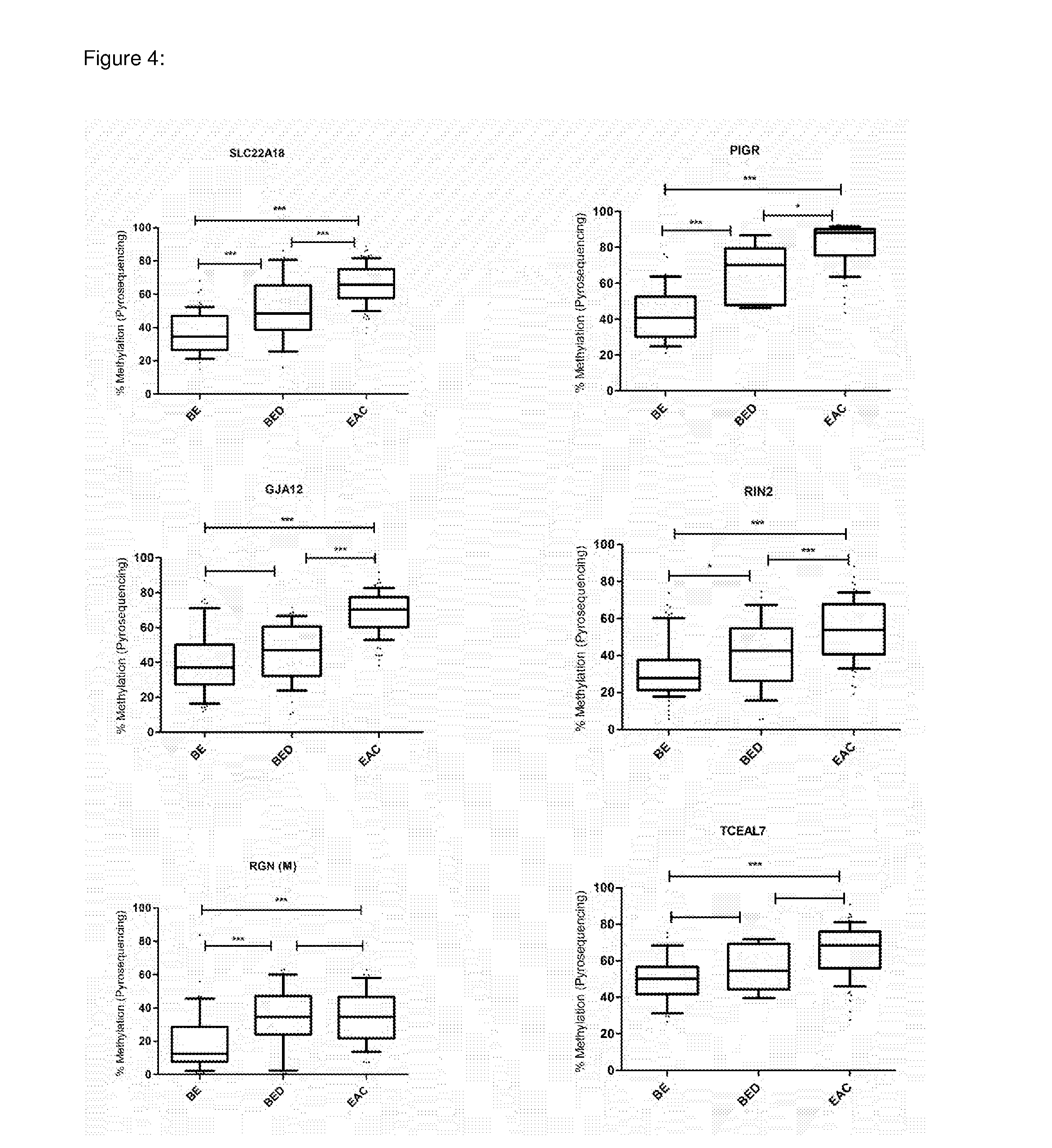
In some embodiments, a method for aiding assessment of the likelihood of dysplasia or esophageal adenocarcinoma being present in a subject can include (a) providing an esophagal sample from said subject (b) determining the methylation status of (i) SLC22A18, (ii) PIGR, (iii) GJA12 and (iv) RIN2 in said sample wherein if 2 or more of said genes are methylated then an increased likelihood of presence of dysplasia or esophageal is determined. The invention also relates to apparatus for same.
Owner:英国研究与创新署
Personalized medicine for the prediction of therapy targeting the hedgehog pathway
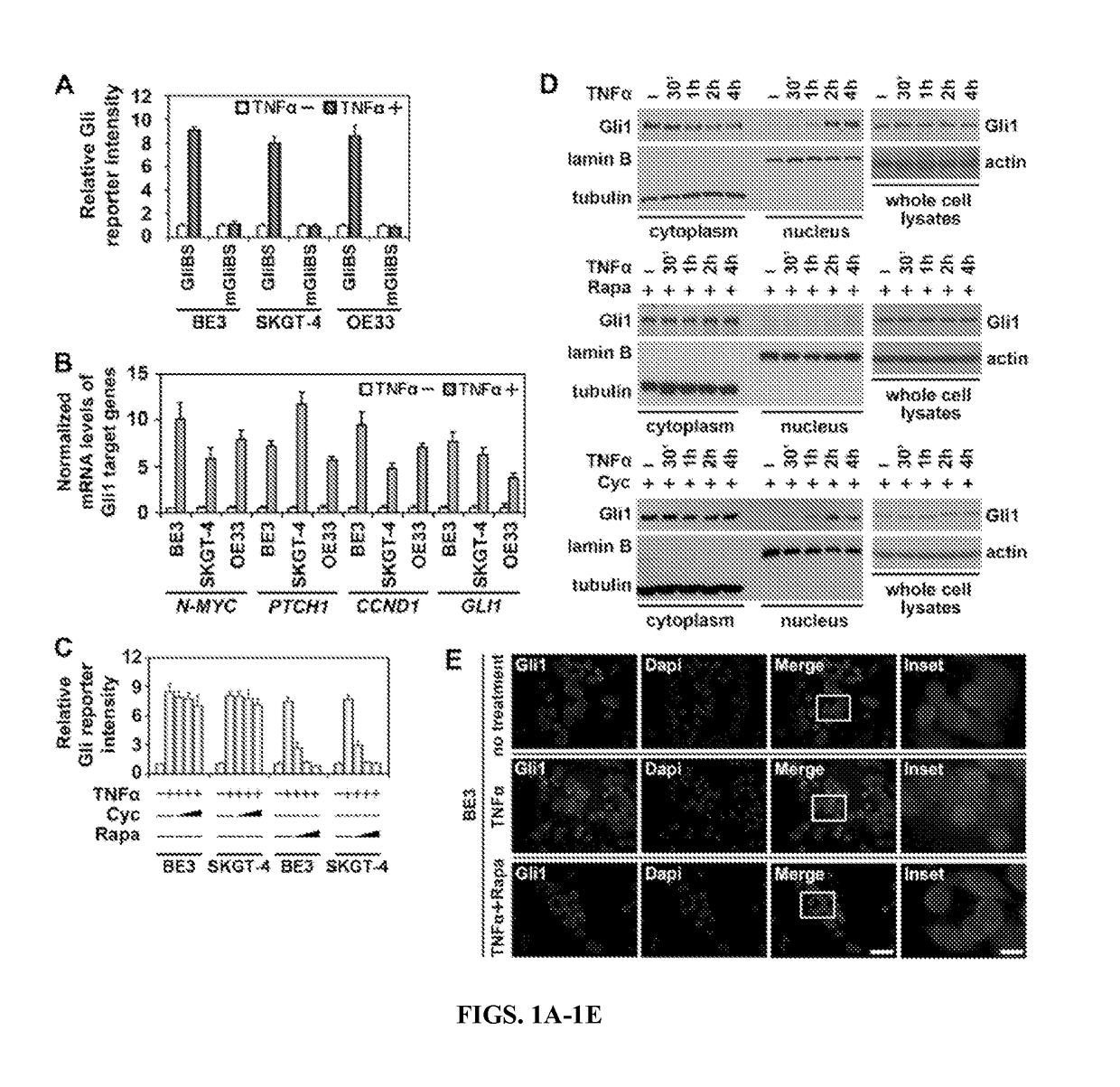
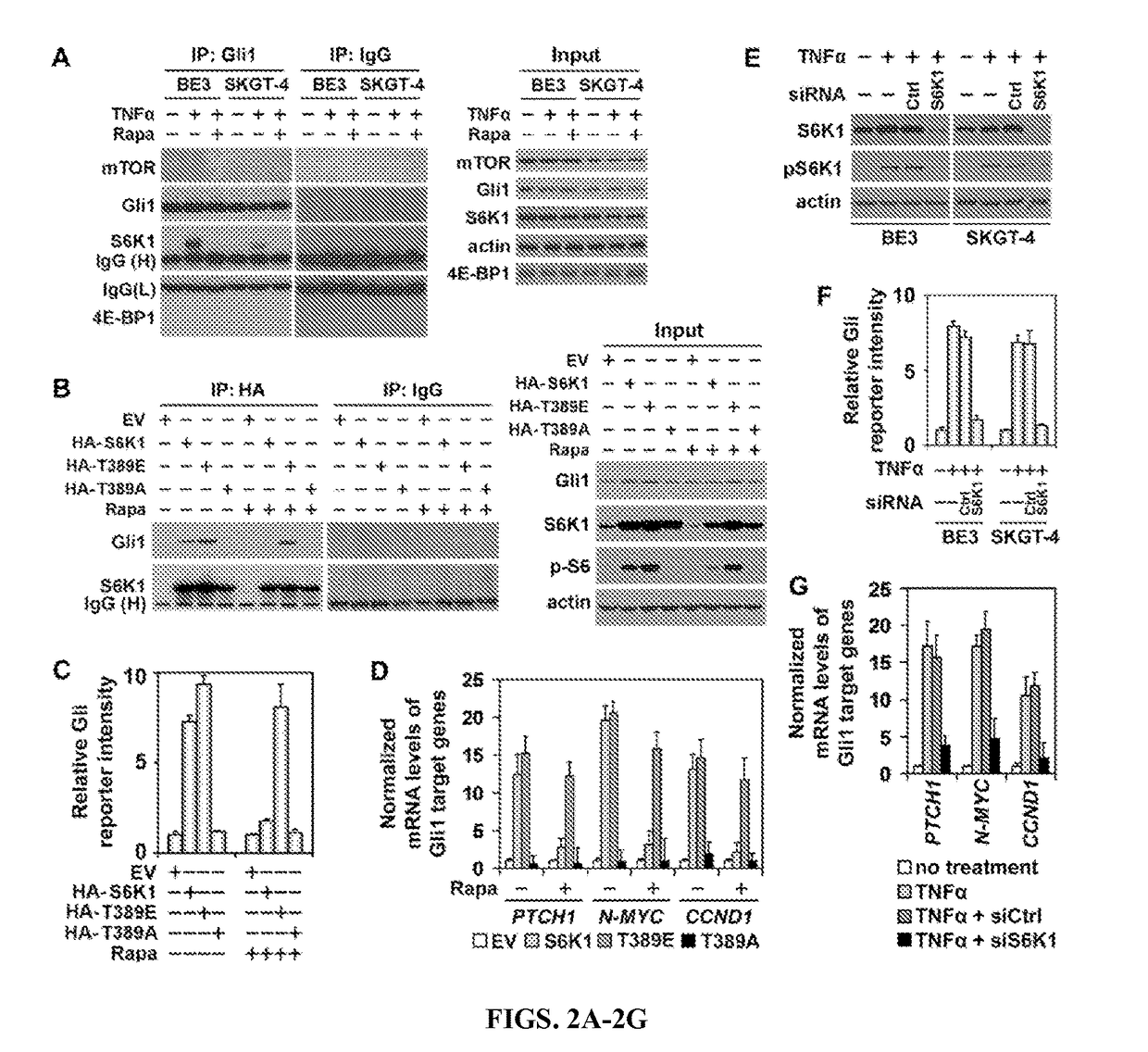
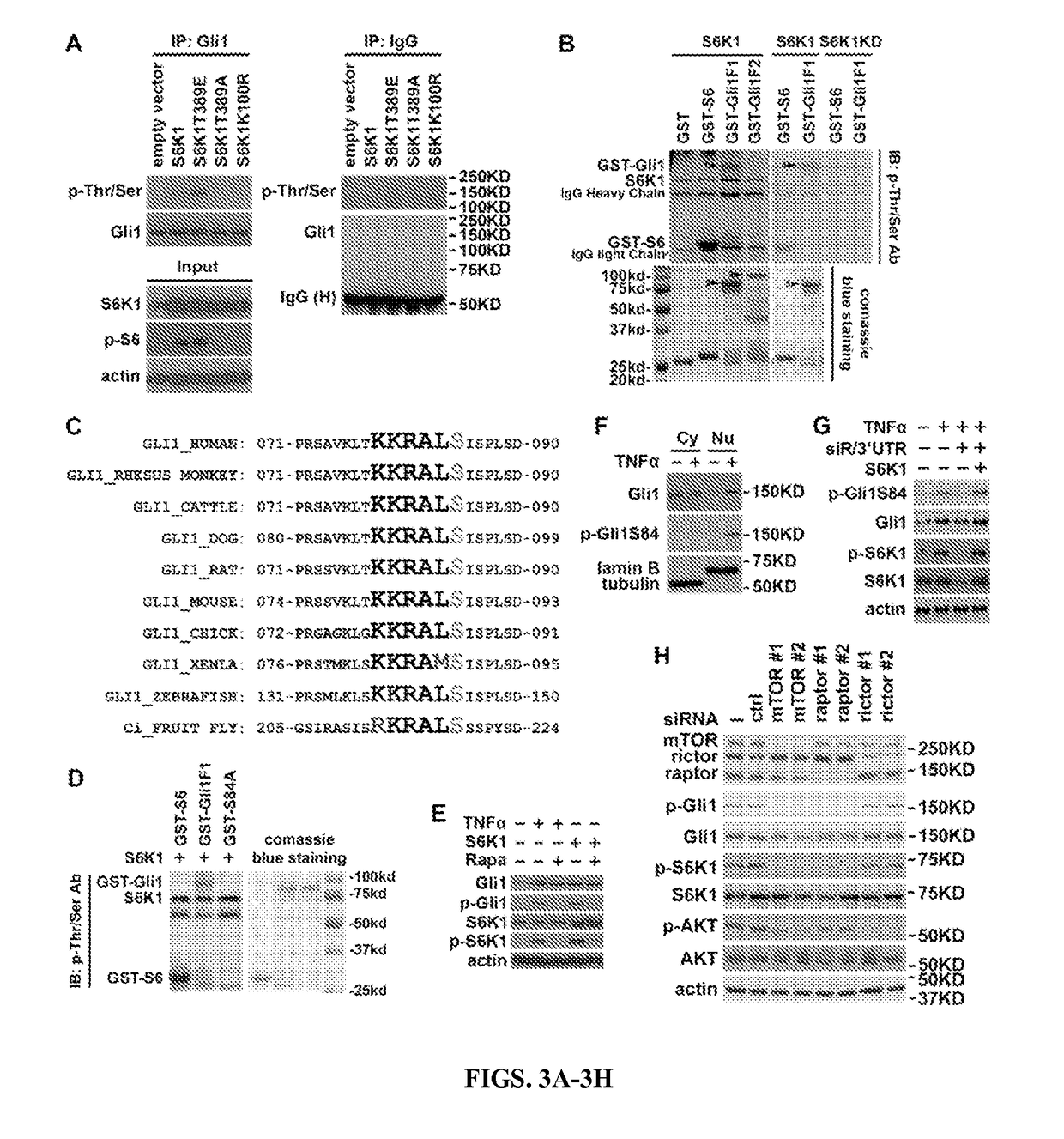
Methods and composition for tumor therapy, especially esophageal adenocarcinoma (EAC), are described. For example, in certain aspects methods for determining Hedgehog and mTOR signaling pathway status to select patients for administering a combination therapy of Hedgehog and mTOR signaling inhibitors are described. Furthermore, the invention provides compositions that involve testing kits for determining signaling status.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
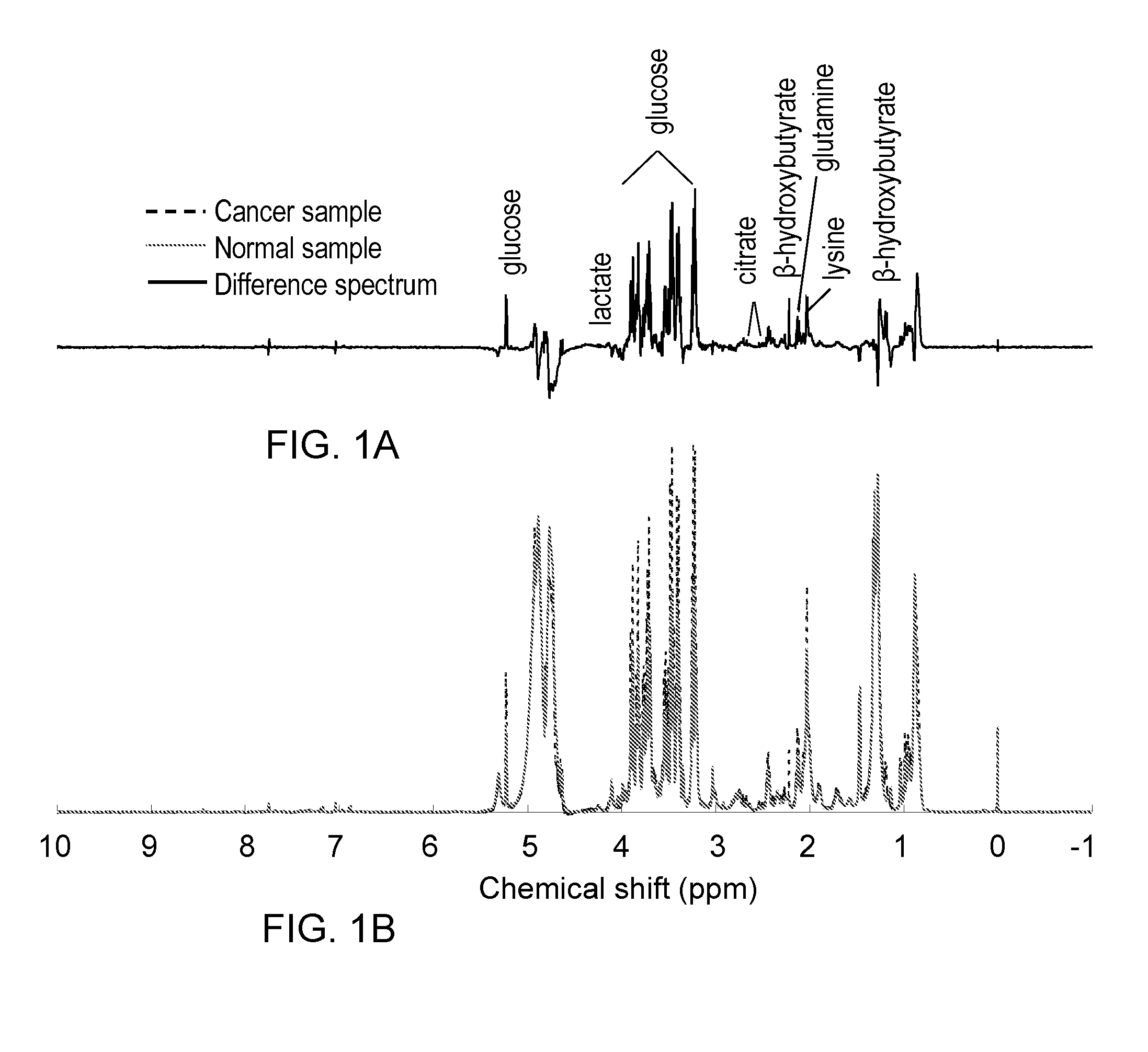
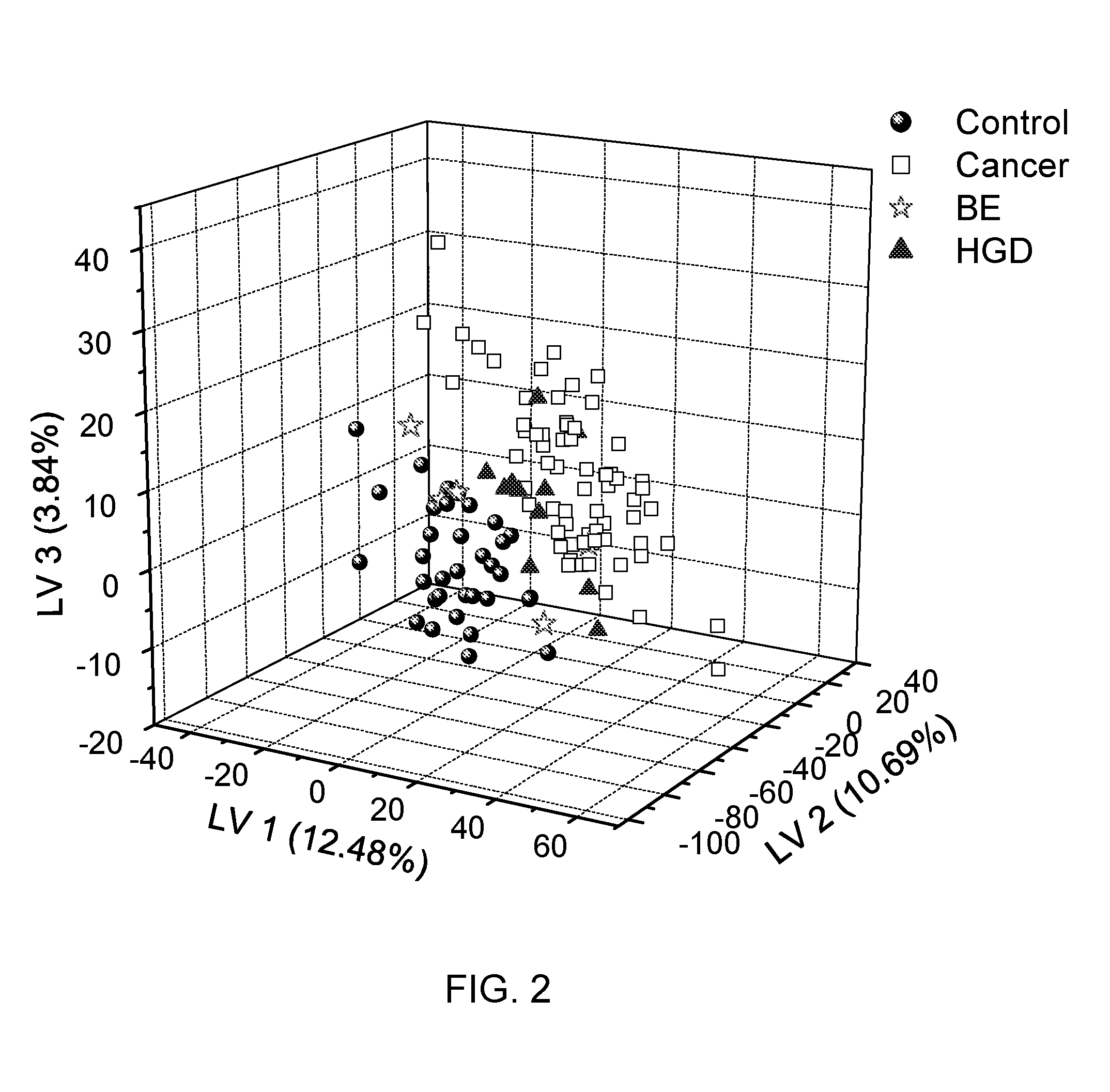
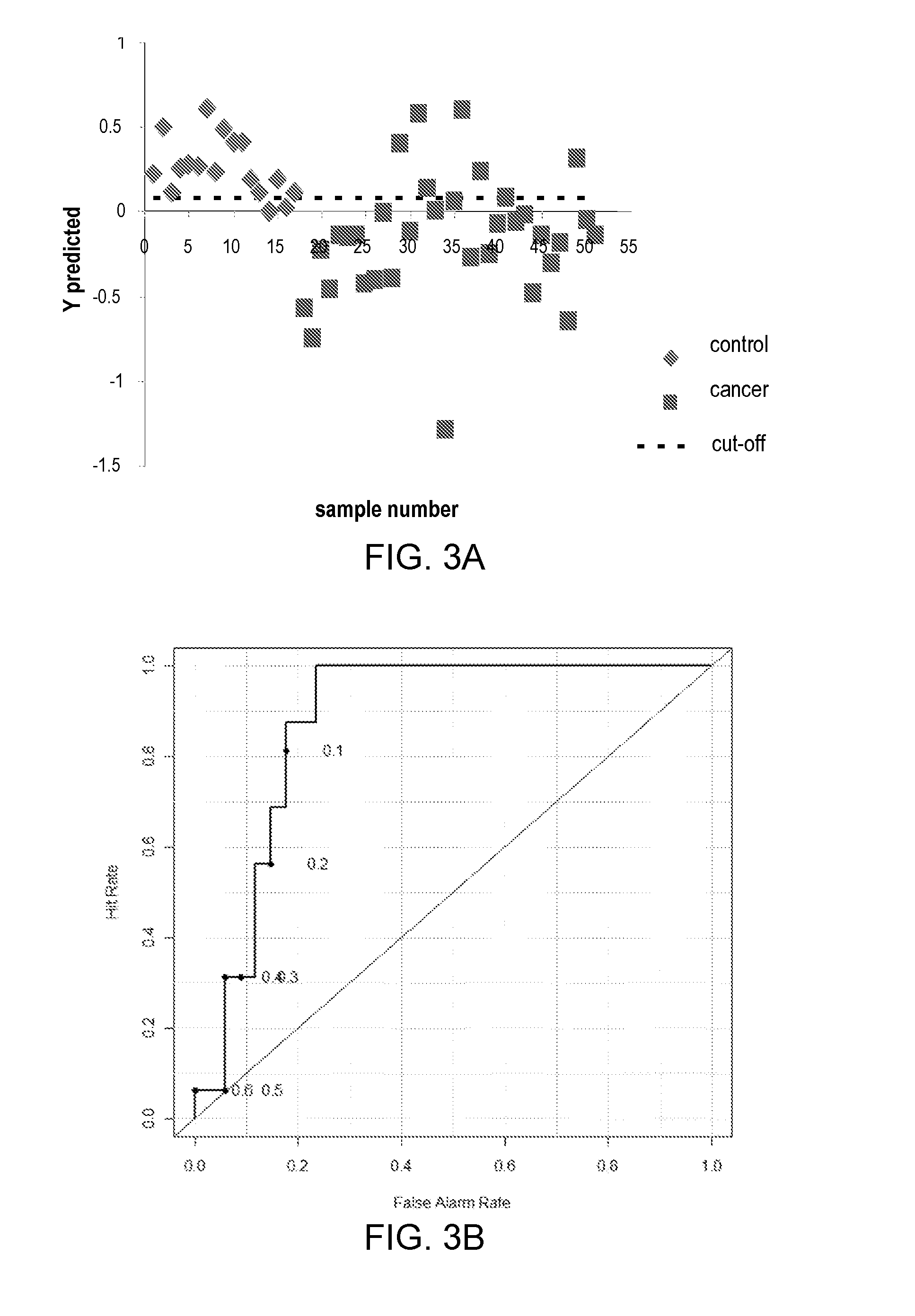
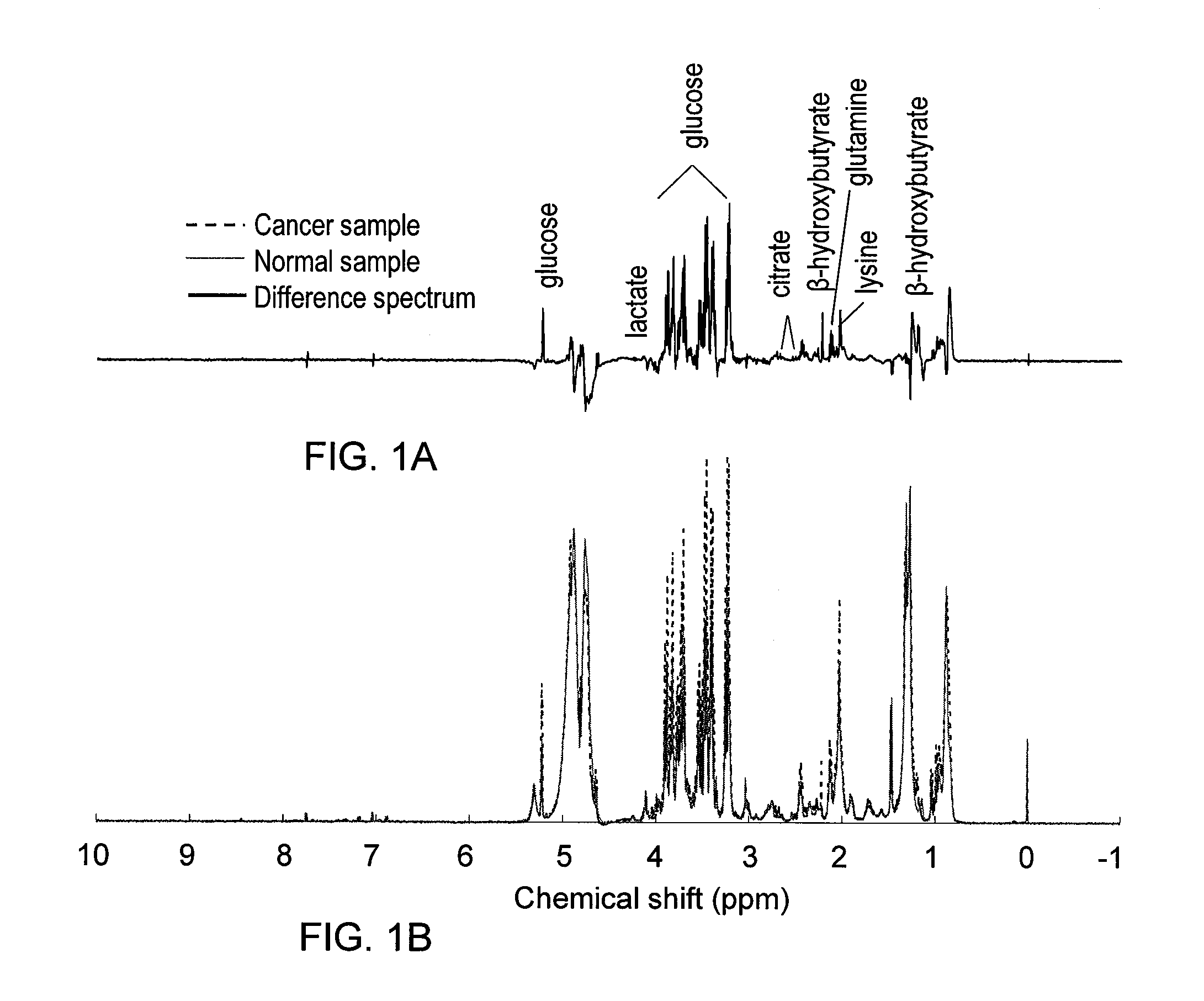
Metabolite Biomarkers for the Detection of Esophageal Cancer Using NMR
Owner:INDIANA UNIV RES & TECH CORP
Methods and probes for detecting esophageal cancer
InactiveUS8034577B2Sugar derivativesMicrobiological testing/measurementEsophageal cancerChromosomal Probes
Probe sets and methods of using probes and probe sets for selectively detecting high grade dysplasia and esophageal adenocarcinoma or low grade dysplasia from biologic samples are described. Methods of the invention include contacting a biological sample obtained from a subject with a set of chromosomal probes to selectively detect an esophageal carcinoma or precursor lesion in the sample, if any, under conditions for specifically hybridizing the probes to their nucleic targets present in the sample. The presence or absence of high grade dysplasia and esophageal adenocarcinoma or low grade dysplasia is thereafter specifically determined from the hybridization pattern detected for the set of chromosomal probes to the biological sample.
Owner:ABBOTT MOLECULAR INC +1
Metabolite Biomarkers for the Detection of Esophageal Cancer Using NMR
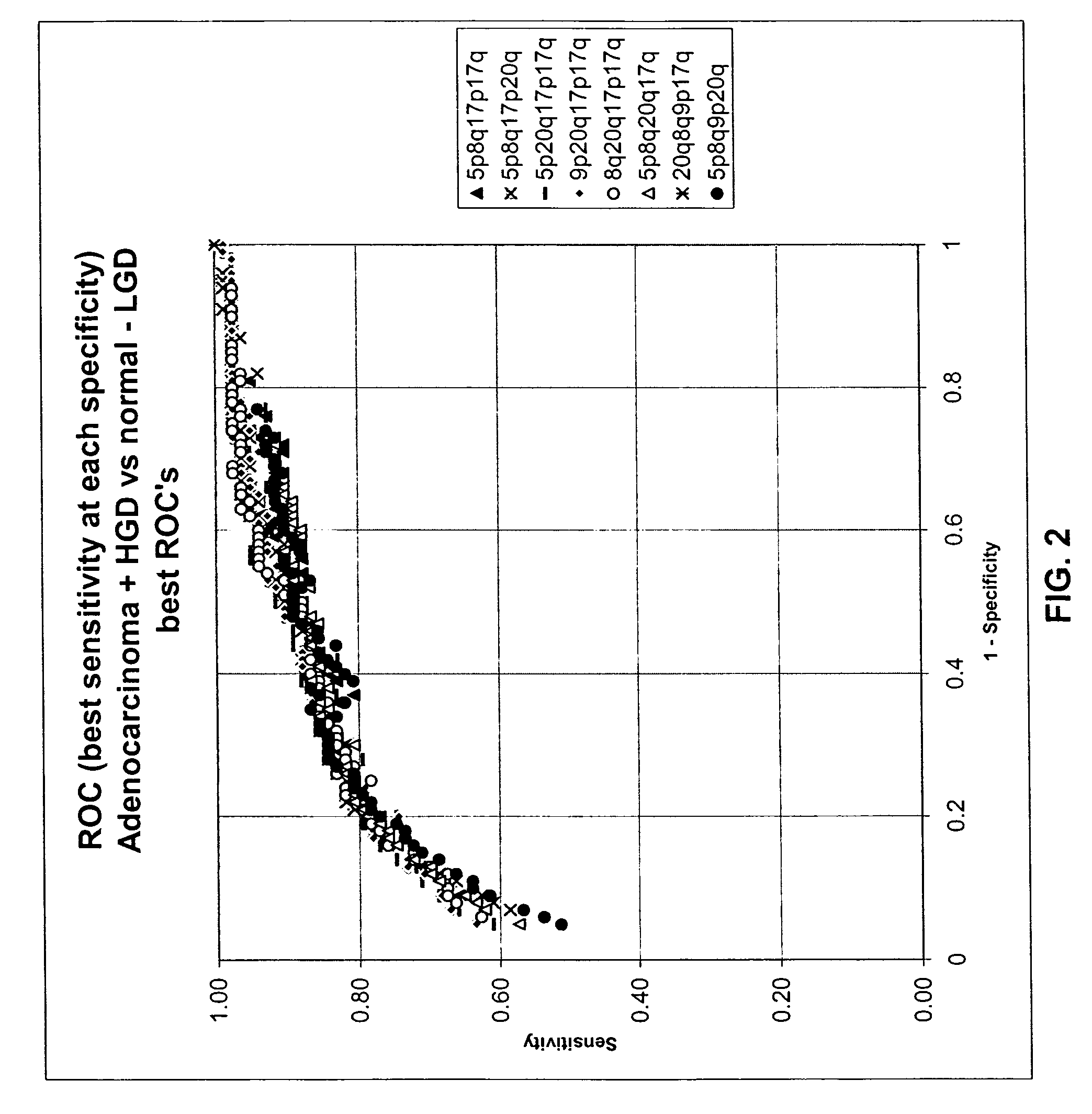
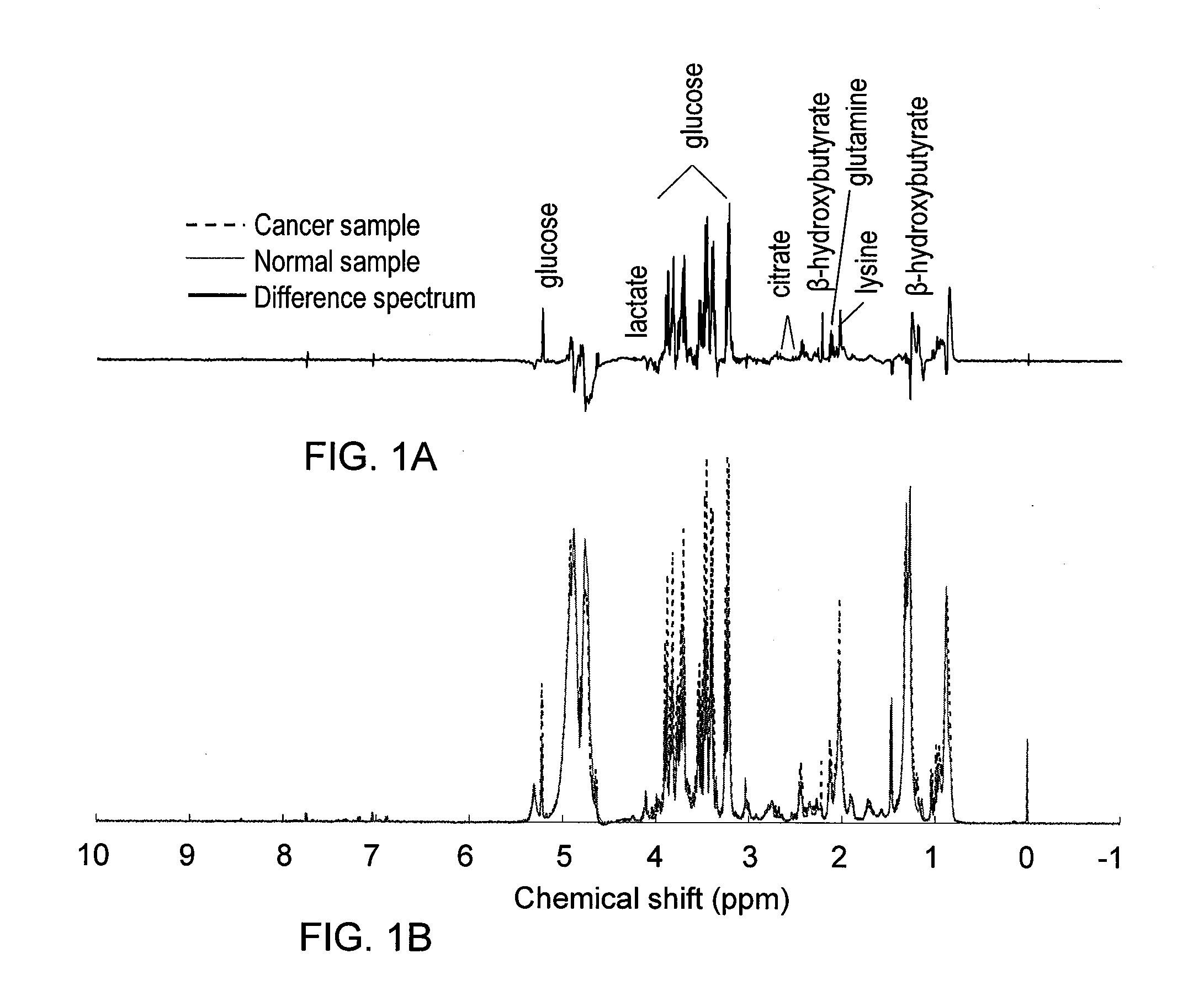
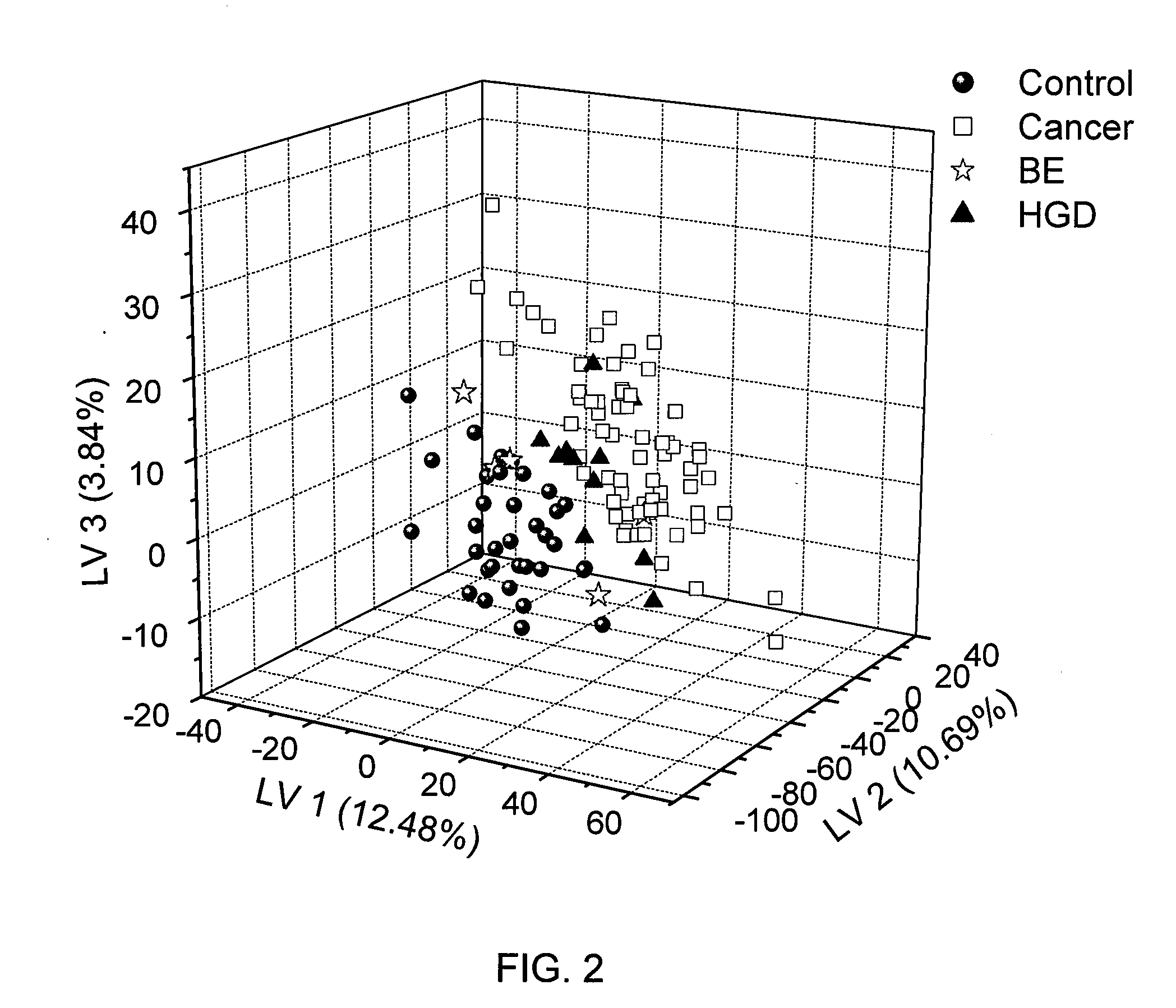
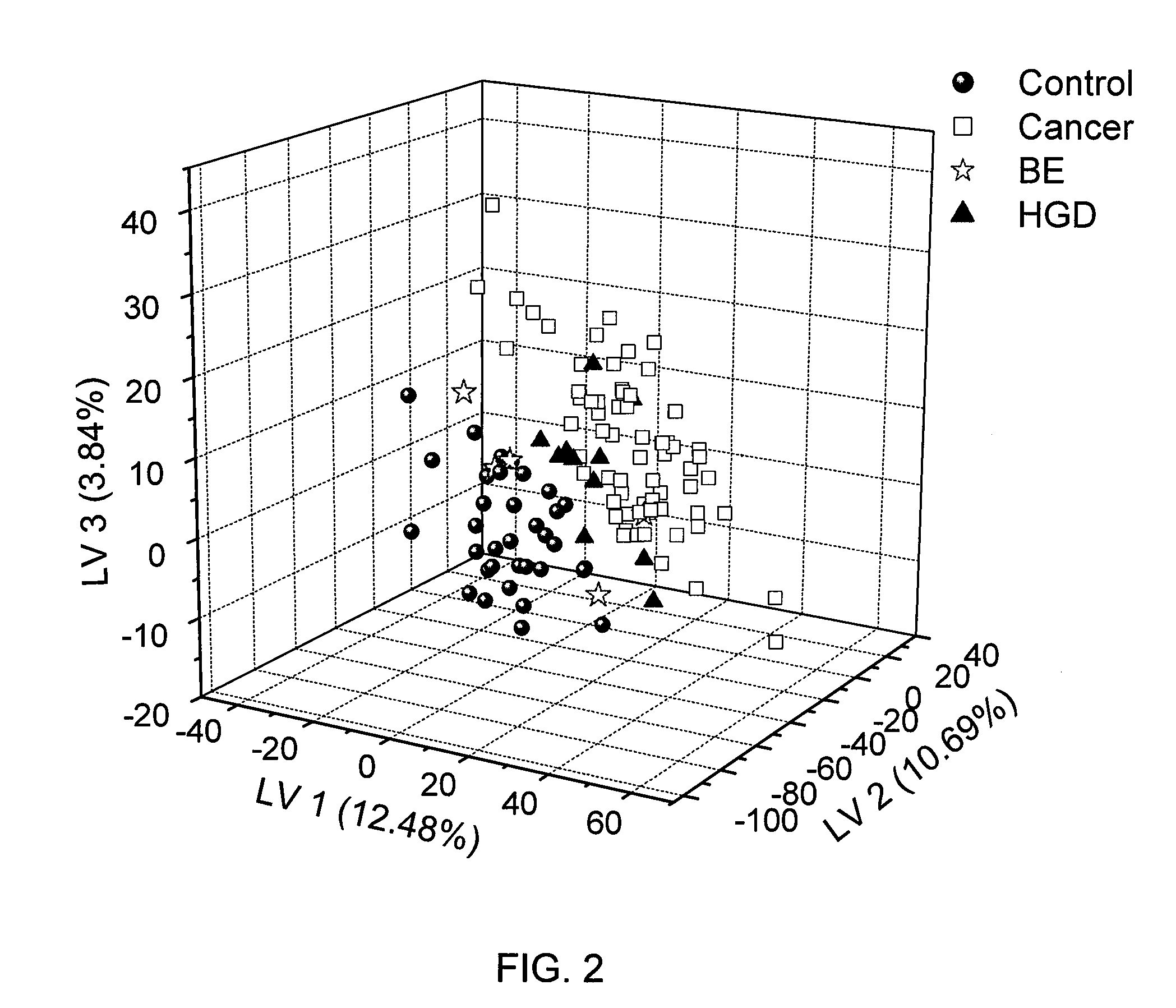
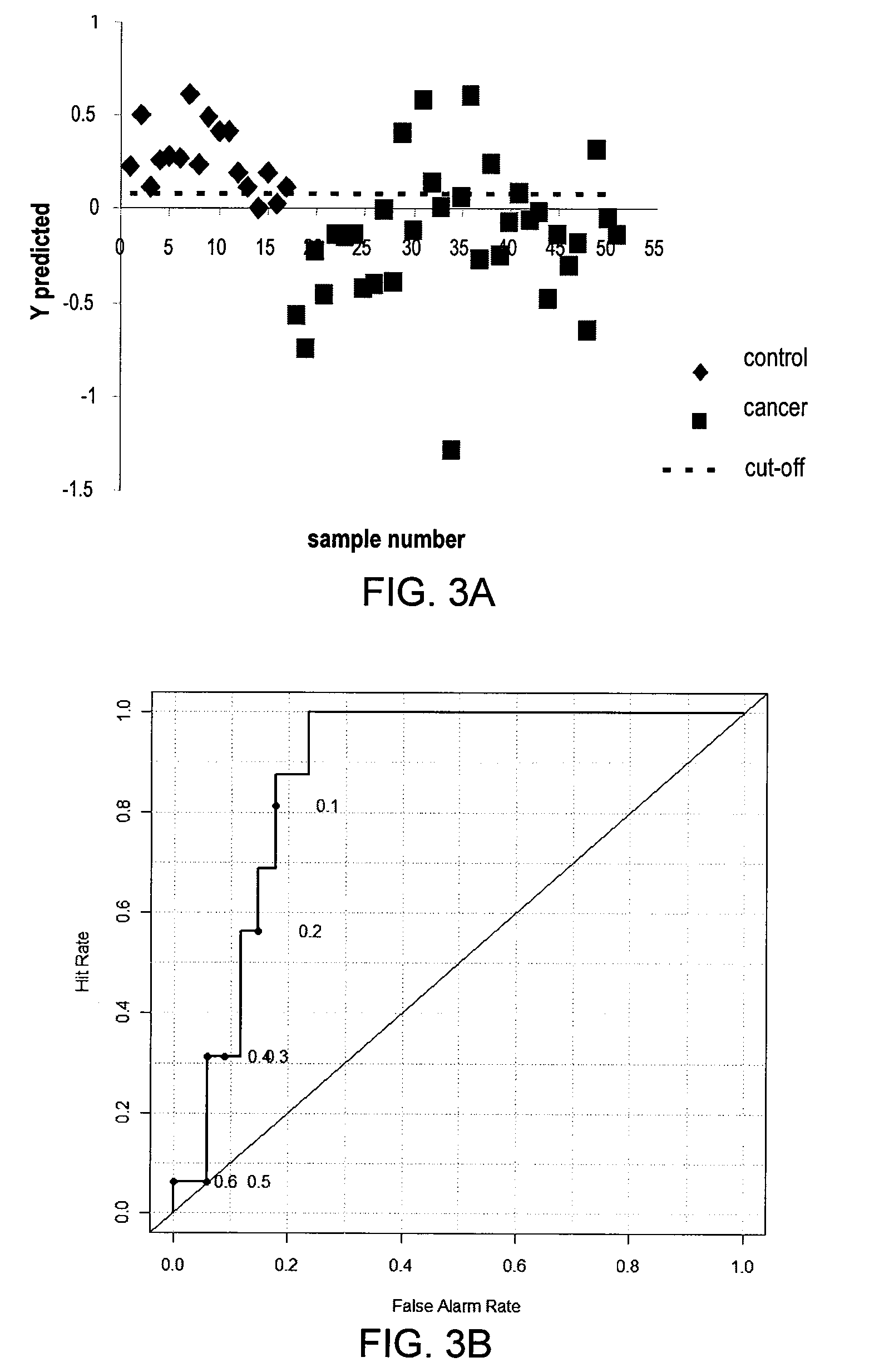
Methods for the detection and screening of esophageal adenocarcinoma (EAC) patients and for the monitoring of EAC treatment using a panel or panels of small molecule metabolite biomarkers are disclosed. In other aspects, methods for detection and screening for the progression of high-risk conditions (BE and HOD) to EAC and to monitoring treatment using a panel or panels of small molecule metabolite biomarkers are disclosed. The biomarkers are sensitive and specific for the detection of EAC, and can also be used to classify Barrett's esophagus (BE) and high-grade dysplasia (HOD), which are widely regarded as precursors of EAC.
Owner:PURDUE RES FOUND INC +1
Metabolite biomarkers for the detection of esophageal cancer using ms
InactiveUS20120107955A1Useful in detectionSugar derivativesMaterial analysis by optical meansDiseaseMetabolite
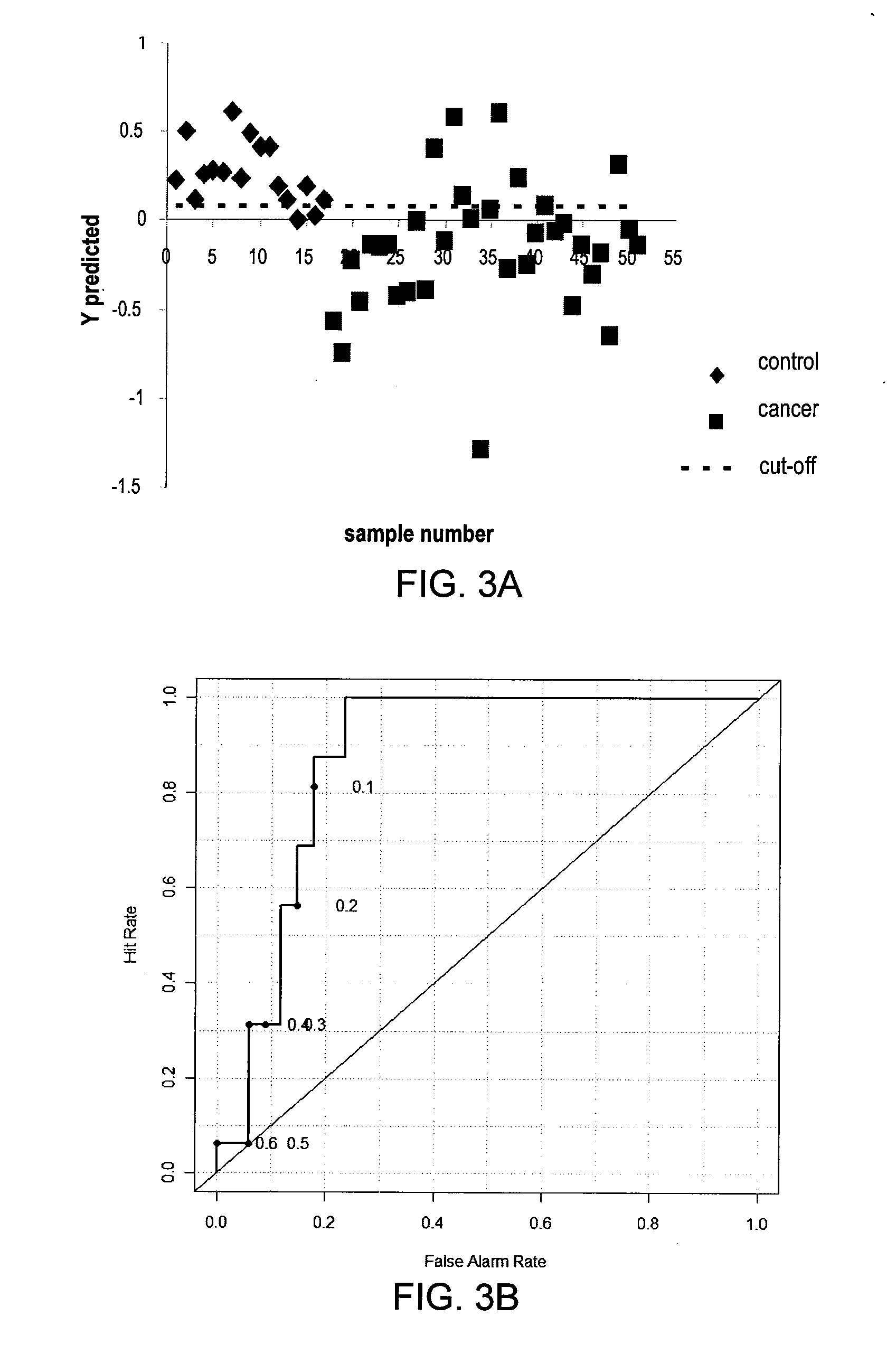
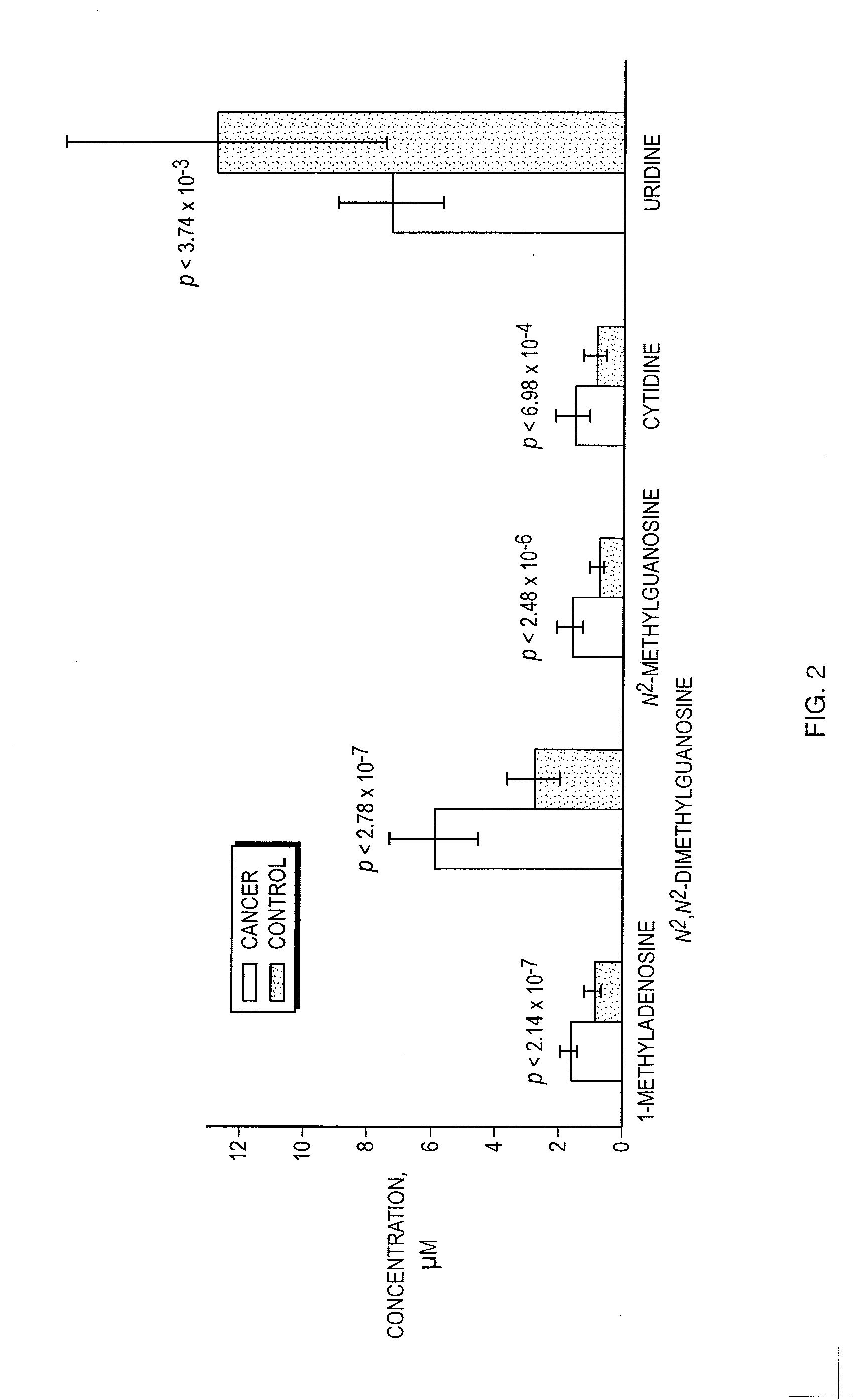
Results of studies of nucleosides in biofluid specimens from patients with esophageal adenocarcinoma and related disorders have identified five biomarkers of the conditions: 1-methyladenosine, N2,N2-dimethylguanosine, N2-methylguanosine, cytidine and uridine. In certain embodiments, methods of measuring these biomarkers and kits for measuring these biomarkers are provided.
Owner:PURDUE RES FOUND INC
Esophageal cancer prognosis biological marker and detection method and application thereof
ActiveCN108753982AStrong correlationEasy to detectMicrobiological testing/measurementBiomarker (petroleum)Amino acid
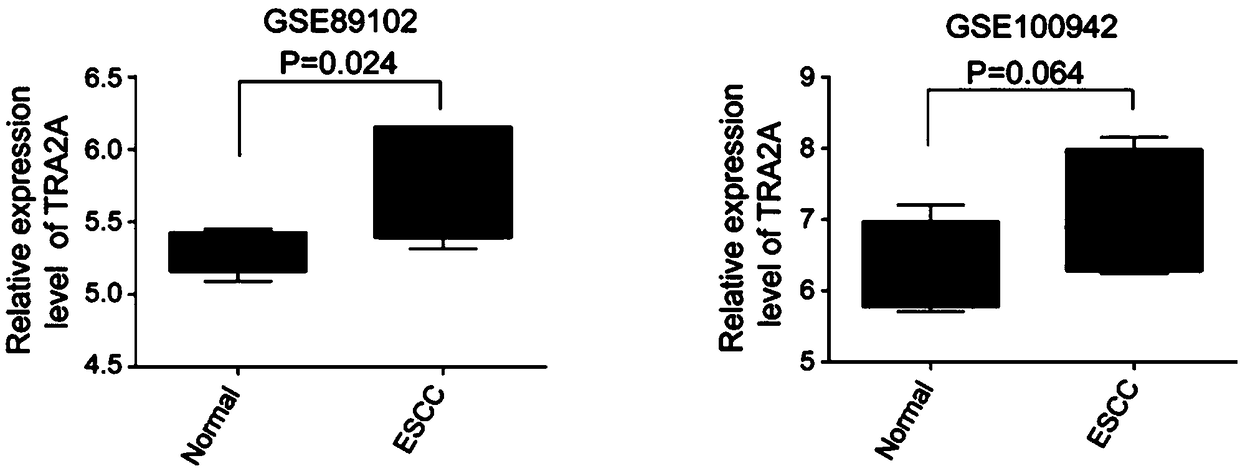
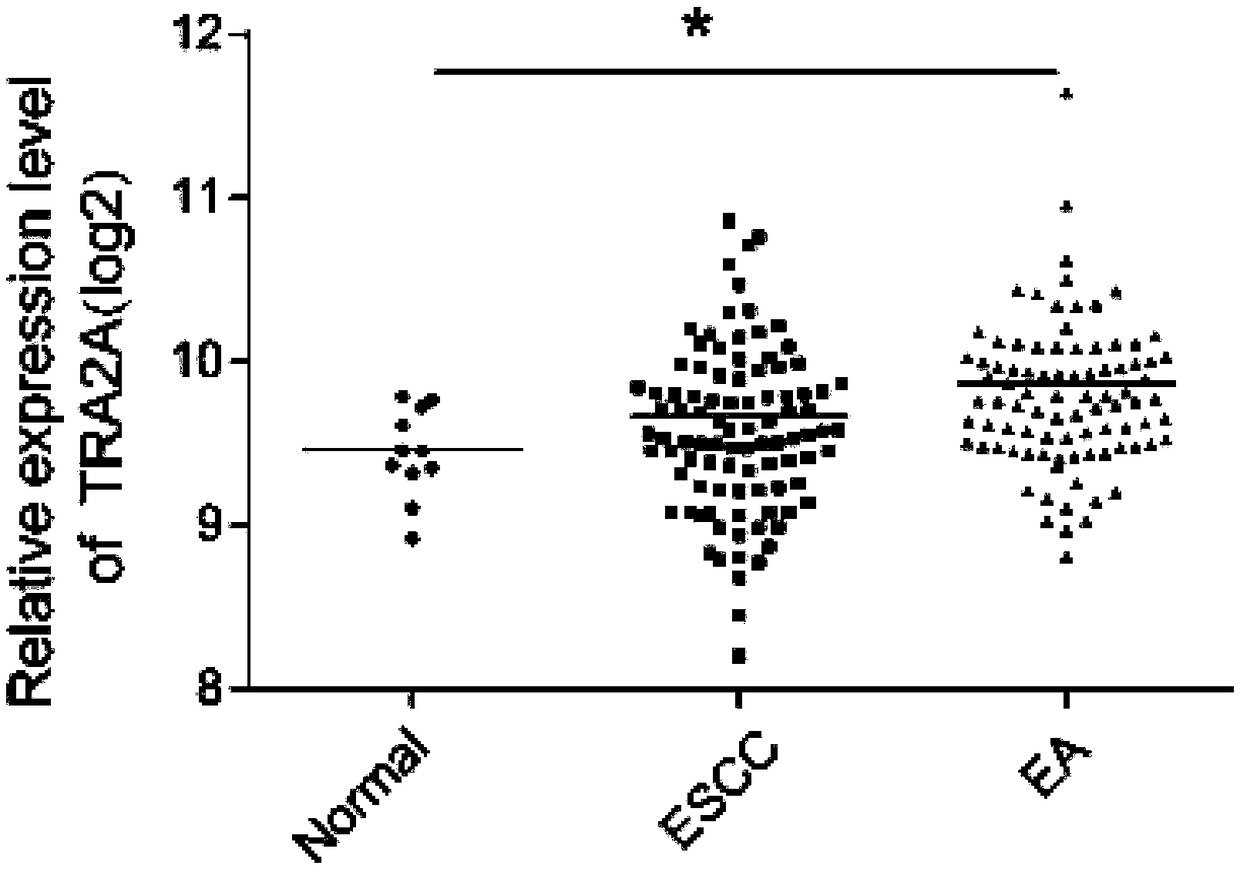
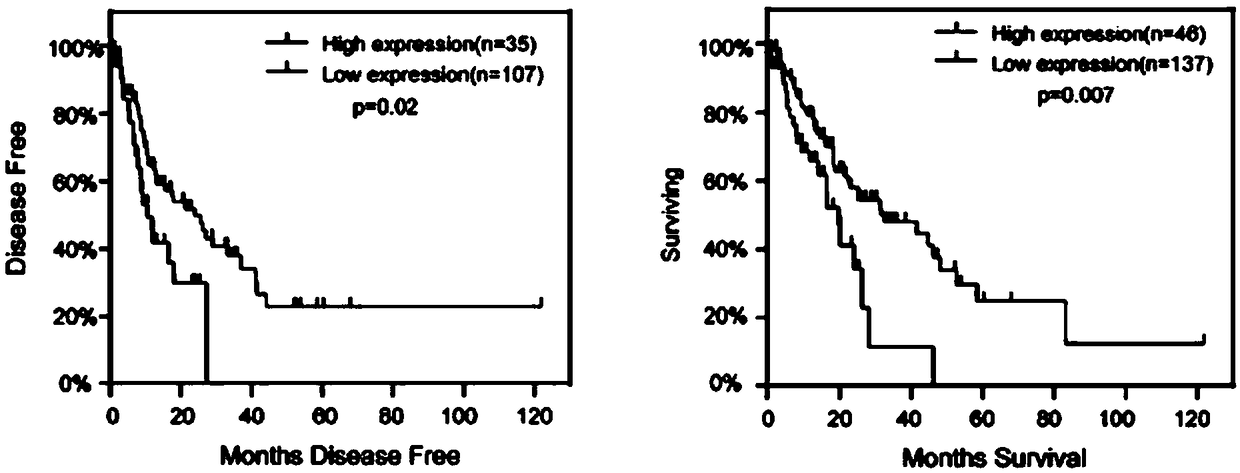
The invention relates to a esophageal cancer prognosis biological marker and a detection method and an application thereof, wherein the biological marker is RNA binding protein TRA2A, and the amino acid sequence is shown in SEQ ID NO: 1; the DNA sequence is shown in SEQ ID NO: 2. The detection method of the esophageal cancer prognostic biological marker mainly comprises two detection methods whichare an mRNA expression change detection and an mRNA expression change detection combined with a gene mutation detection. The detected samples comprise tissues and organs. The biological marker can beused for preparing esophageal cancer diagnostic reagent and / or medicine. The esophageal cancer prognostic biological marker can commonly be used for two main esophageal cancers which are the esophageal adenocarcinoma and the esophageal squamous cell carcinoma, and the detection of the esophageal cancer prognostic biological marker is convenient and rapid, the experiment process is simple and theresult is clear. Except the TRA2A primer, the used reagents are mostly common reagents and the cost is low.
Owner:徐建震
Metabolite biomarkers for the detection of esophageal cancer using NMR
Methods for the detection and screening of esophageal adenocarcinoma (EAC) patients and for the monitoring of EAC treatment using a panel or panels of small molecule metabolite biomarkers are disclosed. In other aspects, methods for detection and screening for the progression of high-risk conditions (BE and HGD) to EAC and to monitoring treatment using a panel or panels of small molecule metabolite biomarkers are disclosed. The biomarkers are sensitive and specific for the detection of EAC, and can also be used to classify Barrett's esophagus (BE) and high-grade dysplasia (HGD), which are widely regarded as precursors of EAC.
Owner:PURDUE RES FOUND INC +1
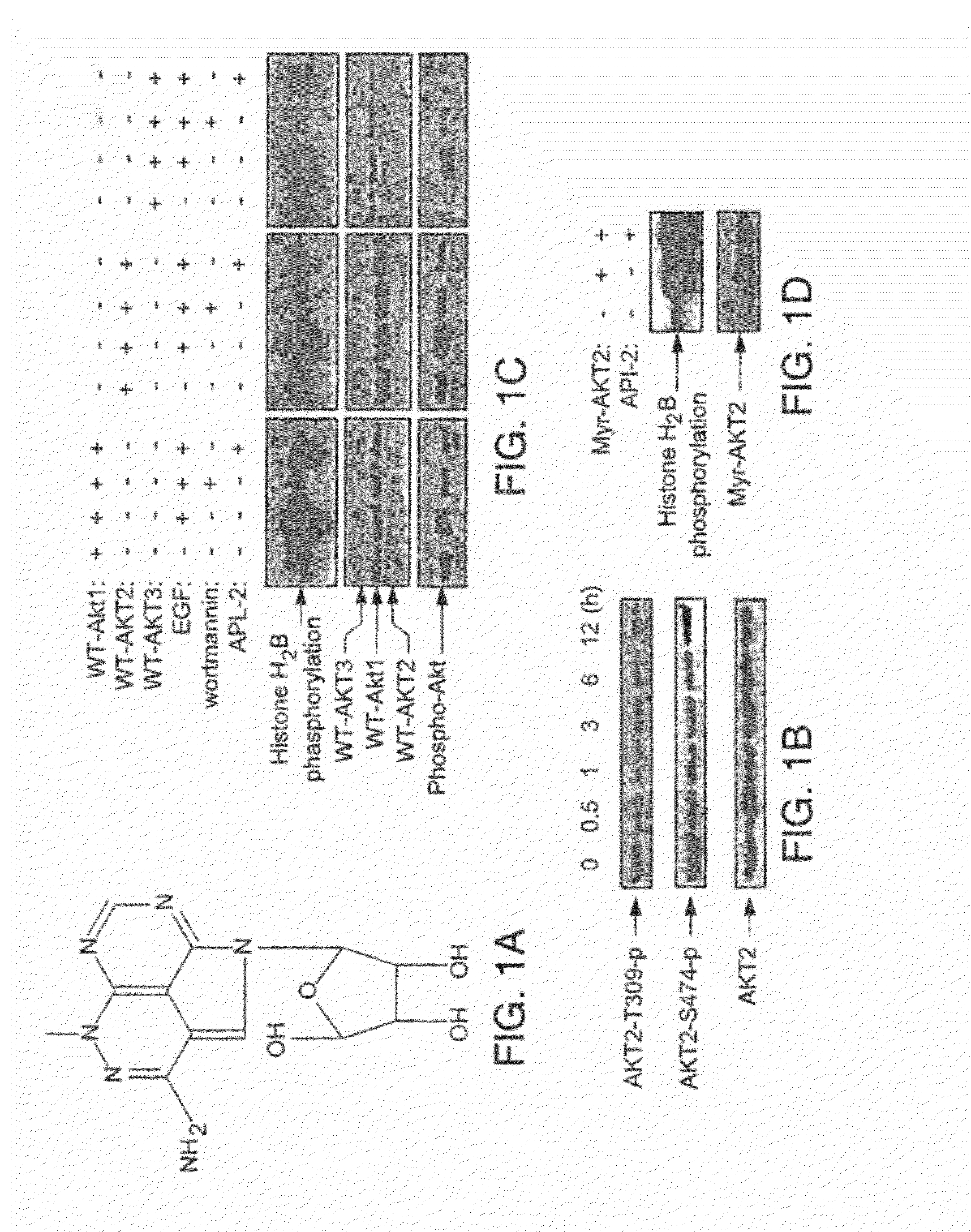
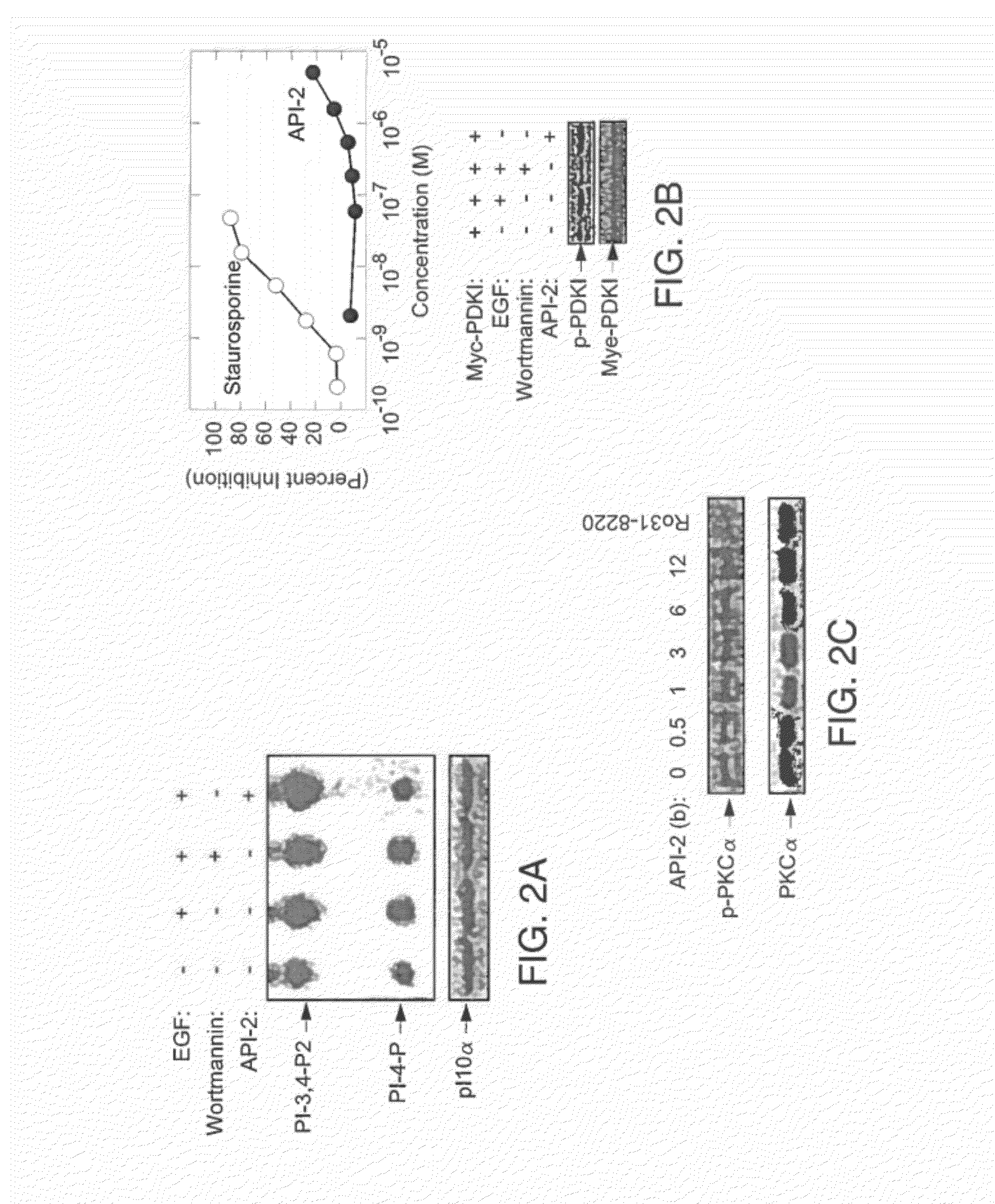
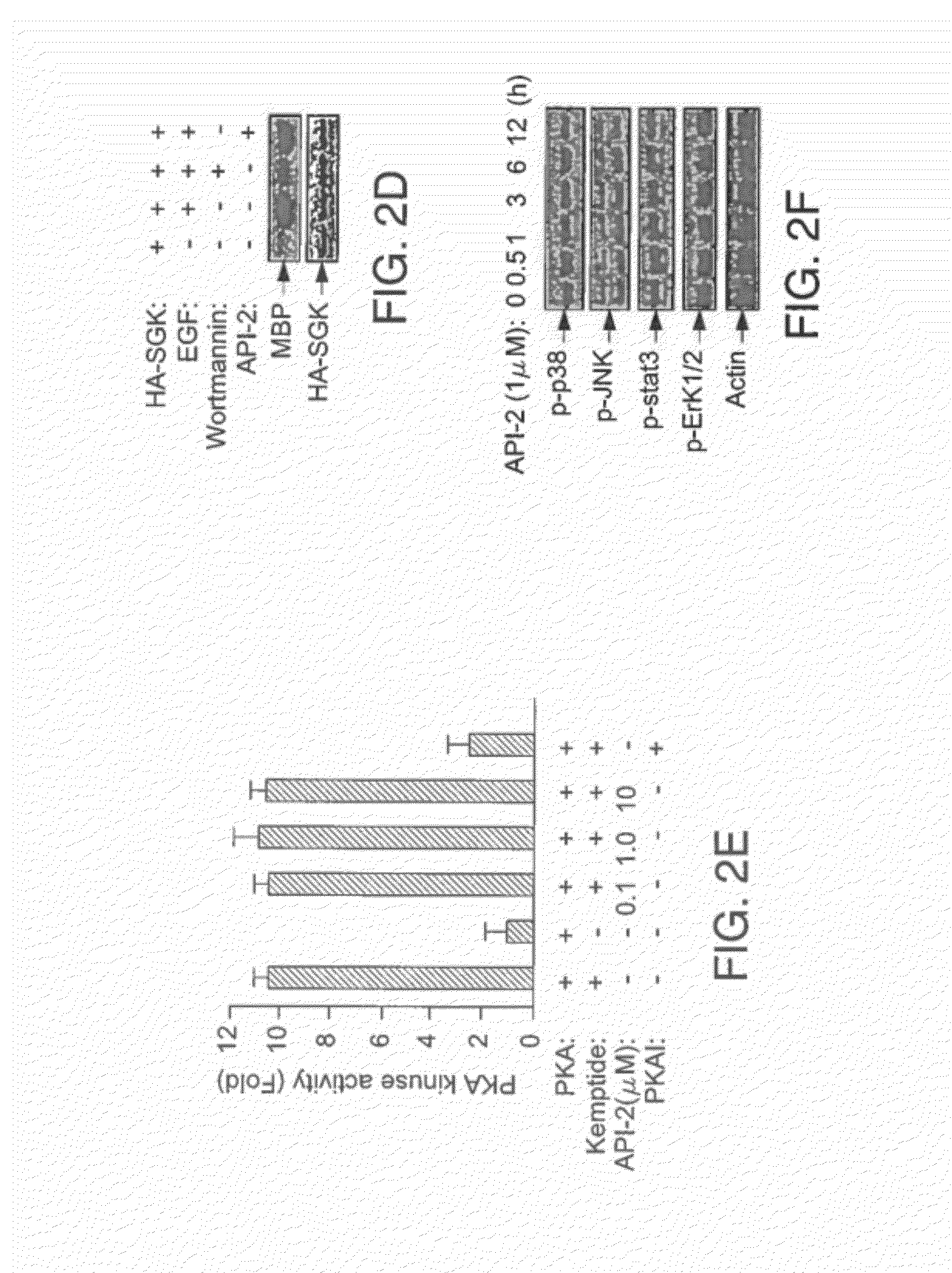
Effective treatment of esophogeal adenocarcinoma using triciribine and related compounds
ActiveUS20100113381A1Reduce systemic toxicityHigh sensitivityBiocideSugar derivativesRegimenMedicine
The inventors have determined, contrary to the prior art and experience, how to successfully use triciribine to treat esophogeal adenocarcinoma by one or a combination of (i) administering triciribine only to patients which according to a diagnostic test described below, exhibit enhanced sensitivity to the drug; (ii) use of a described dosage level that minimizes the toxicity of the drug but yet still exhibits efficacy; or (iii) use of a described dosage regimen that minimizes the toxicity of the drug.
Owner:UNIV OF SOUTH FLORIDA
Barrett's esophagus progression to cancer gene panel and methods of use thereof
InactiveUS20200071767A1High frequencyHealth-index calculationMicrobiological testing/measurementCancer geneOncology
The present invention provides, inter alia, methods of predicting the risk of a subject having Barrett's Esophagus to develop a more severe condition, such as, e.g., low-grade dysplasia (LGD), high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC), methods for supporting the diagnosis of dysplasia or esophageal adenocarcinoma (EAC) in a subject having Barrett's Esophagus. Also provided are kits to implement such methods.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods for predicting esophageal adenocarcinoma (EAC)
ActiveUS20120053066A1Microbiological testing/measurementLibrary screeningMultiple Tumor Suppressor-1Cvd risk
This invention relates, e.g., to methods for predicting a subject's risk for developing esophageal adenocarcinoma (EAC) or high-grade dysplasia (HGD), comprising determining in a sample from the subject the methylation levels of transcriptional promoter regions of various combinations of, among other genes, (a) cadherin 13, H-cadherin (heart) (CDH13); (b) tachykinin-1 (TAC1); (c) nel-like 1 (NELL1); (d) A-kinase anchoring protein 12 (AKAP12); (e) somatostatin (SST); (f) transmembrane protein with EGF-like and two follistatin-like domains (HPP1); (g) CDKN2a, cyclin-dependent kinase inhibitor 2a (p16); or (h) runt-related transcription factor 3 (RUNX3).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
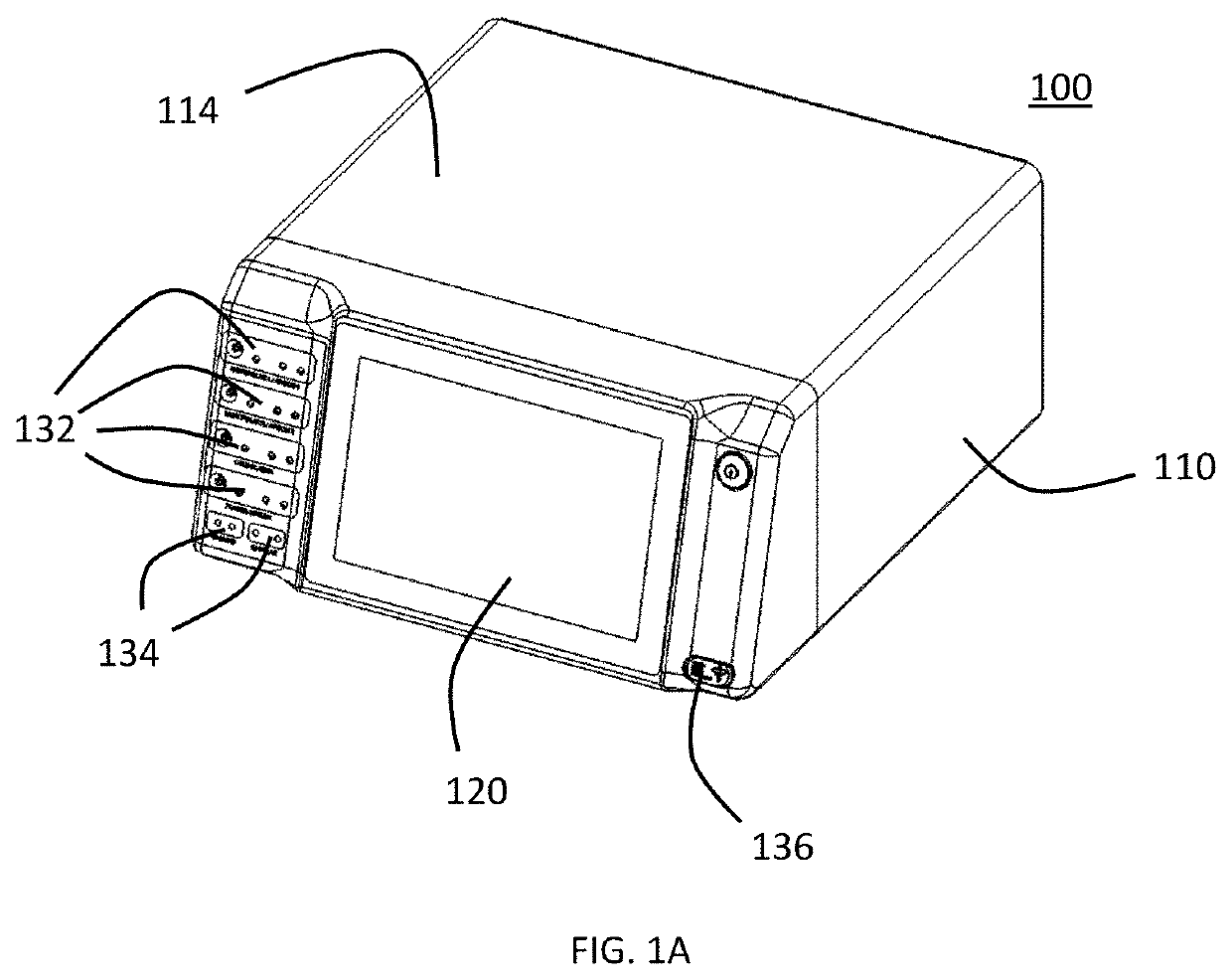
System and Method for Pre-programmed Cold Atmospheric Plasma
To determine appropriate treatment doses of cold atmospheric plasma (CAP), the Canady Helios Cold Plasma Scalpel was tested across numerous cancer cell types including renal adenocarcinoma, colorectal carcinoma, pancreatic adenocarcinoma, ovarian adenocarcinoma, and esophageal adenocarcinoma. Various CAP doses were tested consisting of both high (3 L / min) and low (1 L / min) helium flow rates, several power settings, and a range of treatment times up to 5 minutes. The impact of cold plasma on the reduction of viability was consistently dose dependent, however the anti-cancer capability varied significantly between cell lines. While the lowest effective dose varied from cell line to cell line, in each case an 80-99% reduction in viability was achievable 48 hours after CAP treatment. Therefore, it is critical to select the appropriate CAP dose necessary for treating a specific cancer cell type.
Owner:JEROME CANADY RES INST FOR ADVANCED BIOLOGICAL & TECHCAL SCI
Methods to assess the likelihood of dysplasia or esophageal adenocarcinoma
InactiveUS20150105265A1Saving in surveillance costPromote resultsMicrobiological testing/measurementLibrary screeningPediatricsMethylation
In some embodiments, a method for aiding assessment of the likelihood of dysplasia or esophageal adenocarcinoma being present in a subject can include (a) providing an esophagal sample from said subject (b) determining the methylation status of (i) SLC22A18, (ii) PIGR, (iii) GJA12 and (iv) RIN2 in said sample wherein if 2 or more of said genes are methylated then an increased likelihood of presence of dysplasia or esophageal is determined. The invention also relates to apparatus for same.
Owner:UK RES & INNOVATION LTD
Methods for early detection of esophageal cancer
InactiveUS20140296079A1Microbiological testing/measurementLibrary screeningEsophageal cancerOncology
The present invention relates to a method for screening, predicting or prognosing esophageal adenocarcinoma / high grade dysplasia in a subject.
Owner:NEOGENOMICS LAB
Effective treatment of esophogeal adenocarcinoma using triciribine and related compounds
The inventors have determined, contrary to the prior art and experience, how to successfully use triciribine to treat esophogeal adenocarcinoma by one or a combination of (i) administering triciribine only to patients which according to a diagnostic test described below, exhibit enhanced sensitivity to the drug; (ii) use of a described dosage level that minimizes the toxicity of the drug but yet still exhibits efficacy; or (iii) use of a described dosage regimen that minimizes the toxicity of the drug.
Owner:UNIV OF SOUTH FLORIDA
Personalized medicine for the prediction of therapy targeting the hedgehog pathway
Methods and composition for tumor therapy, especially esophageal adenocarcinoma (EAC), are described. For example, in certain aspects methods for determining Hedgehog and mTOR signaling pathway status to select patients for administering a combination therapy of Hedgehog and mTOR signaling inhibitors are described. Furthermore, the invention provides compositions that involve testing kits for determining signaling status.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Biomarker for esophageal cancer typing and application thereof
PendingCN114214409ARealize typing diagnosisPathological diagnosis is correctMicrobiological testing/measurementCharacter and pattern recognitionFOXP1MAP2K4
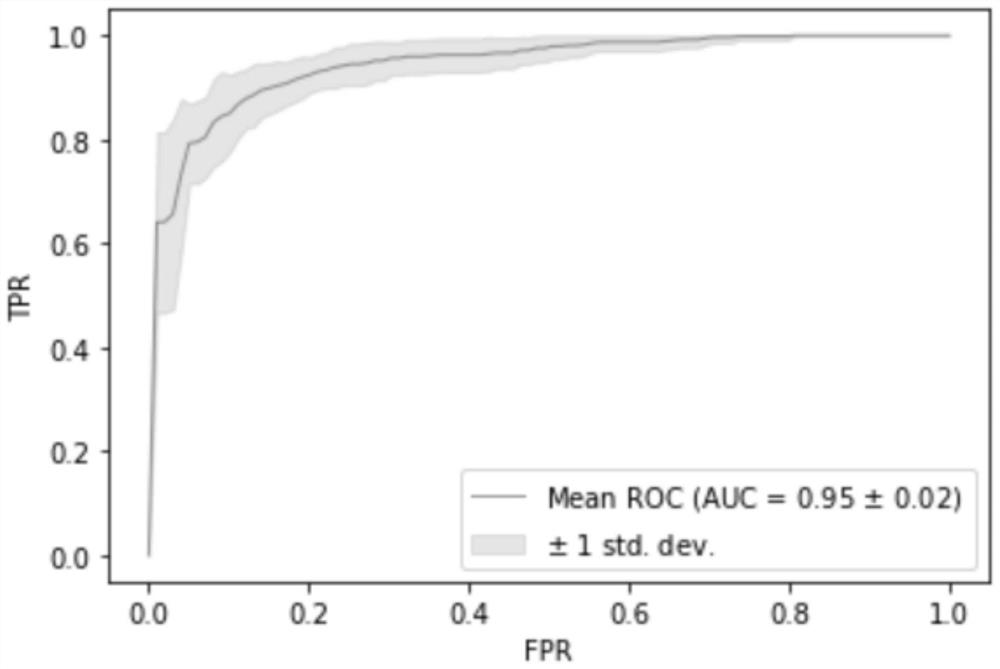
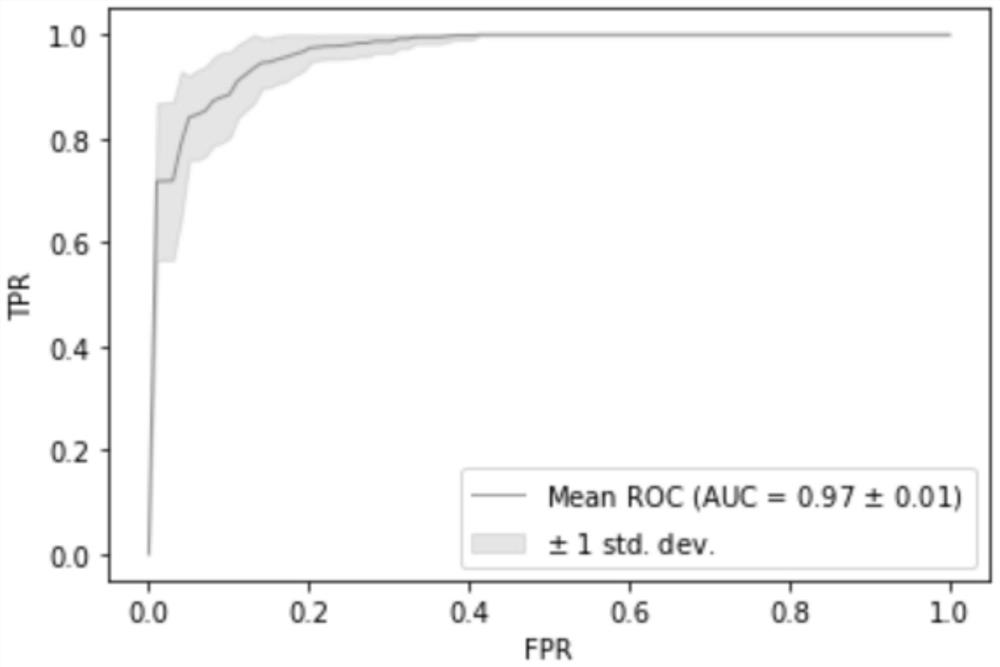
The invention relates to a biomarker for esophageal cancer typing and application thereof, and relates to the technical field of medical detection. The biomarker comprises at least five kinds of genes such as GAS7, LRP1B, MAP2K4, FOXP1, WWOX and the like. When the biomarkers are used for carrying out typing diagnosis on esophageal squamous carcinoma and esophageal adenocarcinoma, the diagnostic power AUC can reach 0.9447 when 5 markers are used, the diagnostic power AUC can reach 0.9789 when 10 markers are used, the diagnostic power AUC can reach 0.9714 when 20 markers are used, and the diagnostic power AUC can reach 0.9489 when all the markers are used, so that the biomarkers have excellent diagnostic power and can be used for diagnosis of esophageal squamous carcinoma and esophageal adenocarcinoma. The invention provides a method for discriminating two pathological subtypes based on molecular level, provides mutual verification for pathological diagnosis results, ensures that the case diagnosis results are correct, and is convenient for subsequent precise treatment.
Owner:深圳康华君泰生物科技有限公司
Device and method to treat esophageal disorders
ActiveUS11149275B2Function increaseExtended half-lifeSugar derivativesSpecial deliveryDiseaseExtracellular vesicle
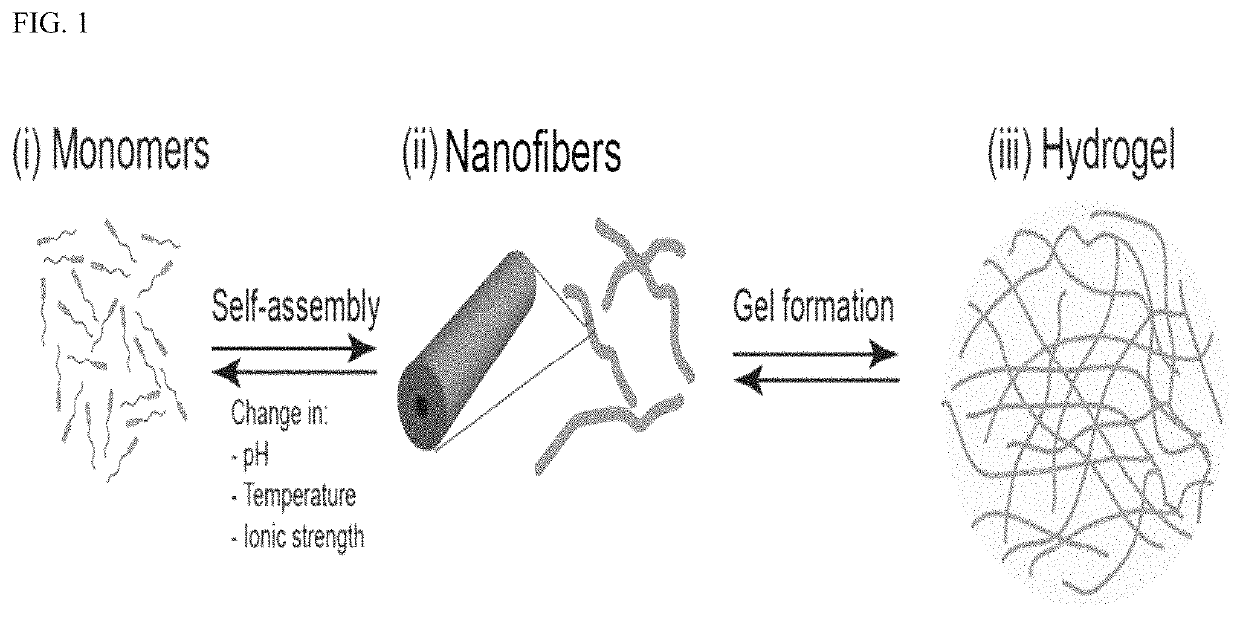
Described are devices for treating disease in subjects wherein the devices include a sponge comprising a hydrogel, extracellular vesicles (EVs), and an agent. Methods of using the devices to treat or prevent disease such as esophageal adenocarcinoma are also described.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods for predicting esophageal adenocarcinoma (EAC)
ActiveUS9758833B2Microbiological testing/measurementLibrary screeningMultiple Tumor Suppressor-1Omodysplasia
This invention relates, e.g., to methods for predicting a subject's risk for developing esophageal adenocarcinoma (EAC) or high-grade dysplasia (HGD), comprising determining in a sample from the subject the methylation levels of transcriptional promoter regions of various combinations of, among other genes, (a) cadherin 13, H-cadherin (heart) (CDH13); (b) tachykinin-1 (TAC1); (c) nel-like 1 (NELL1); (d) A-kinase anchoring protein 12 (AKAP12); (e) somatostatin (SST); (f) transmembrane protein with EGF-like and two follistatin-like domains (HPP1); (g) CDKN2a, cyclin-dependent kinase inhibitor 2a (p16); or (h) runt-related transcription factor 3 (RUNX3).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
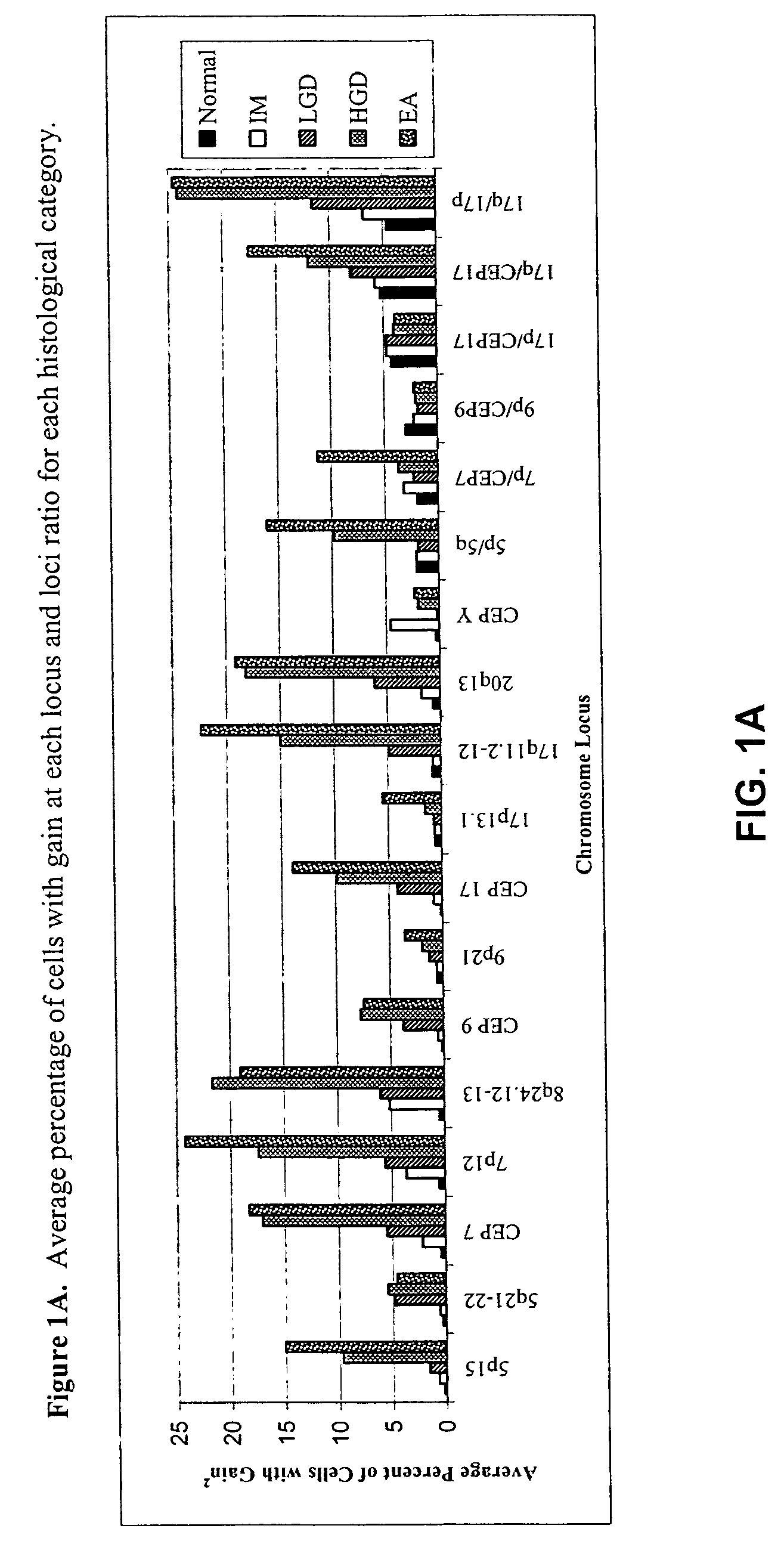
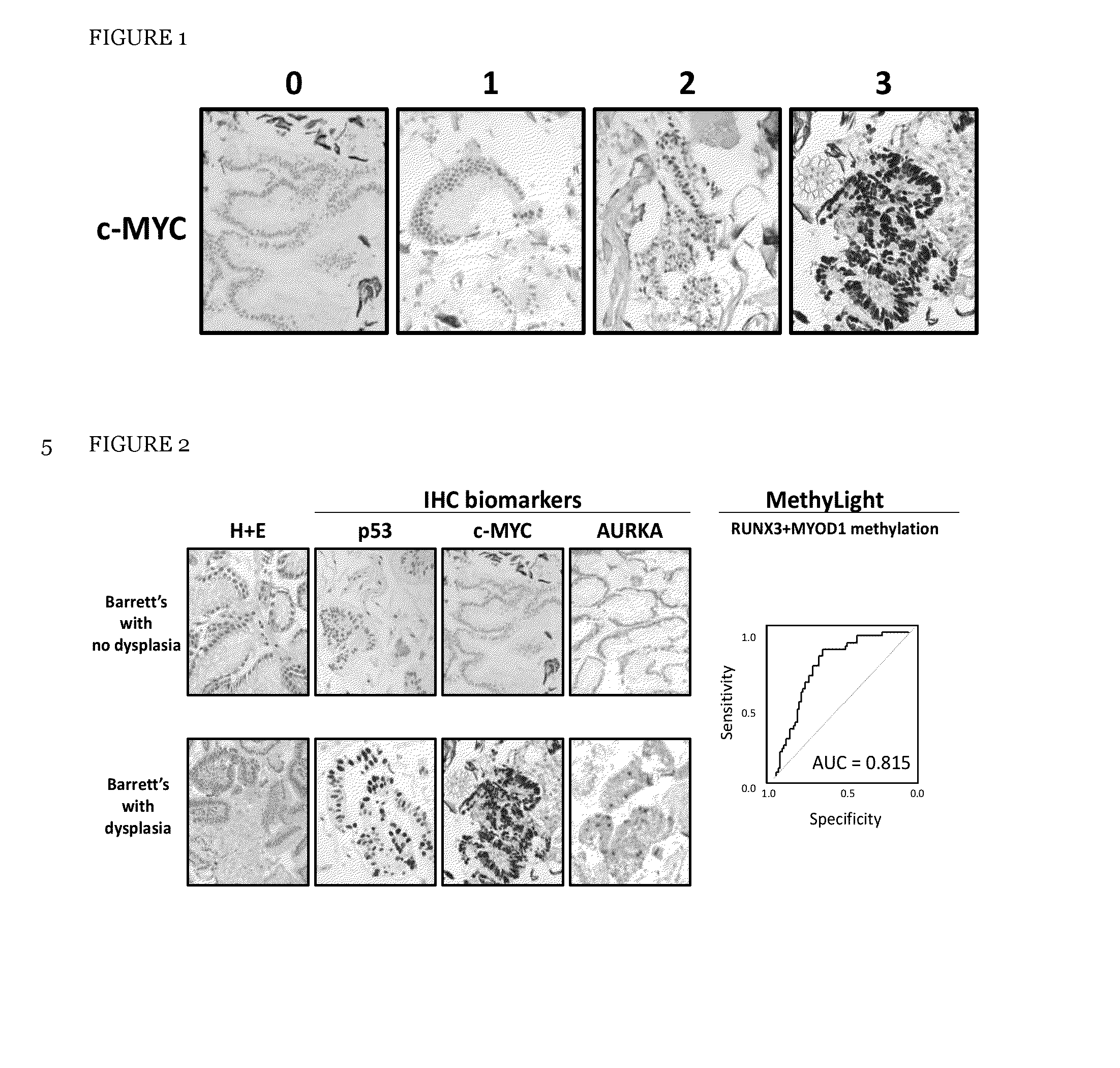
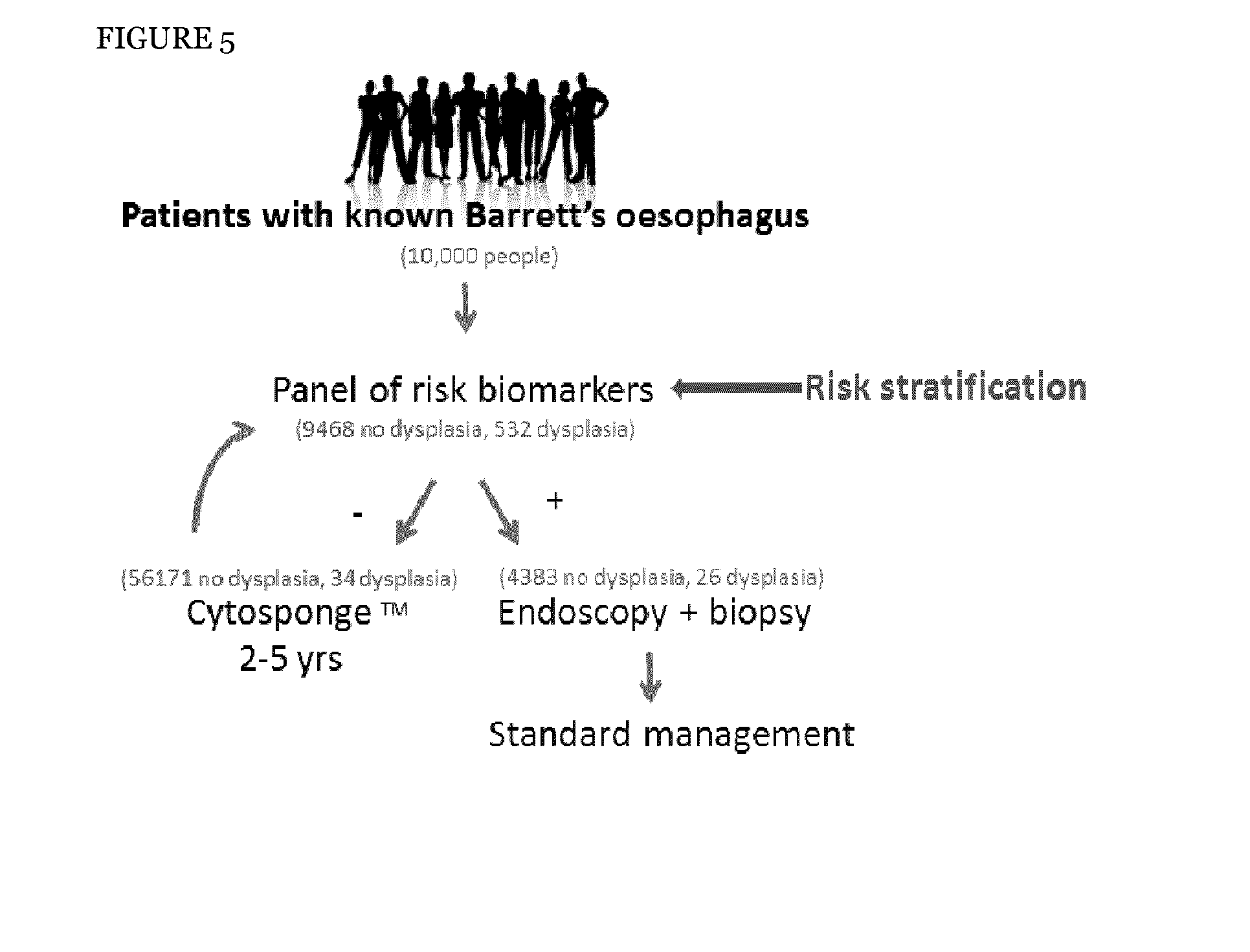
Method and apparatus of aiding detection of surface abnormality in the oesophagus
InactiveUS20160003827A1Great proportionPeptide librariesNucleotide librariesBiologyEsophageal adenocarcinoma
The invention relates to a method of aiding detection of a surface abnormality in the oesophagus of a subject, wherein said surface abnormality is selected from the group consisting of low-grade dysplasia (LGD), high-grade dysplasia (HGD), asymptomatic oesophageal adenocarcinoma (OAC) and intra-mucosal cancer (IMC), the method comprising:a) providing a sample of cells from said subject, wherein said sample comprises cells collected from the surface of the subject's oesophagus;b) assaying said cells for at least two markers selected from(i) p53;(ii) c-Myc;(iii) AURKA or PLK1, preferably AURKA; and(iv) methylation of MyoD and Runx3;wherein detection of abnormal levels of at least two of said markers infers that the subject has an increased likelihood of a surface abnormality in the oesophagus. The invention also relates to certain kits, apparatus and uses.
Owner:UK RES & INNOVATION LTD +1
Esophageal ablation technology
An esophageal ablation system including a positioner, an elongated, flexible shaft extending from the positioner, and a microwave emitter, assembly disposed near the distal end of the shaft. The emitter assembly includes one or more microwave antennas and a balloon for spacing the antennas relative to target tissue. The device may have an inner balloon for deploying the antenna. The systems, devices and methods disclosed are useful for treating Barrett's Esophagus, Esophageal Adenocarcinoma, and Squamous Cell Carcinoma.
Owner:SYMPLE SURGICAL
Systems and methods for barrett's esophagus pathogenesis and esophageal adenocarcinoma progression revealing markers
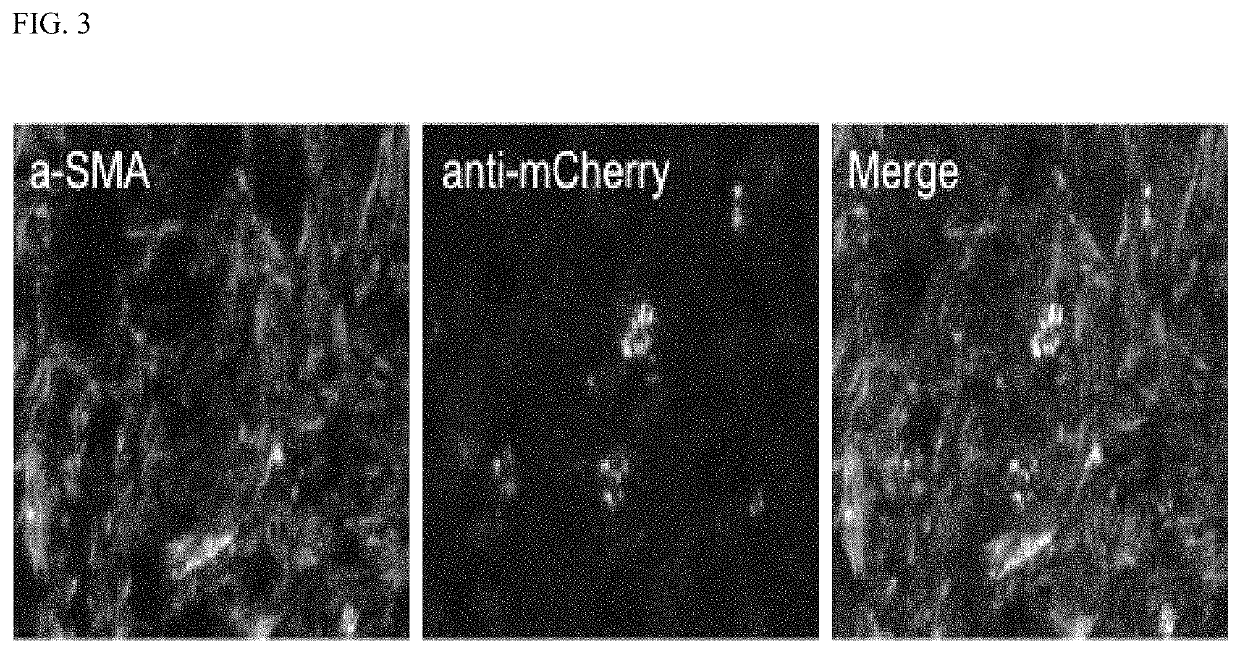
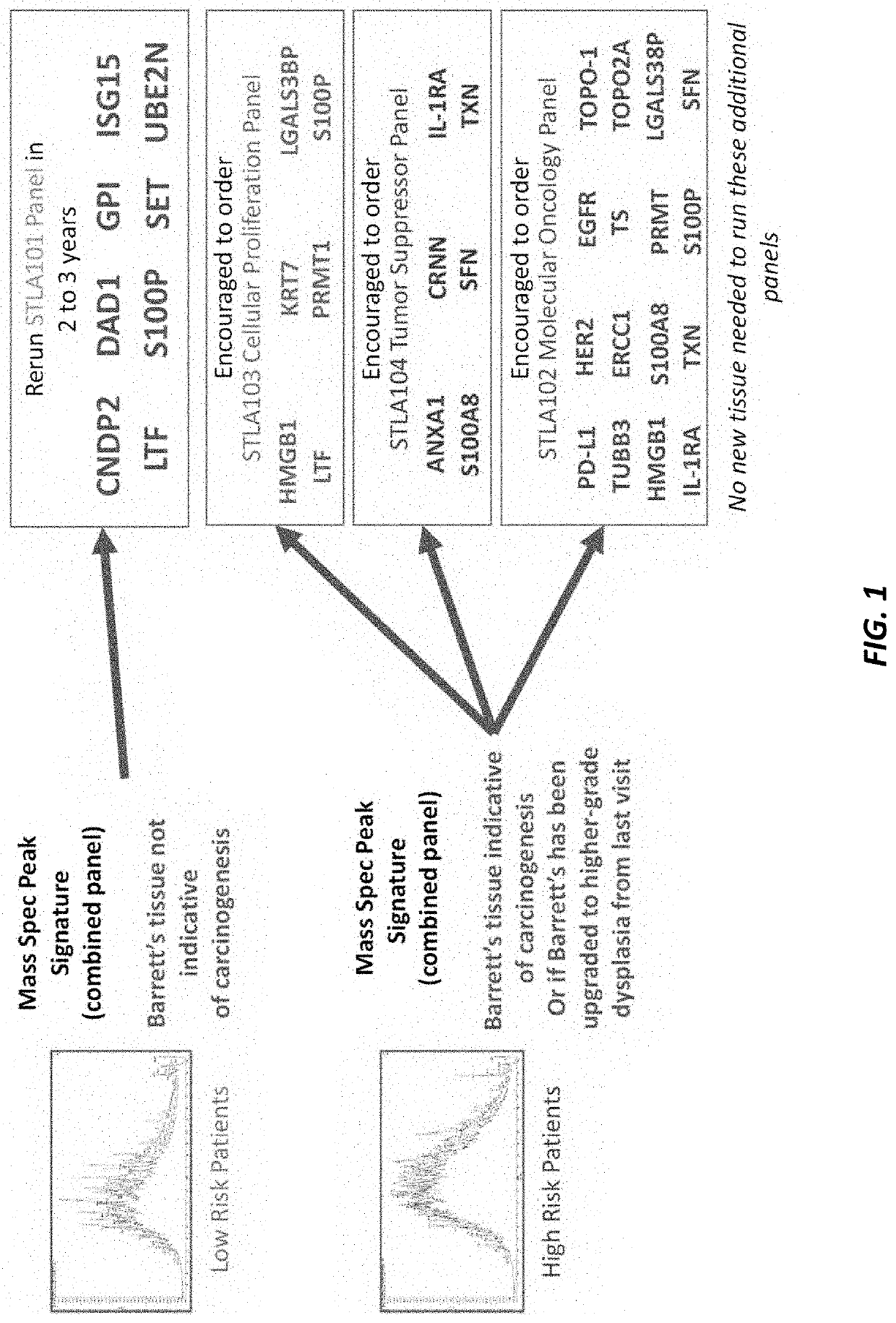
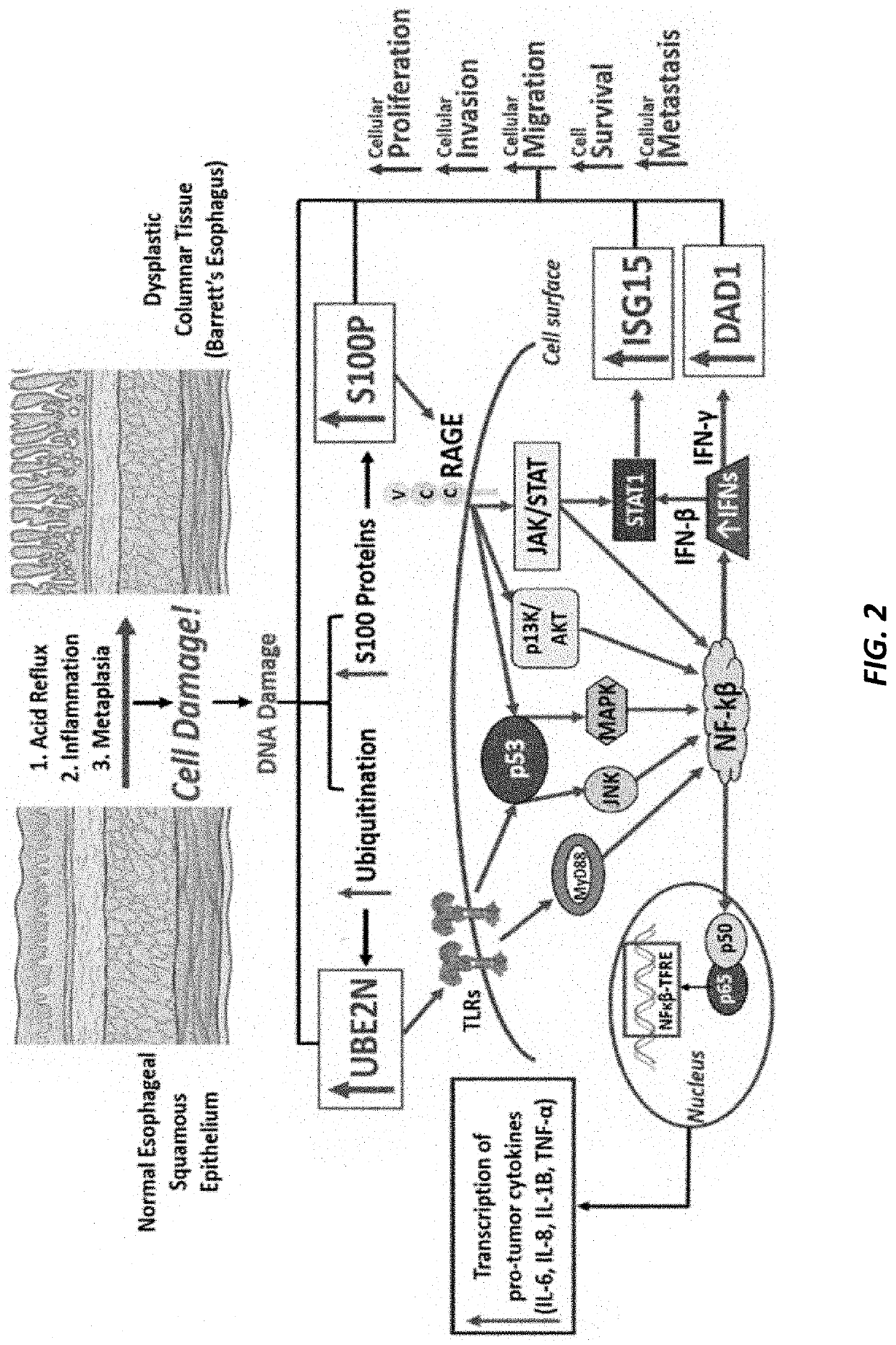
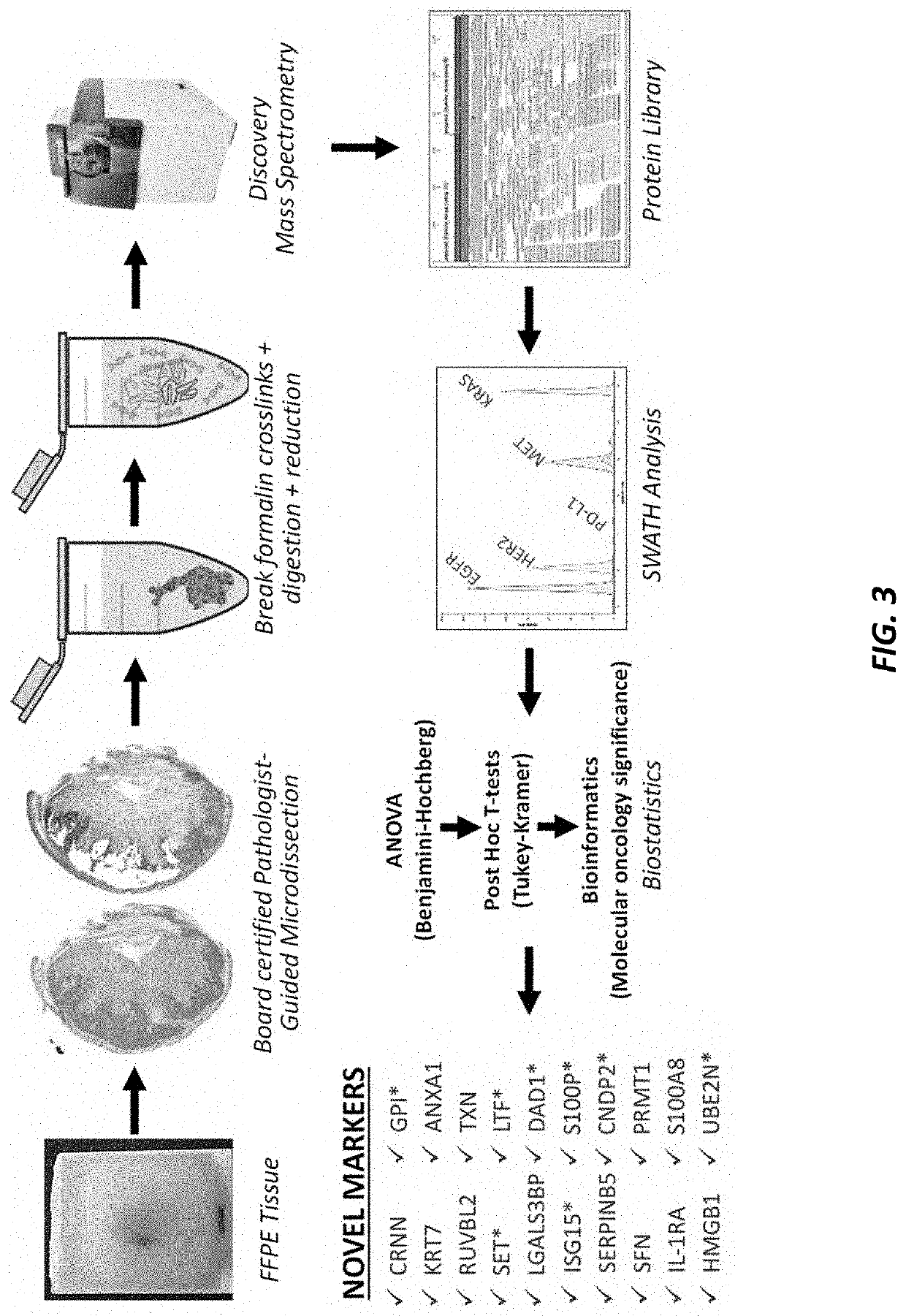
PendingUS20210364521A1High expressionIncreased riskMicrobiological testing/measurementBiological testingISG15Inducer Cells
Methods are provided for assessing risk of developing esophageal adenocarcinoma in a subject using one or more of the following marker genes / proteins: ISG15, LTF, CNDP2, DAD1, SET, UBE2N, S100P, and GPI. Methods are also provided for determining expression of one or more esophageal adenocarcinoma risk factors in a subject. Methods are also provided for treating esophageal adenocarcinoma in a subject, for preventing esophageal adenocarcinoma in a subject, for inhibiting or decreasing proliferation of esophageal adenocarcinoma cells, for inhibiting or decreasing migration of esophageal adenocarcinoma cells, or for increasing susceptibility to cytotoxicity or inducing cell death of esophageal adenocarcinoma cells.
Owner:STELLA DIAGNOSTICS INC
Marker for predicting response of esophageal adenocarcinoma patient to combined treatment and application thereof
The invention belongs to the field of biomedicine, and particularly relates to a marker for predicting response of an esophageal adenocarcinoma patient to combined treatment and application thereof. The combined treatment provided by the invention is neoadjuvant radiotherapy and chemotherapy combined with attezumab treatment. In particular, the marker includes RORB, IGKV19, FGD5P1, or a combination thereof.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Application of combined pyroptosis related genes to esophageal adenocarcinoma prognosis model
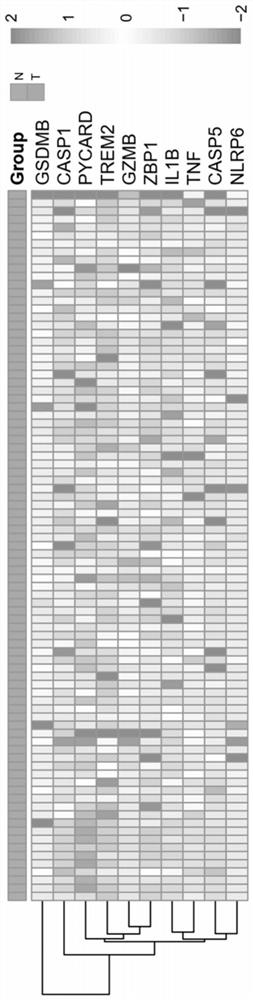
PendingCN113969318AImprove accuracyHealth-index calculationMicrobiological testing/measurementDiseaseZBP1
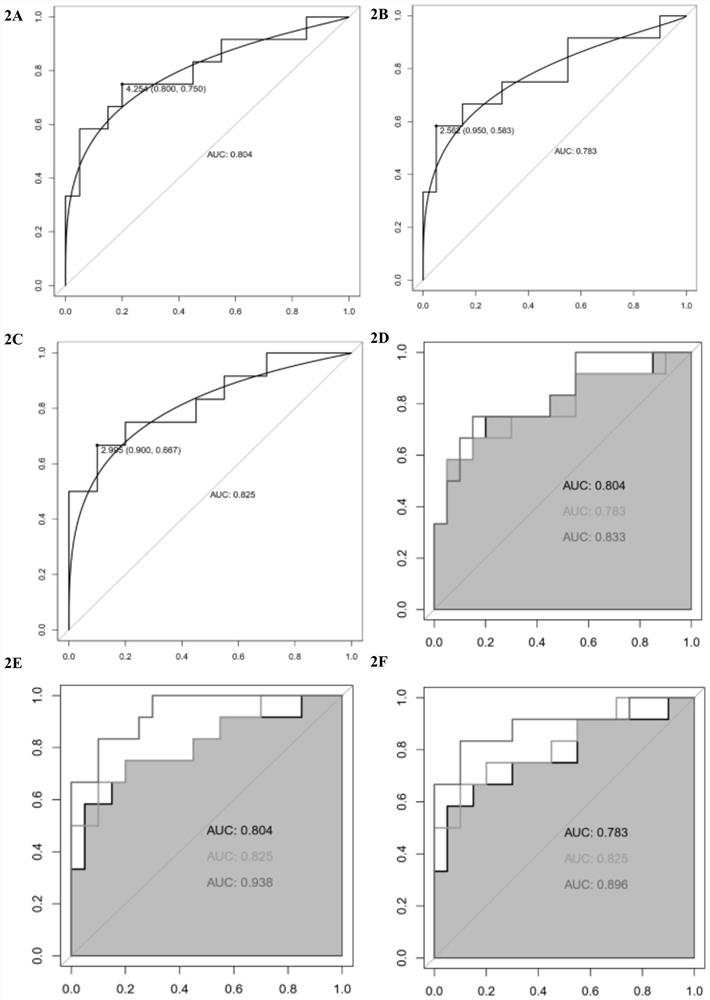
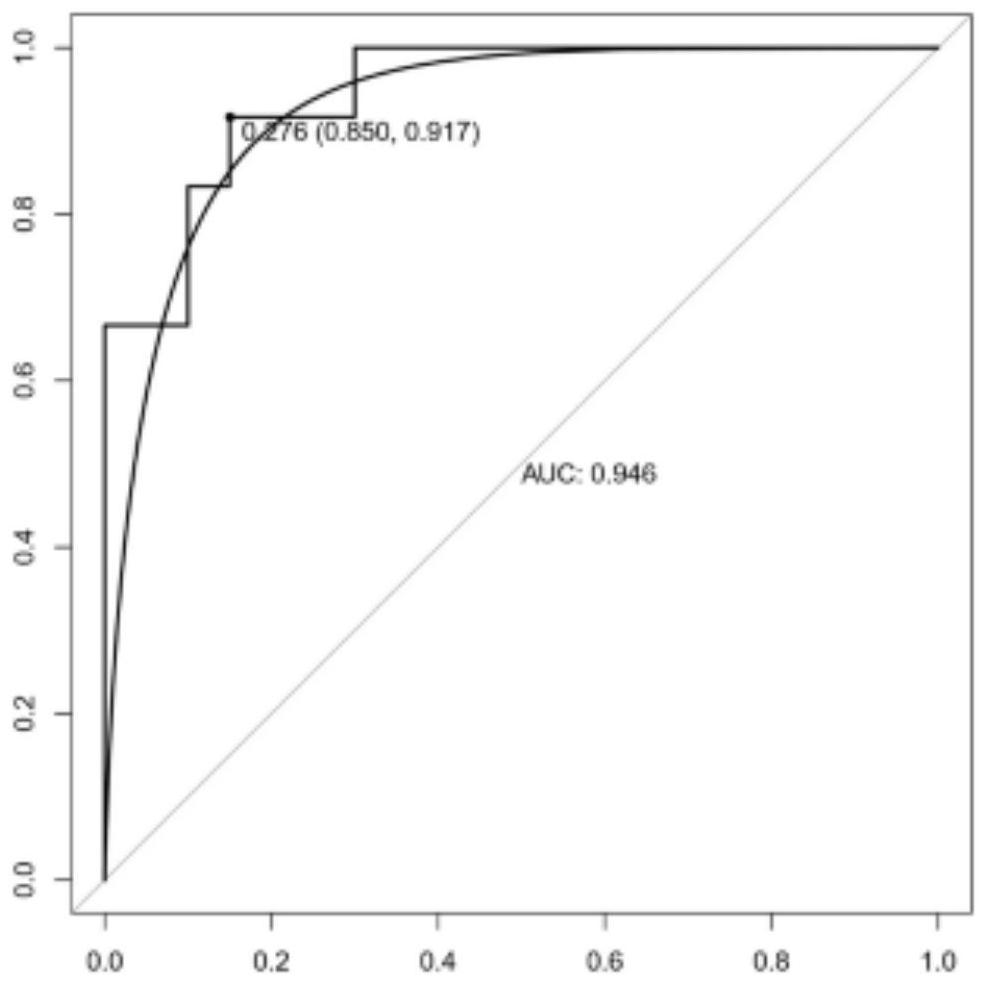
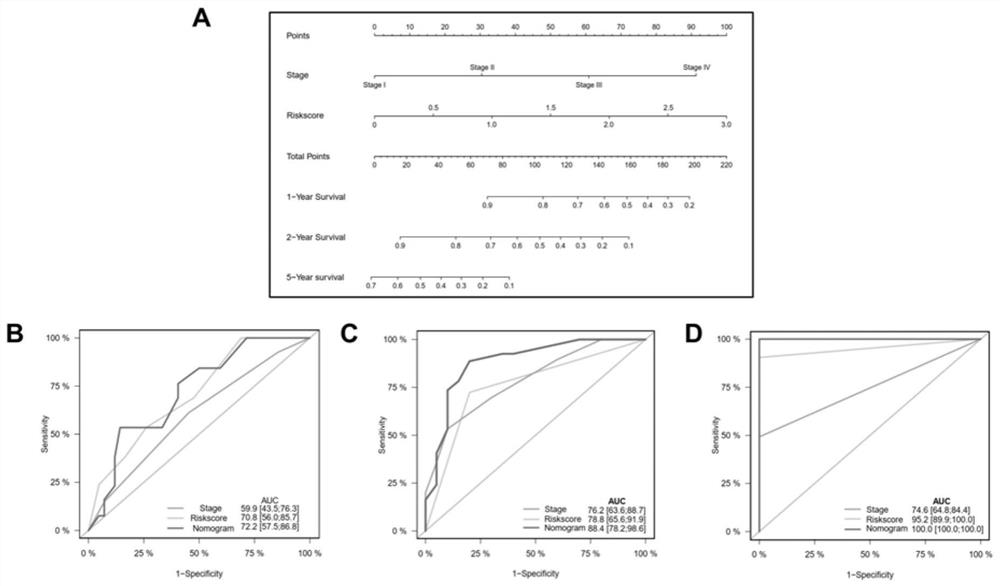
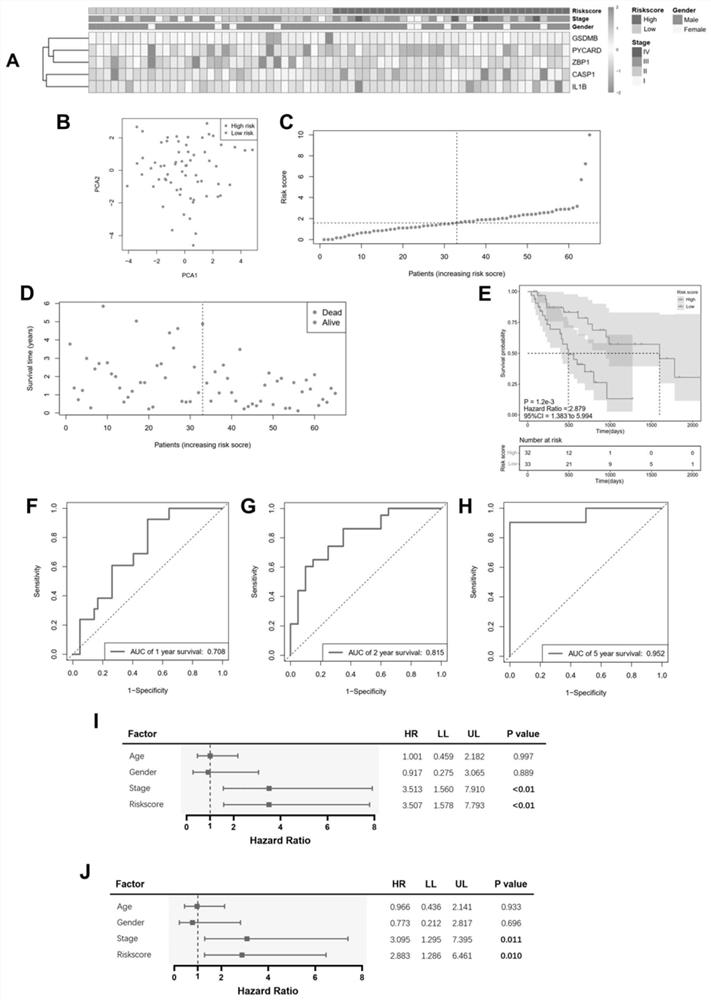
The invention provides an application of combined pyroptosis related genes to an esophageal adenocarcinoma prognosis model. The combined pyroptosis related genes are CASP1, GSDMB, IL1B, PYCARD and ZBP1. An establishment method of the esophageal adenocarcinoma prognosis model comprises the following steps: step 1, collecting and sorting data; step 2, screening differentially expressed pyroptosis related genes; step 3, reanalyzing the differentially expressed pyroptosis related genes; step 4, establishing a risk index evaluation model; and step 5, constructing an alignment diagram. The prognosis model provided by the invention has the advantage of high accuracy, and can provide a new method for disease diagnosis and prognosis for esophageal adenocarcinoma patients clinically.
Owner:GUANGDONG GENERAL HOSPITAL
Esophageal ablation technology
An esophageal ablation system including a positioner, an elongated, flexible shaft extending from the positioner, and a microwave emitter assembly disposed near the distal end of the shaft. The emitter assembly includes one or more microwave antennae and a balloon for spacing the antennae relative to target tissue. The device may have an inner balloon for deploying the antenna. The systems, devices and methods disclosed are useful for treating Barrett's Esophagus, Esophageal Adenocarcinoma, and Squamous Cell Carcinoma.
Owner:SYMPLE SURGICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com