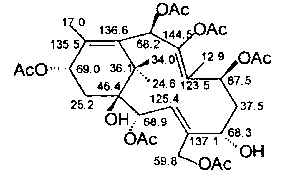
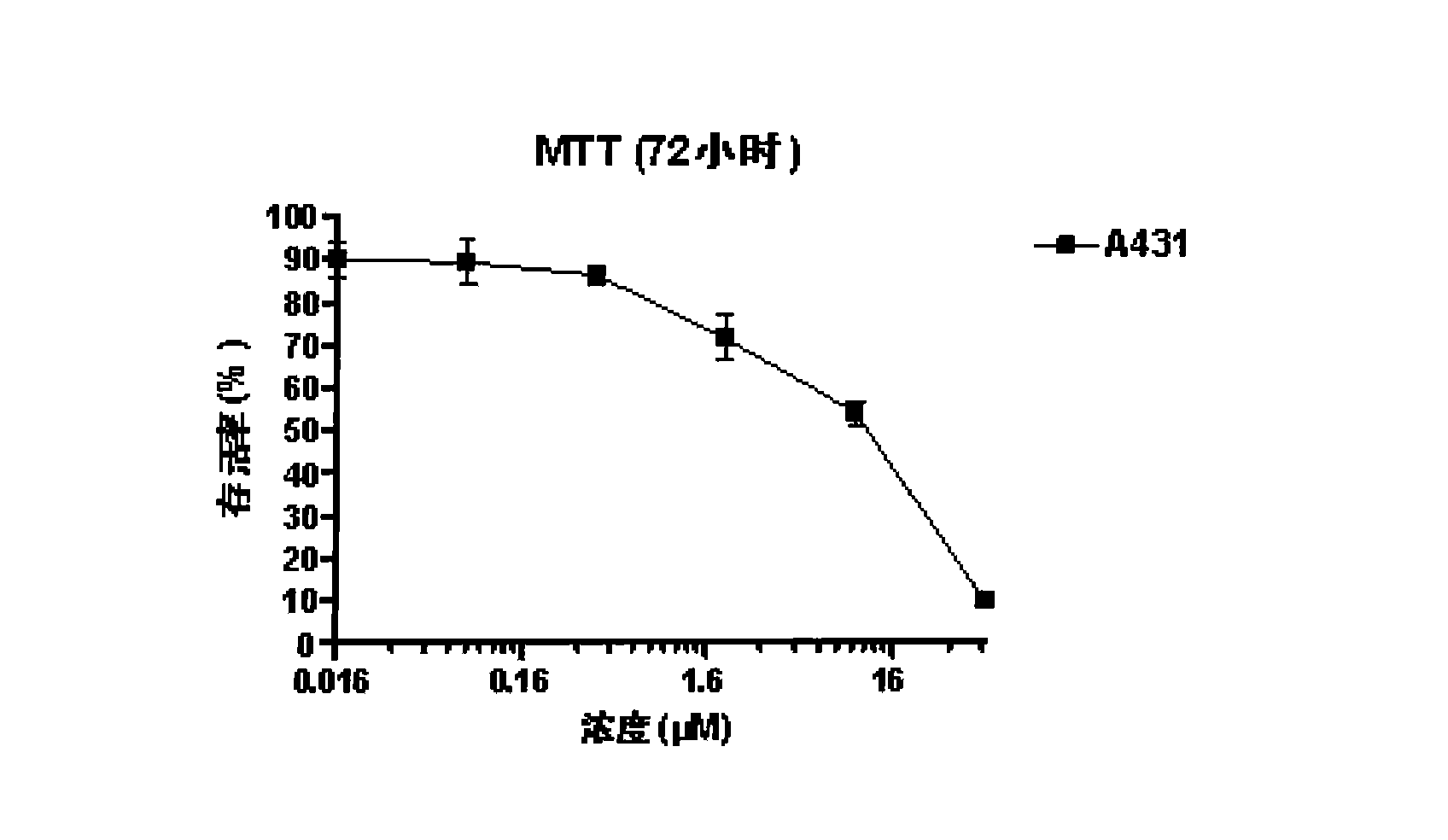
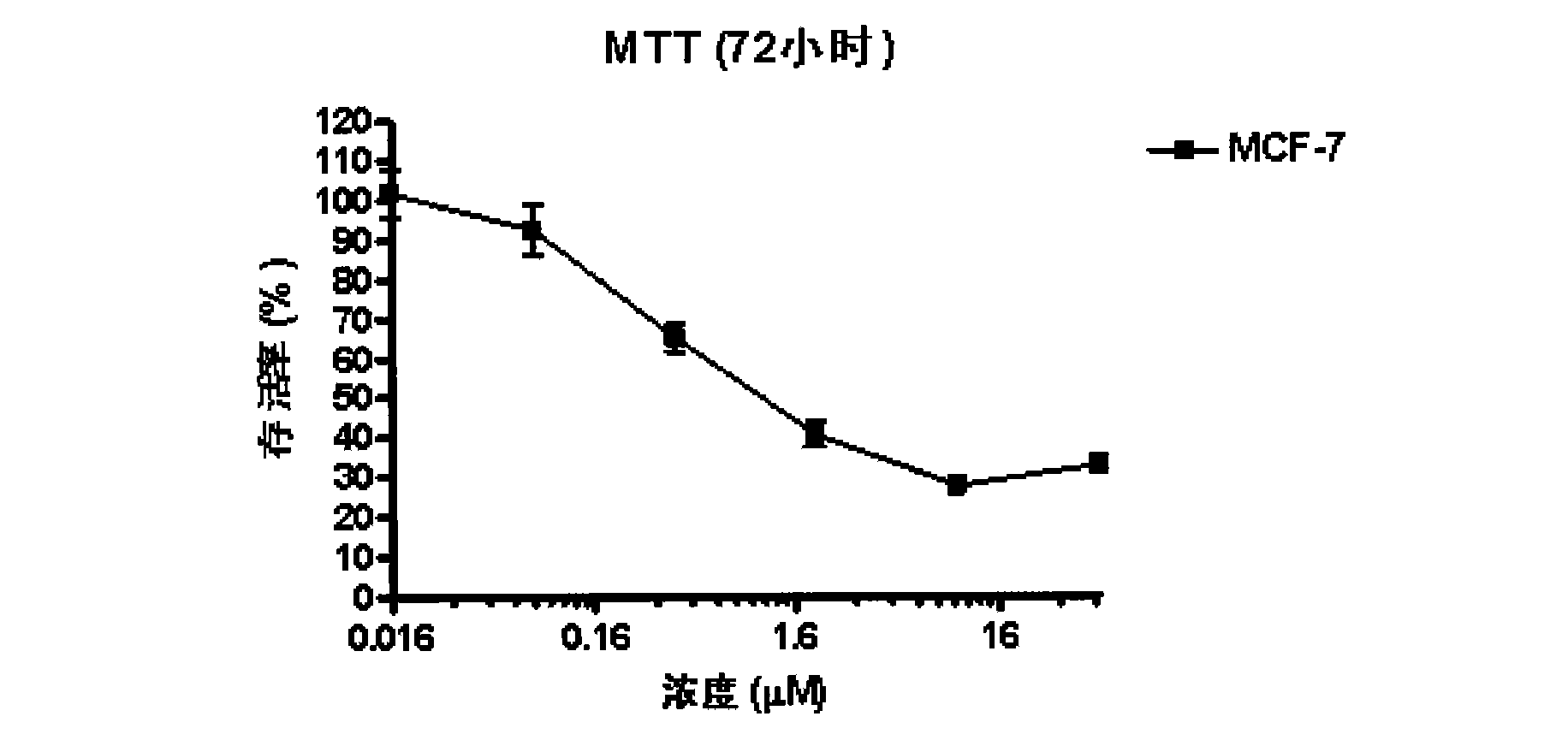
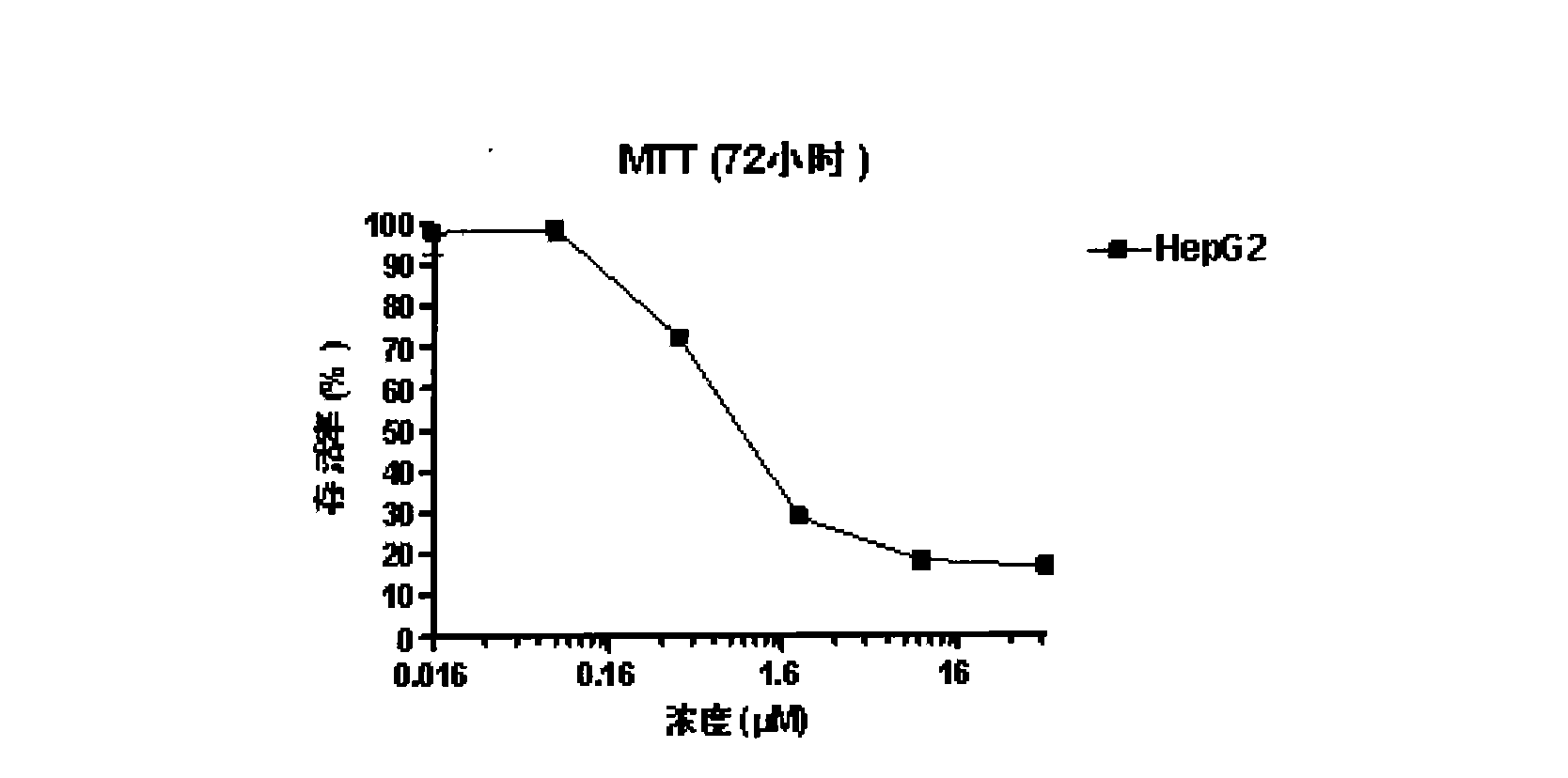
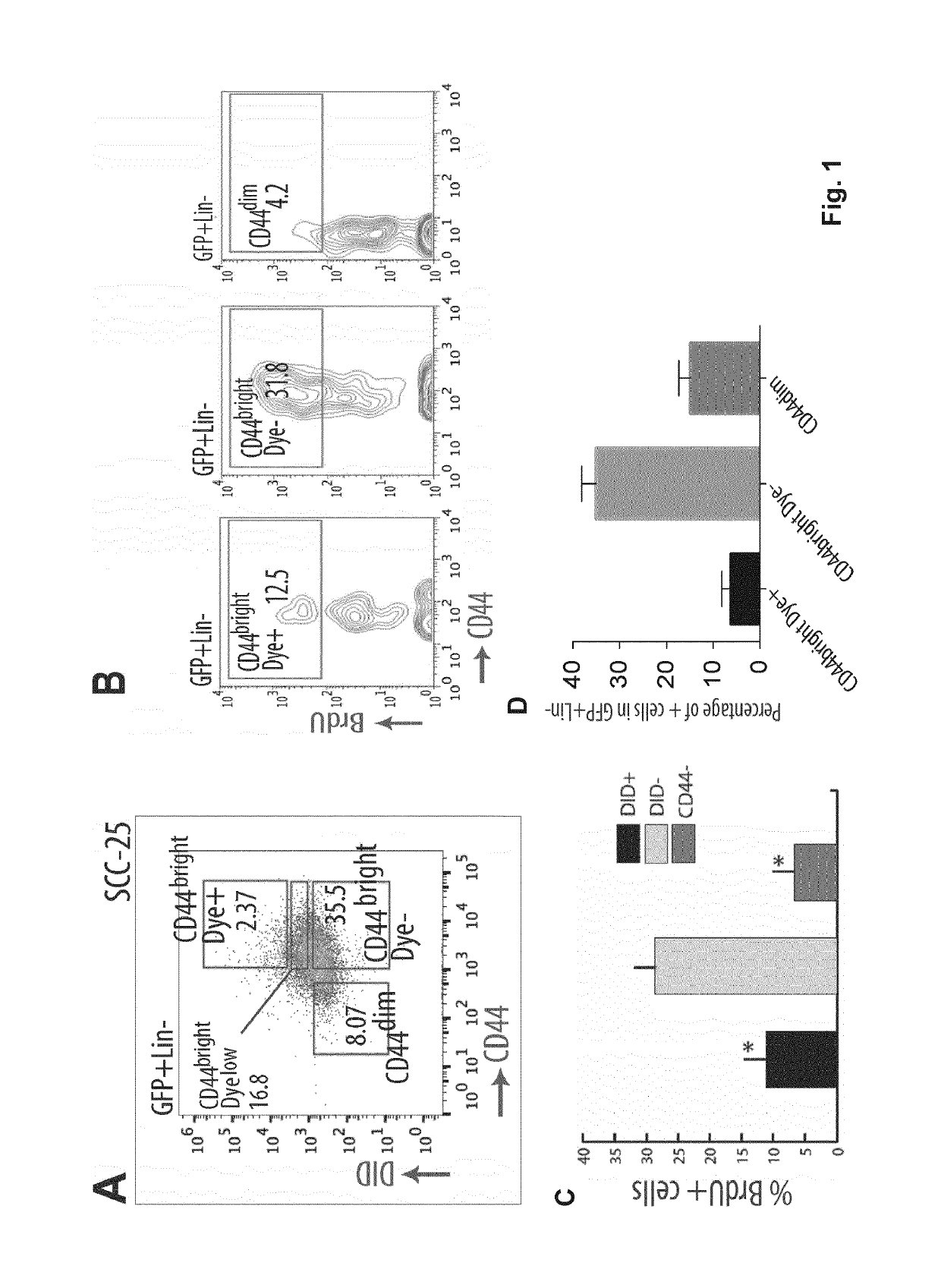
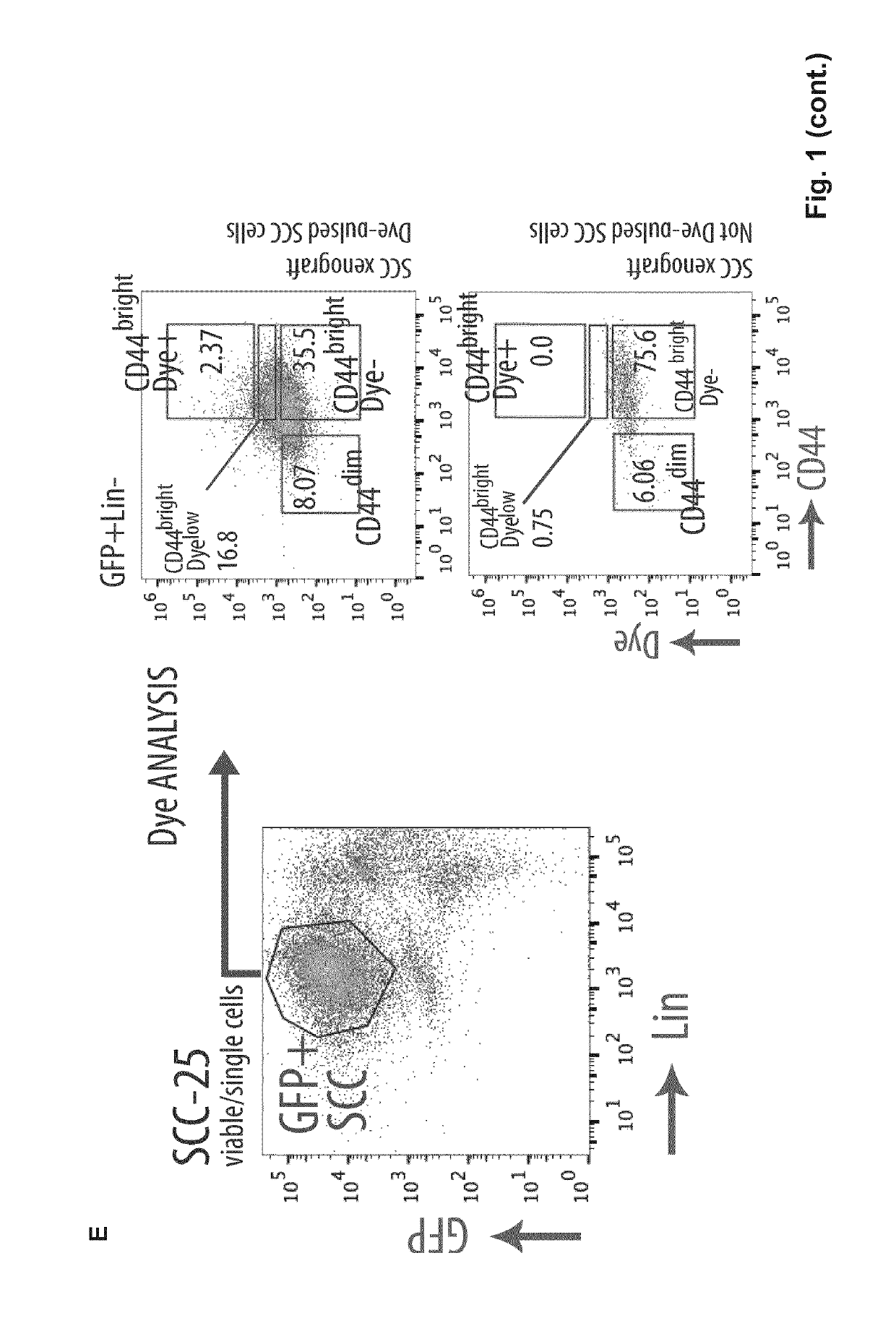
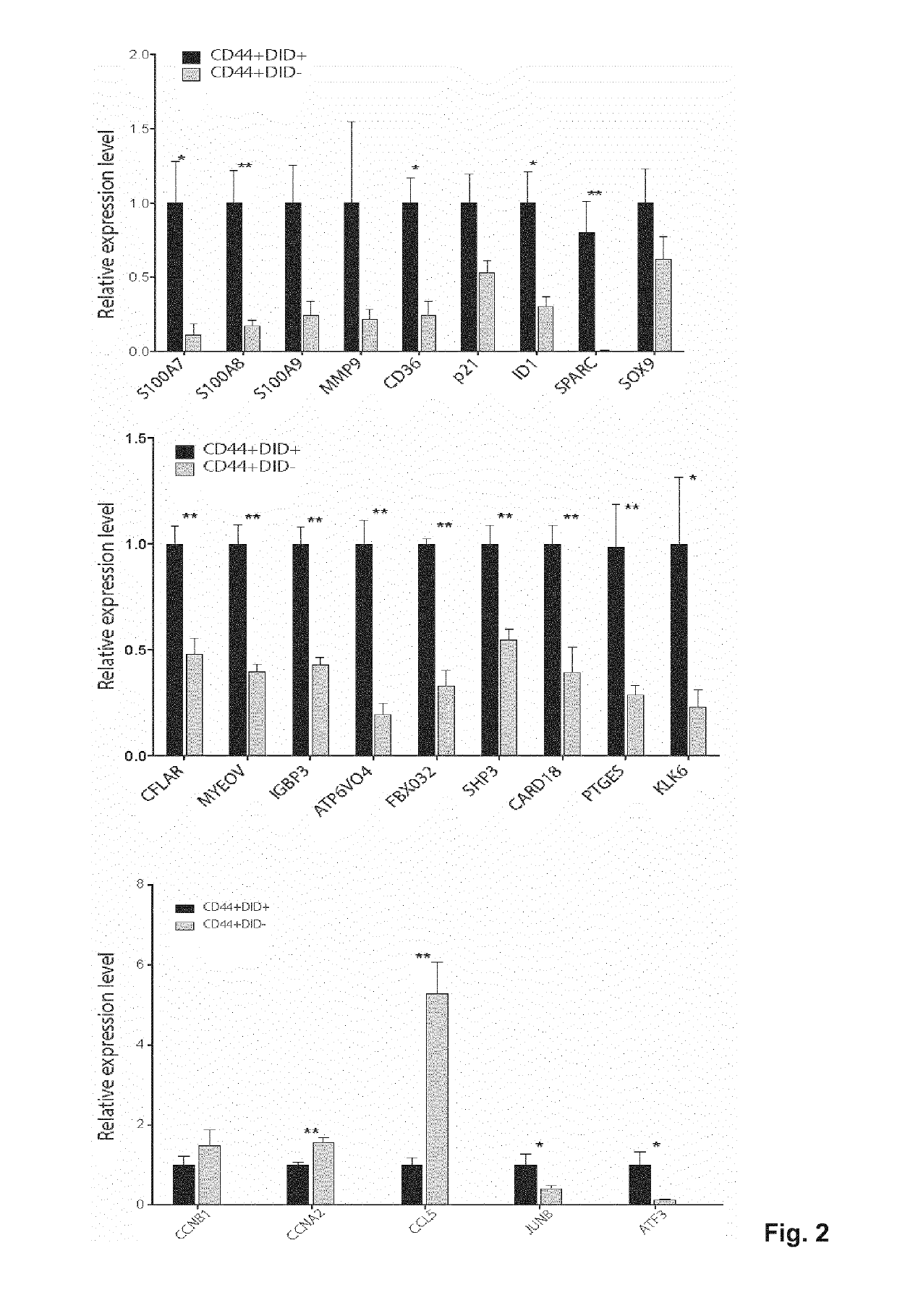
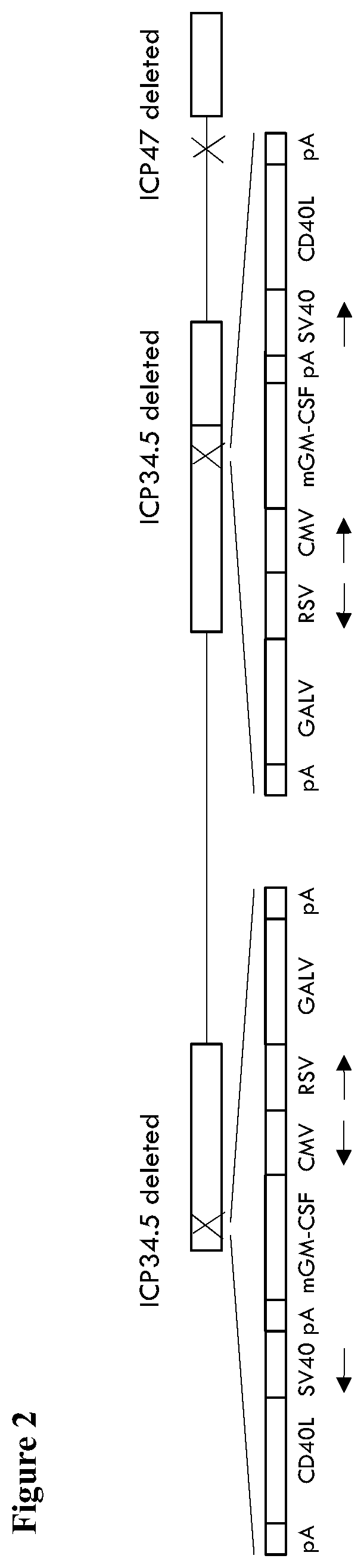
Patents
Literature
140 results about "Squamous Cell Cancers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical apparatus for determination of biological conditions using impedance measurements
ActiveUS20070135729A1Accurate and reliableAccurate and reliable wayDiagnostic recording/measuringSensorsDiseaseBasal cell carcinoma
Medical apparatus for diagnosing of a diseased condition of the skin of a subject. The apparatus comprises an electrically conducting probe including a plurality of electrodes, each electrode comprising a plurality of micro-needles, wherein each electrode comprises a base substrate, said micro-needles being integrally formed with said substrate and arranged in a laterally spaced relationship apart from each other and having a length being sufficient to penetrate the stratum corneum, said micro-needles being arranged with an at least partially oblique shape. Furthermore, the present invention relates to an electrode for use in the device, arrays of micro-needle and method for the diagnostics of biological conditions using impedance measurements. The diagnostics is in particular related to cancer, and preferably skin cancer, wherein skin cancer is a basal cell carcinoma, a malignant melanoma, a squamous cell carcinoma, or precursors of such lesions.
Owner:SCIBASE
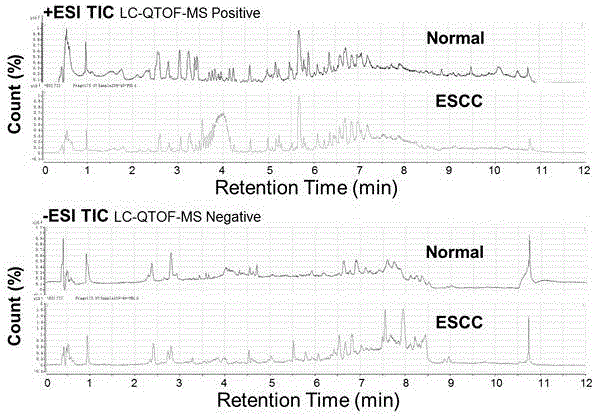
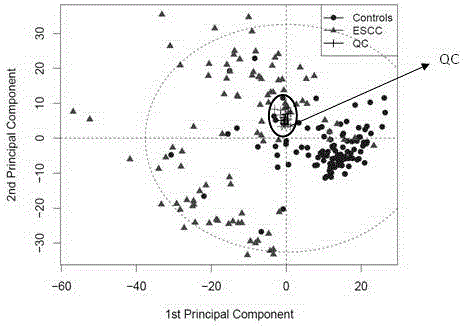
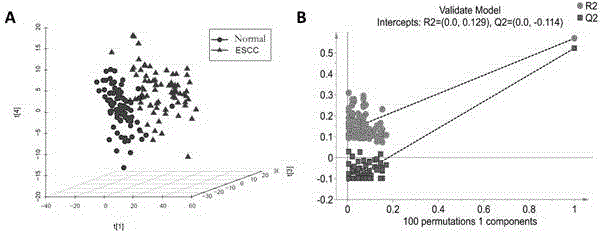
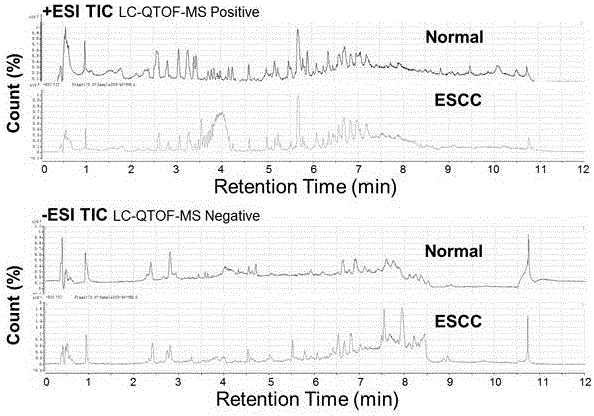
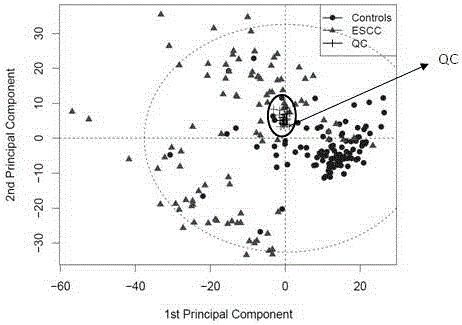
Diagnosis marker suitable for early-stage esophageal squamous cell cancer diagnosis and screening method of diagnosis marker
ActiveCN105044361AImproved prognosisReduce mortalityComponent separationBiological testingDiagnostic modalitiesScreening method
The invention discloses a diagnosis marker suitable for an early-stage esophageal squamous cell cancer diagnosis and a screening method of the diagnosis marker. Twenty five kinds of serum metabolic markers and ten relevant metabolic pathways are discovered; through the combination of the twenty five kinds of serum metabolic markers, the diagnosis marker used for an esophagus cancer diagnosis can be obtained. The screening method of the diagnosis marker has high operability; the diagnosis marker can be used for building a diagnosis model; the diagnosis model has the advantages of good effect, high sensitivity and good specificity, is suitable for a late-stage esophagus cancer diagnosis and is also suitable for the early-stage esophagus cancer diagnosis. The diagnosis model built by adopting the diagnosis marker provided by the invention has the advantages that the diagnosis can be realized only through blood sampling; the noninvasive effect is achieved; the cost is low; the modern internal invasive diagnosis mode can be well replaced; the pain of a patient is greatly reduced; in addition, the diagnosis speed is high; convenience is realized; the required time is short; the work efficiency is improved; the early discovery and early treatment of an esophagus cancer can be favorably realized; good clinic use and popularization value is realized.
Owner:SHANDONG RES INST OF TUMOUR PREVENTION TREATMENT
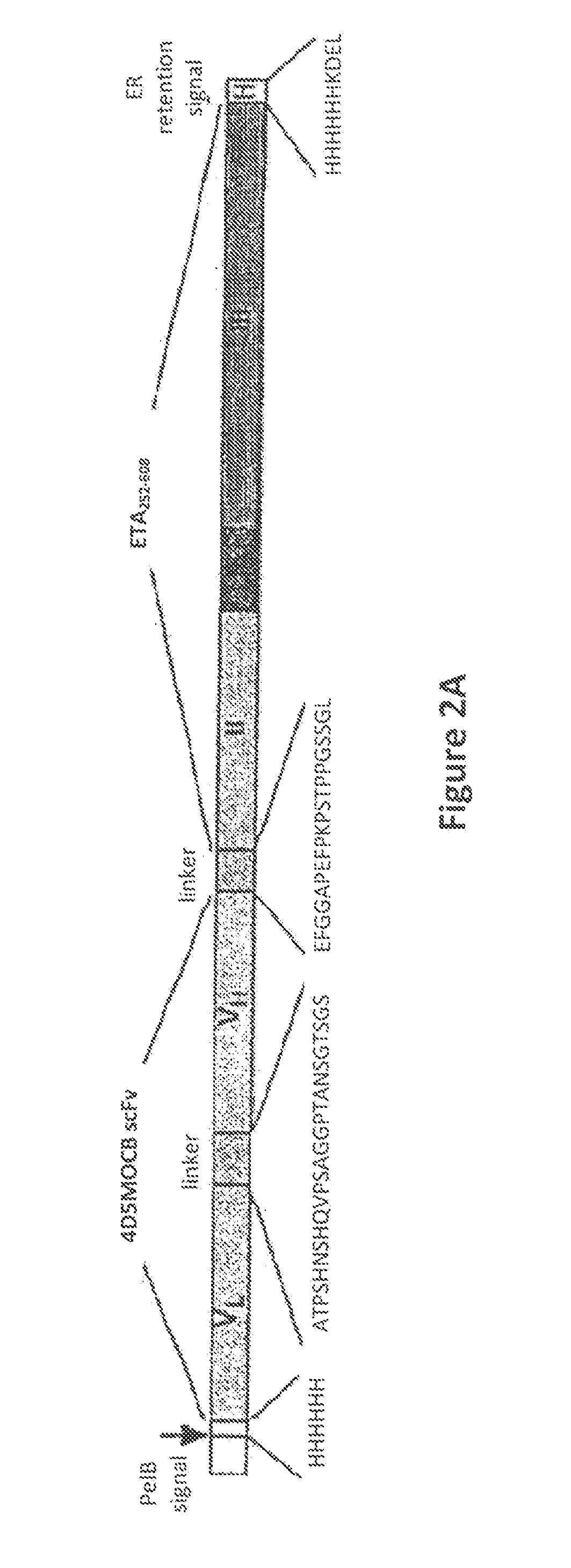
Methods for Treating Cancer Using an Immunotoxin
ActiveUS20100249039A1Peptide/protein ingredientsAntibody mimetics/scaffoldsBladder cancerSingle-Chain Antibodies
The present invention relates to methods for preventing or treating head and neck squamous cell cancer and bladder cancer using an immunotoxin comprising (a) a ligand that binds to a protein on the cancer cell attached to; (b) a toxin that is cytotoxic to the cancer cell. In a specific embodiment, the invention is directed to the prevention or treatment of head and neck squamous cell cancer or bladder cancer using VB4-845, which is a recombinant immunotoxin comprising a humanized, MOC31-derived, single-chain antibody fragment that is fused to a truncated form of Pseudomonas exotoxin A. Also encompassed by the invention are combination therapy methods, including the use of reduced dosages of chemotherapeutic agents, for the prevention or treatment of cancer. Also encompassed by the invention are formulations and methods for direct administration of the recombinant immunotoxin to the carcinoma, for the prevention or treatment of cancer.
Owner:UNIV ZURICH
Methods and compositions for the diagnosis and treatment of esphageal adenocarcinomas
InactiveCN101861401ASugar derivativesMicrobiological testing/measurementSquamous CarcinomasSquamous Cell Cancers
Methods and compositions for the diagnosis, prognosis and / or treatment of esophageal adenocarcinoma and Barrett's esophagus associated adenocarcinoma are disclosed, along with more markers where a difference is indicative of esophageal adenocarcinoma and squamous cell carcinoma, and / or Barrett's esophagus associated adenocarcinomas or a predisposition thereto. The invention also provides methods and compositions of identifying anti-cancer agents therefor.
Owner:THE OHIO STATE UNIV RES FOUND +1
Method for distinguishing between head and neck squamous cell carcinoma and lung squamous cell carcinoma
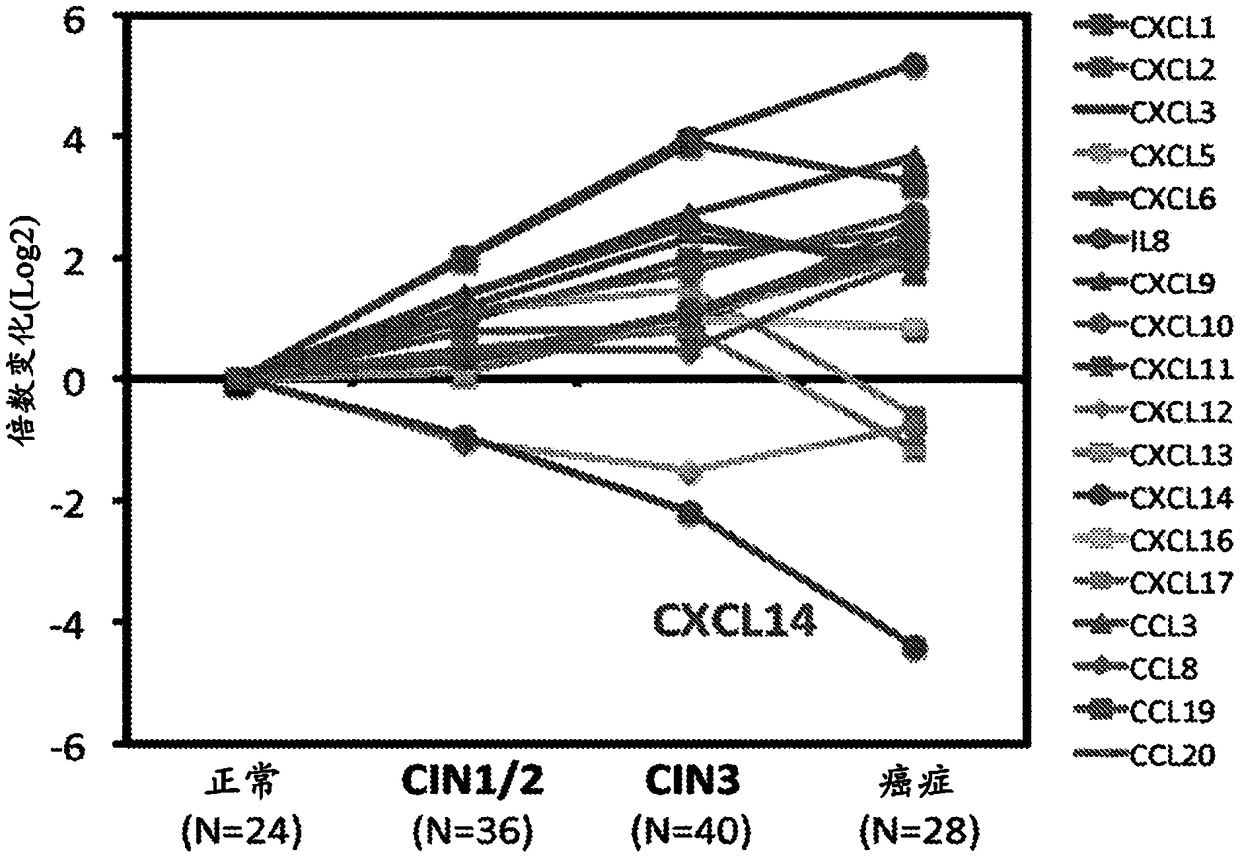
ActiveUS20070264644A1Sugar derivativesMicrobiological testing/measurementLung squamous cell carcinomaCXCL13
The present invention is a method distinguishing between head and neck squamous cell carcinoma and lung squamous cell carcinoma. In particular, a 10-gene classifier has been identified which can be used to distinguish between primary squamous cell carcinoma of the lung and metastatic head and neck squamous cell carcinoma. These genes include CXCL13, COL6A2, SFTPB, KRT14, TSPYL5, TMP3, KLK10, MMP1, GAS1, and MYH2. A panel of one or more of these genes, or proteins encoded thereby, can be used for early diagnosis and selection of an appropriate therapeutic treatment.
Owner:WISTAR INST THE A CORP OF PA +1
RNA from cytology samples to diagnose disease
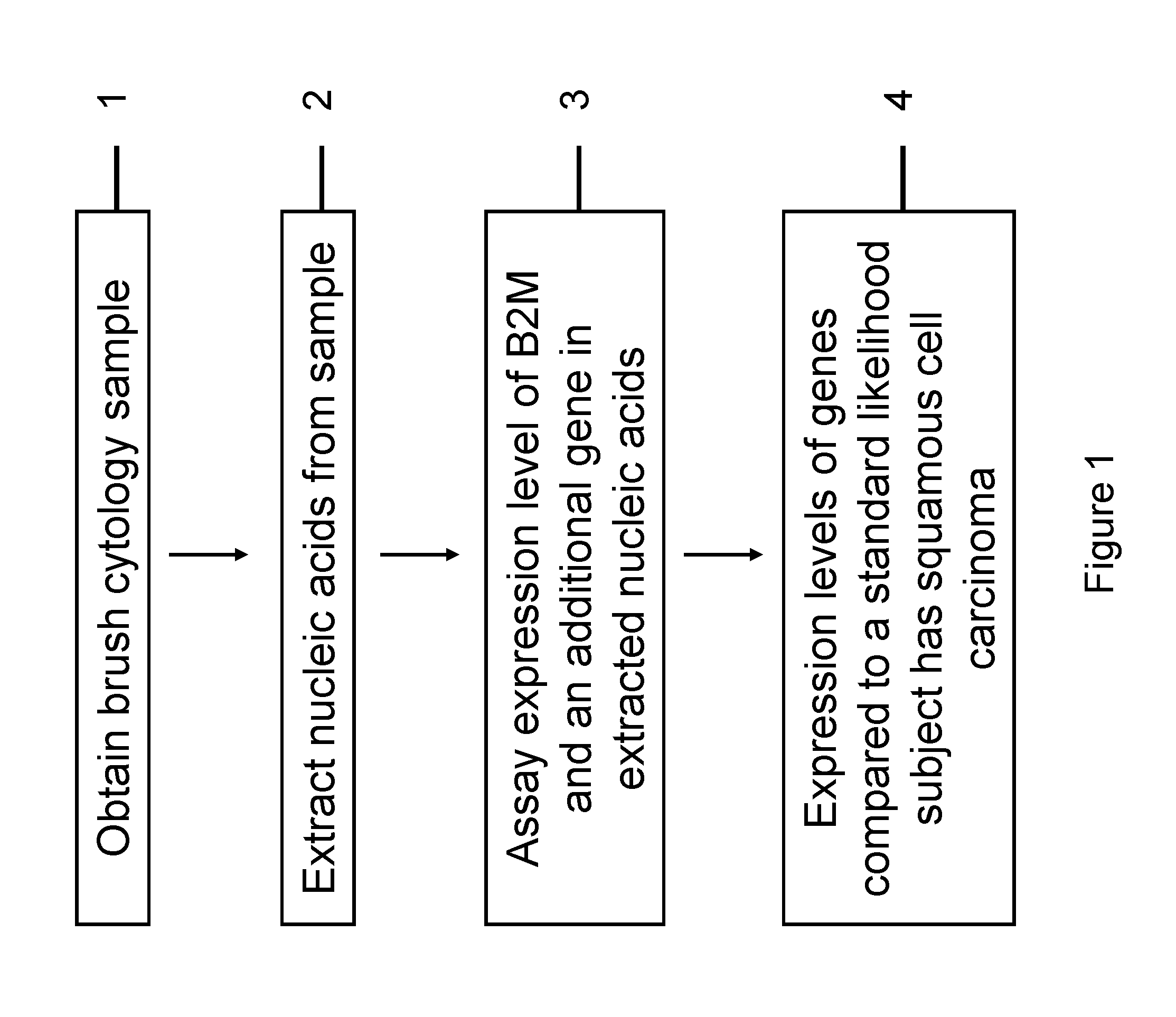
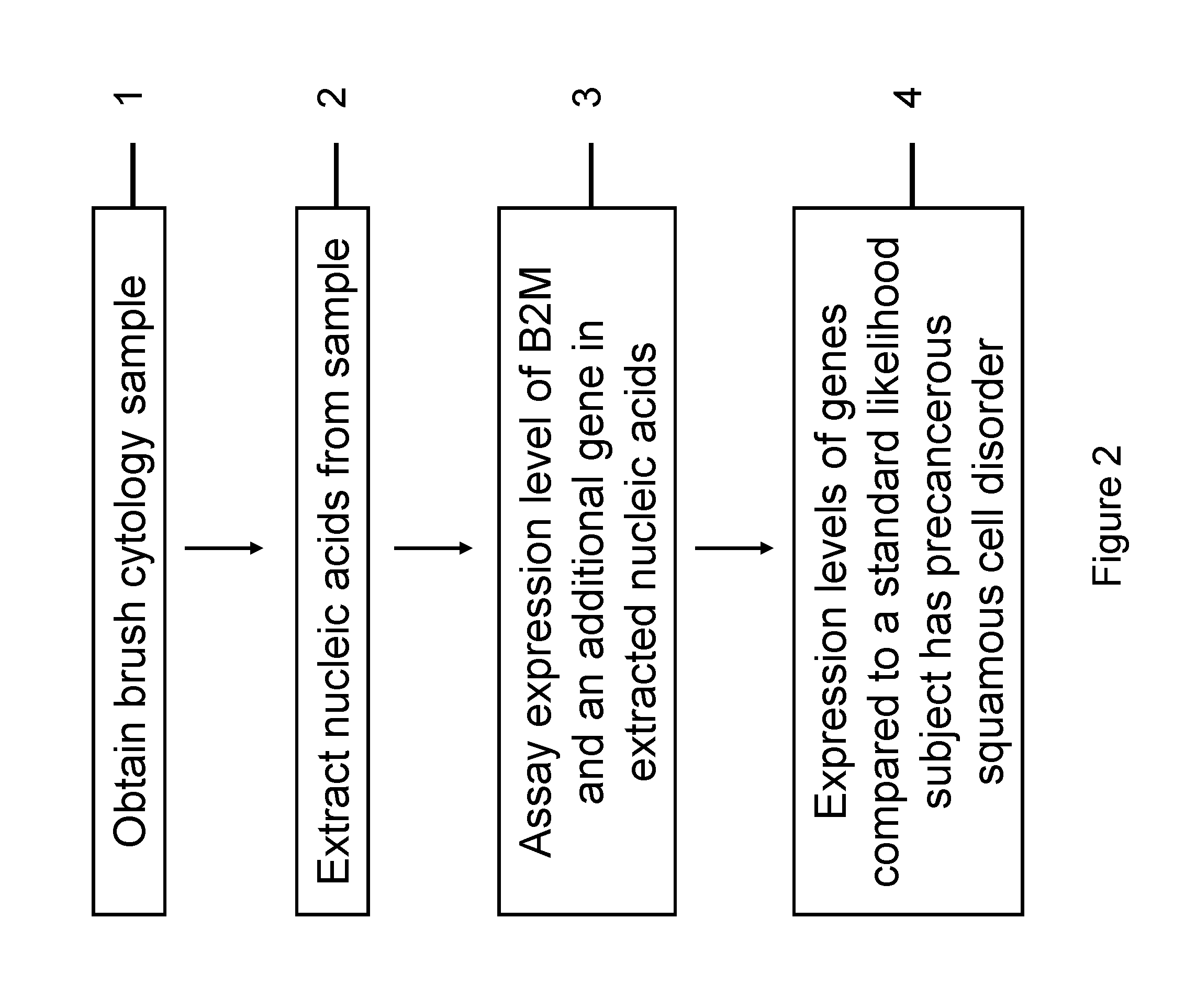
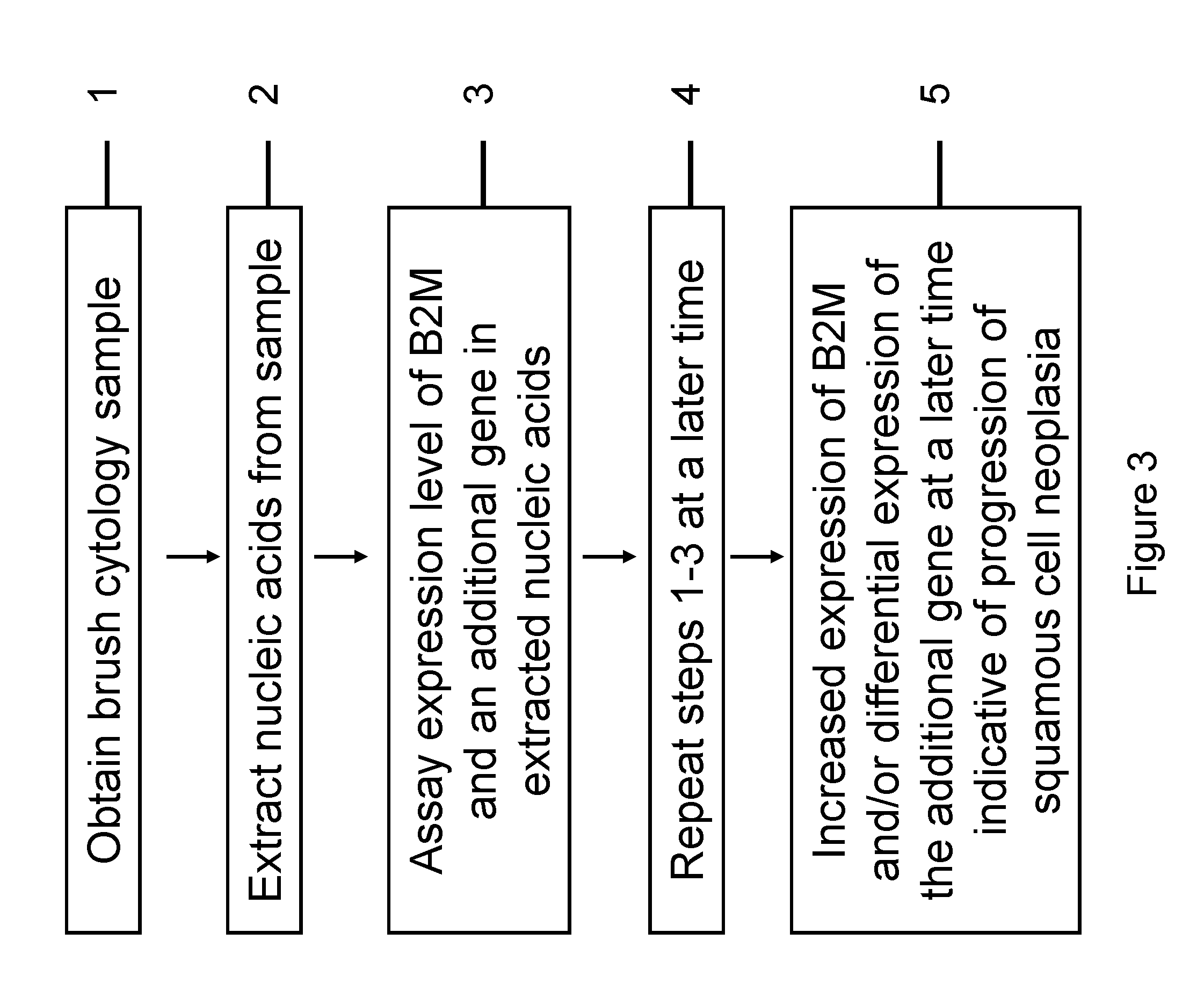
InactiveUS20120231468A1Monitor progressMicrobiological testing/measurementSquamous CarcinomasWhite blood cell
The invention relates to methods and kits for detecting the likelihood that a subject has cancer, e.g., squamous cell carcinoma, by assaying the expression levels of tumor associated genes. More specifically, the expression levels of nucleic acids or proteins can be assayed in the tumor associated genes, e.g., over-expression of beta-2 microgobulin (B2M), keratin 17 (KRT17), interleukin 8 (IL8), or annexin A2 (ANXA2), and under-expression of cytochrome p450 1B1 (CYP1B1) or laminin gamma-2 (LAMC2) can be indicative of the likelihood a subject has squamous cell carcinoma or a precancerous squamous cell disorder. The expression levels compared to standards can be indicative of the likelihood a subject has squamous cell carcinoma. The expression levels of B2M, CYP1B1, KRT17, IL8, ANXA2, or LAMC2 can also be repeatedly assayed to monitor the progression of a squamous cell neoplasia.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
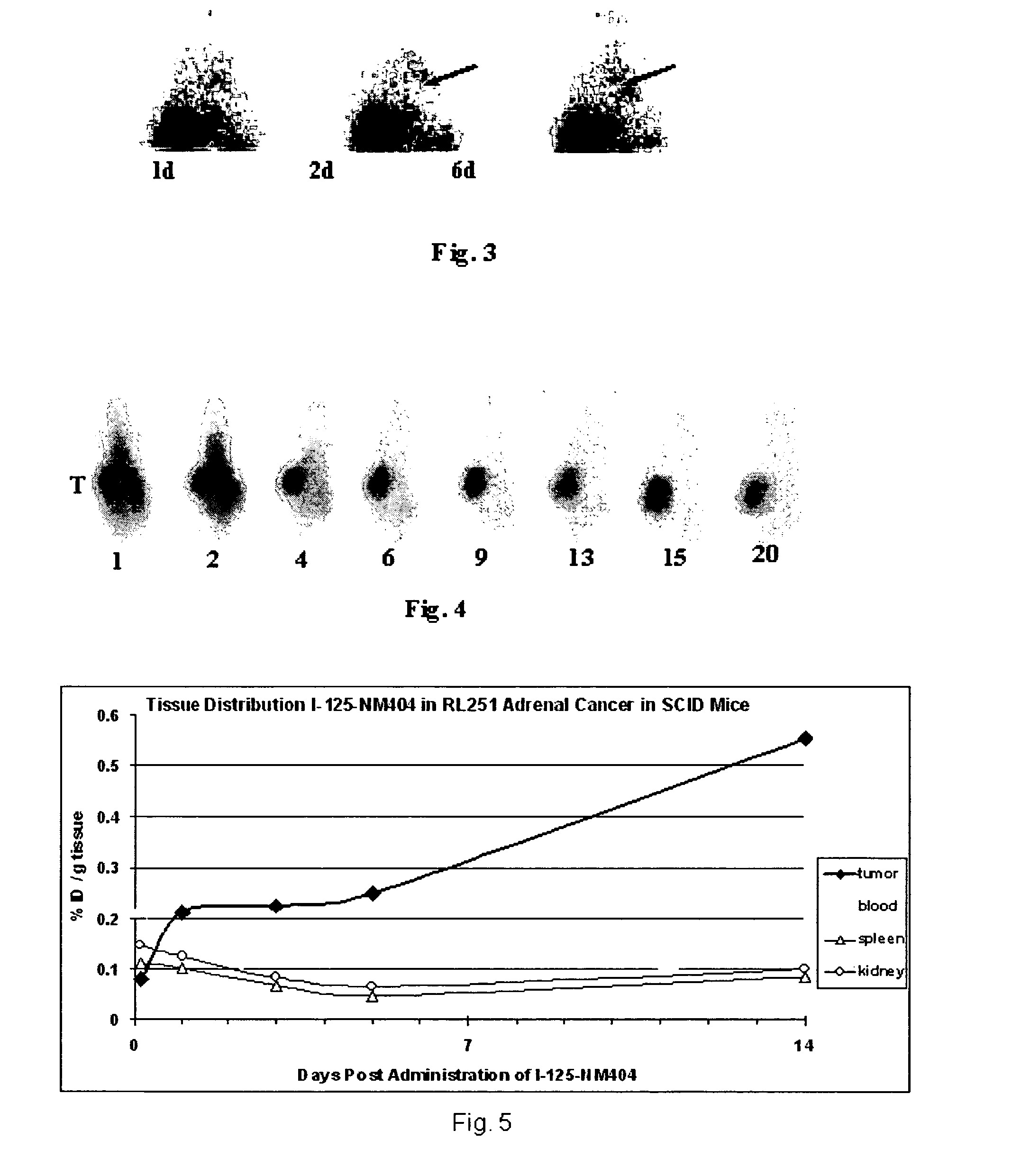
Phospholipid ether analogs as agents for detecting and locating cancer, and methods thereof
ActiveUS8540968B2X-ray constrast preparationsRadioactive preparation carriersMelanomaLymphatic Spread
The present invention provides methods for treating, detecting and locating recurrence of cancer, radiation and chemo insensitive cancer or metastasis of cancer selected from the group consisting of Lung cancer, Adrenal cancer, Melanoma, Colon cancer, Colorectal cancer, Ovarian cancer, Prostate cancer, Liver cancer, Subcutaneous cancer, Squamous cell cancer, Intestinal cancer, Hepatocellular carcinoma, Retinoblastoma, Cervical cancer, Glioma, Breast cancer and Pancreatic cancer in subject using phospholipid ether analogs.
Owner:CELLECTAR LLC
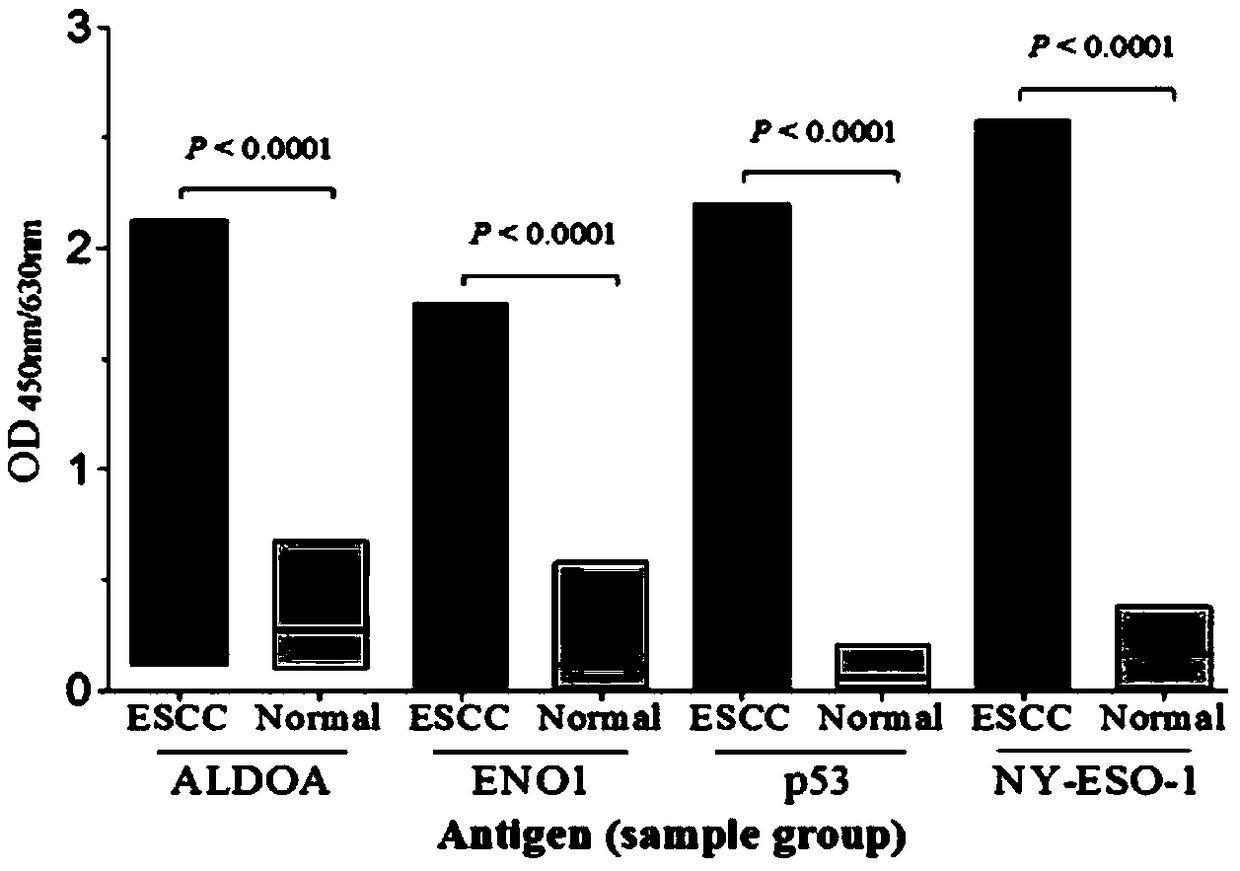
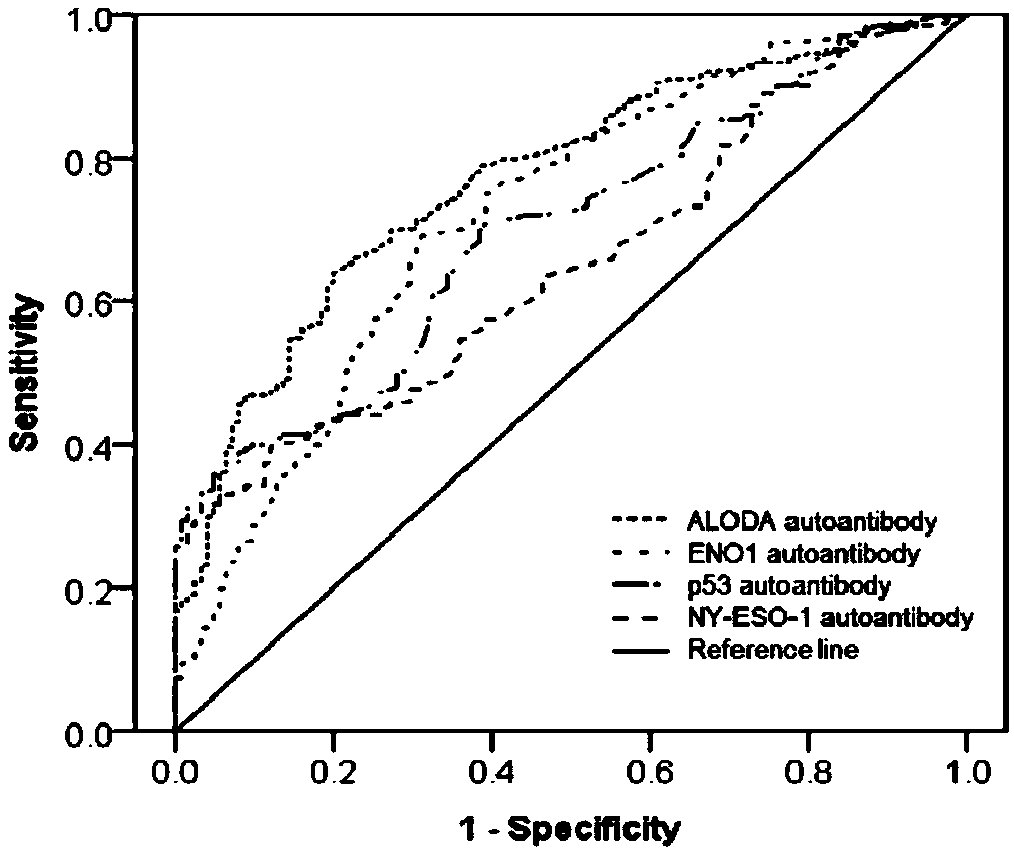
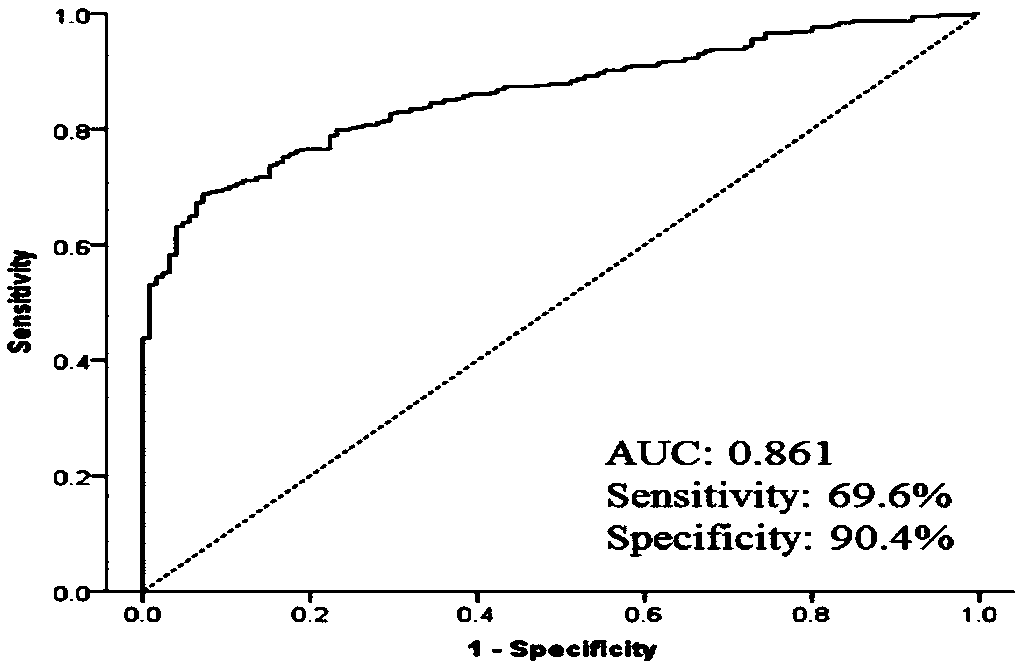
Esophageal squamous cell carcinoma autoantibody molecular marker model and application thereof
ActiveCN109342727AGood distinctionIncreased sensitivityDisease diagnosisStage I Esophageal Squamous Cell CarcinomaAutoantibody production
The invention relates to an esophageal squamous cell carcinoma autoantibody molecular marker model and an application thereof. The molecular model mainly comprises an ALDOA autoantibody, an ENO1 autoantibody, a p53 autoantibody, and an NY-ESO-1 autoantibody, and can be used for preparing a kit for distinguishing esophageal squamous carcinoma patients and healthy medical examiners. The kit for detecting esophageal squamous cell carcinoma patients mainly comprises recombinant ALDOAD protein, recombinant ENO1 protein, recombinant p53 protein, and recombinant NY-ESO-1 protein. According to the esophageal squamous cell carcinoma autoantibody molecular marker model and the application thereof, the ENO1 autoantibody is found to be elevated in serum level in the esophageal squamous carcinoma patients for the first time, and is jointly detected with the ALDOA autoantibody, the p53 autoantibody, and the NY-ESO-1 autoantibody for distinguishing the esophageal squamous cell carcinoma patients andthe healthy medical examiners, and has a better distinguishing effect than a single index detection; and in addition, the detection method adopted by the invention is an enzyme-linked immunosorbent assay indirect method, is simple and convenient to implement, has good sensitivity and specificity, and is a method which is mature and reliable and can be widely used in base layer hospitals.
Owner:汕头市颂美恩生物科技有限公司
Duocarmycin ADCS showing improved in vivo antitumor activity
ActiveUS9421278B2Organic active ingredientsDipeptide ingredientsHaematological malignancyProstate cancer
The present invention relates to duocarmycin-containing antibody-drug conjugates (ADCs) for use in the treatment of human solid tumors and haematological malignancies expressing HER2, in particular breast cancer, gastric cancer, bladder cancer, ovarian cancer, lung cancer, prostate cancer, pancreatic cancer, colorectal cancer, head and neck squamous cell cancer or osteosarcoma, and acute lymphoblastic leukaemia. In particular, the present invention relates to duocarmycin-containing ADCs for use in the treatment of human solid tumors with HER2 IHC 2+ or 1+ and HER2 FISH negative tissue status. Advantageously, the present invention relates to duocarmycin-containing ADCs for use in the treatment of triple negative breast cancer (TNBC).
Owner:BYONDIS BV
Yew branch and leaf extract, extraction method and applications thereof
InactiveCN102949385AEasy to operateOrganic active ingredientsDermatological disorderTonsilSquamous Carcinomas
The present invention relates to a yew branch and leaf extract, an extraction method and applications thereof. The yew branch and leaf extract is extracted by adopting branches and leaves of Taxus chinensis var. mairei as raw materials, and comprises cephalomannine and 7-epitaxol, wherein the active ingredients provide an inhabitation effect for squamous cells in skin cancers, and especially provide significant treatment effects for anal squamous cell carcinoma, esophagus squamous carcinoma and tonsil squamous cell carcinoma. According to the present invention, resource advantages of the yew are utilized to deep develop, utilize and research the Taxus chinensis var. mairei; and a yew anti-skin cancer component extraction method with a characteristic of simple operation is provided, and an effect of the component in anti-skin cancer application is determined.
Owner:JIANGSU HONGDOUSHAN BIOLOGICAL TECH
Application of compound as JAK-STAT3 signal passage inhibitor
InactiveCN101537001AGood curative effectImproved prognosisOrganic active ingredientsAntineoplastic agentsProstate cancerTherapeutic effect
The invention discloses an application of a compound as a JAK-STAT3 signal passage inhibitor, and particularly relates to an application of a compound with a formula I or pharmaceutically acceptable salt of the compound in the process of preparing an anti-tumor medicament. The application provides a novel treating candidate medicament for a tumor patient, thereby probably further improving the treatment effect on the patient and improving the prognosis of the patient. The compound with the formula I or the pharmaceutically acceptable salt of the compound has the effect on various tumor cells, which indicates that the compound can be used for treating various cancers including cerebral tumor, genitourinary system tumor, lymphatic system tumor, stomach cancer, laryngeal cancer, nasopharyngeal cancer, skin cancer, bone cancer, blood cancer, leukemia, breast cancer and histiocytic lymphoma, non-small cell lung cancer, small-cell lung cancer, lung adenocarcinoma, lung squamous cell cancer, pancreatic cancer, prostatic cancer, liver cancer, epithelial cell cancer and the like. The definition of genes in the formula refers to description for details.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Targeting metastasis stem cells through a fatty acid receptor (CD36)
ActiveUS20190106503A1Diminish of metastasis growthPromote growthCompound screeningApoptosis detectionLipid formationLymphatic Spread
Metastasis stem cells are targeted through a fatty acid receptor. Blockers or inhibitors of CD36 activity or expression are for the treatment of oral squamous cell cancer (OSCC), particularly for the treatment of generated metastases and for diminishing generation from primary tumors. Apart from shRNAs, anti-CD36 antibodies are provided as blockers or inhibitors, especially those that block the binding of CD36 to oxidized LDL and fatty acids and their incorporation into cells, because the promotion of their transport is indicated as the mechanism by which CD36 promote metastases dissemination and growth. A method identifies candidates to anticancer agents, particularly for OSCC metastasis, among those that promote in CD36+ cells, in vivo or in vitro, effects associated to CD36 depletion or blocking such as decrease of growth accumulation of lipid droplets and decrease of size in the case of metastases.
Owner:FUNDACIO INST DE RECERCA BIOMEDICA (IRB BARCELONA) +1
Medical apparatus for determination of biological conditions using impedance measurements
ActiveUS9636035B2Accurate and reliable wayDiagnostic recording/measuringSensorsMelanomaSquamous Carcinomas
Medical apparatus for diagnosing of a diseased condition of the skin of a subject. The apparatus comprises an electrically conducting probe including a plurality of electrodes, each electrode comprising a plurality of micro-needles, wherein each electrode comprises a base substrate, said micro-needles being integrally formed with said substrate and arranged in a laterally spaced relationship apart from each other and having a length being sufficient to penetrate the stratum corneum, said micro-needles being arranged with an at least partially oblique shape. Furthermore, the present invention relates to an electrode for use in the device, arrays of micro-needle and method for the diagnostics of biological conditions using impedance measurements. The diagnostics is in particular related to cancer, and preferably skin cancer, wherein skin cancer is a basal cell carcinoma, a malignant melanoma, a squamous cell carcinoma, or precursors of such lesions.
Owner:SCIBASE
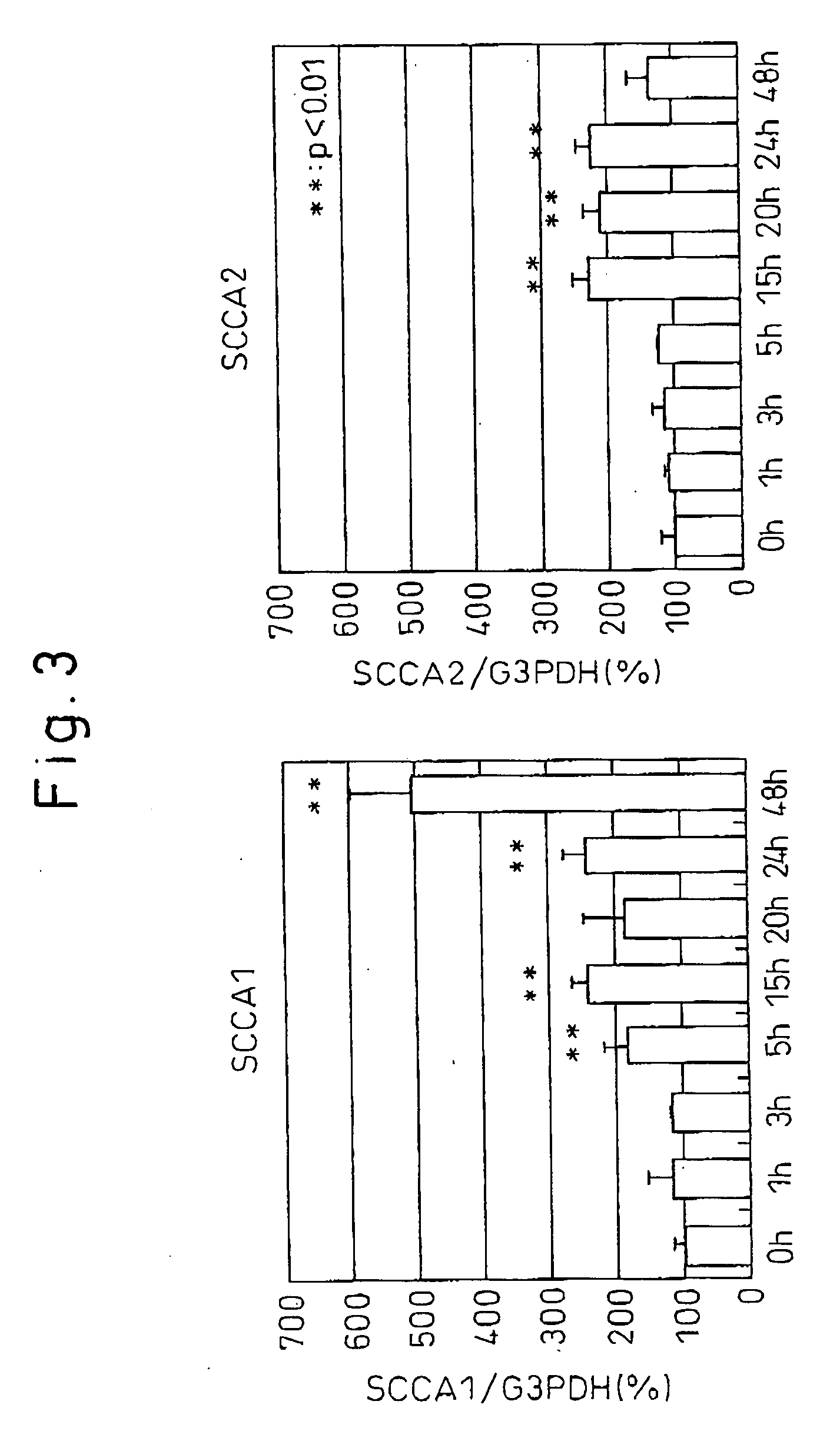
Method and Pharmaceutical Composition for Treating Psoriasis, Squamous Cell Carcinoma and/or Parakeratosis by Inhibiting Expression of Squamous Cell Carcinoma-Related Antigen
In a first aspect thereof, the present invention provides a method for treatment and / or prevention of a disease selected from the group consisting of psoriasis and squamous cell carcinoma by inhibiting the expression of squamous cell carcinoma antigen (SCCA) by cells. In another aspect thereof, the present invention provides a method for screening for substances that inhibit epidermal parakeratosis, wherein the activity of a candidate substance that inhibits cysteine protease inhibitory activity possessed by squamous cell carcinoma antigen 1 (SCCA-1) is used as an indicator.
Owner:SHISEIDO CO LTD
Reagent for auxiliarily diagnosing lung cancer lymph node metastasis
InactiveCN102445543AIncrease credibilityPracticalBiological testingStainingPolymeric immunoglobulin receptor
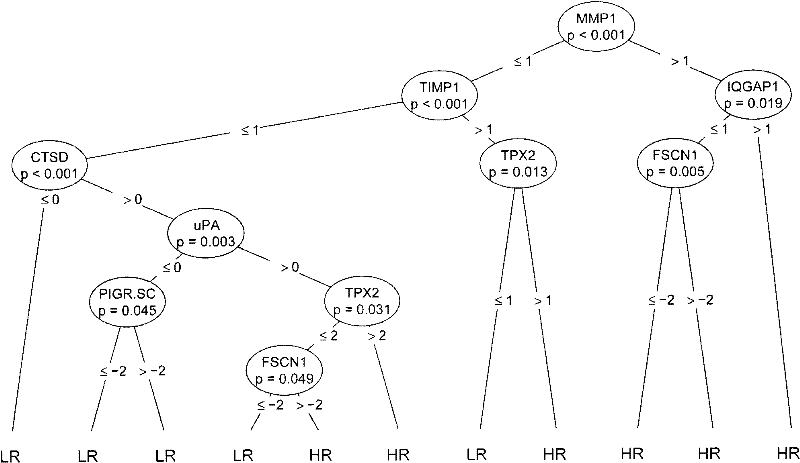
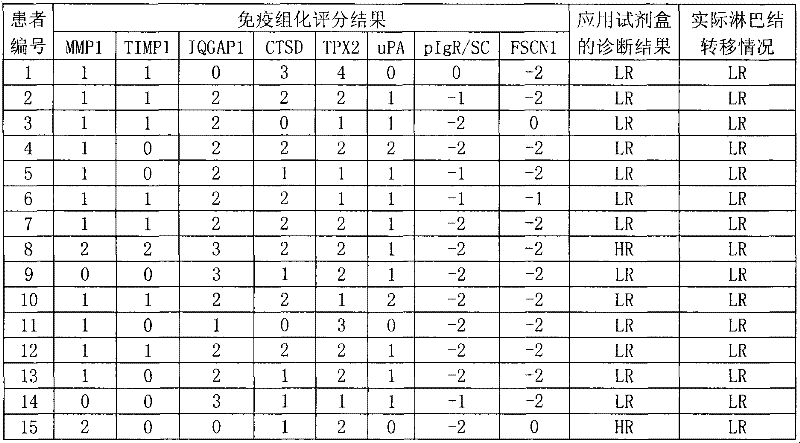
The invention discloses a reagent for auxiliarily diagnosing lung cancer lymph node metastasis, comprising 8 antibodies which are used for detecting 8 protein markers which are MMP1 (matrix metalloproteinase-1), TIMP1 (tissue inhibitor of metalloproteinases metallopeptidase inhibitor 1), IQGAP1 (IQ motif containing GTPase activating protein 1), TPX2 (targeting protein for Xklp2), uPA (Urokinase-type plasminogen activator), Cathepsin-D, Fascin and pIgR / SC (polymeric immunoglobulin receptor / secretory component). By adopting the 8 antibodies and an immunohistochemical staining result, lung cancer lymph node metastasis can be auxiliarily diagnosed, and the reagent is expected to be used for the risk estimation of lung squamous cell cancer lymph node metastasis and the prognostic prediction. The reagent has high creditability, strong practicability and clinical use value based on the clinical routine immunohistochemical staining technology when the reagent is used for auxiliary diagnosis.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Duocarmycin adcs showing improved in vivo antitumor activity
ActiveUS20160008486A1Organic active ingredientsDipeptide ingredientsHaematological malignancyProstate cancer
The present invention relates to duocarmycin-containing antibody-drug conjugates (ADCs) for use in the treatment of human solid tumours and haematological malignancies expressing HER2, in particular breast cancer, gastric cancer, bladder cancer, ovarian cancer, lung cancer, prostate cancer, pancreatic cancer, colorectal cancer, head and neck squamous cell cancer or osteosarcoma, and acute lymphoblastic leukaemia. In particular, the present invention relates to duocarmycin-containing ADCs for use in the treatment of human solid tumours with HER2 IHC 2+ or 1+ and HER2 FISH negative tissue status. Advantageously, the present invention relates to duocarmycin-containing ADCs for use in the treatment of triple negative breast cancer (TNBC).
Owner:BYONDIS BV
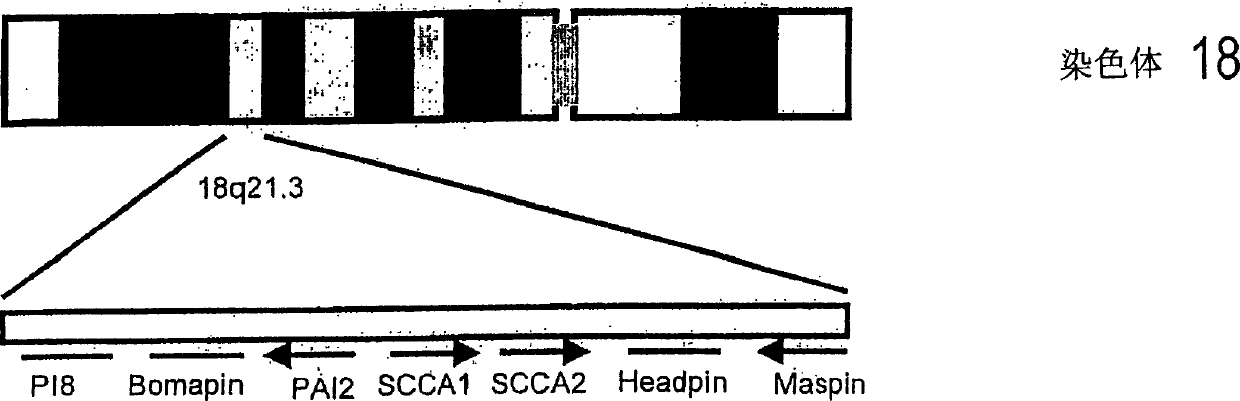
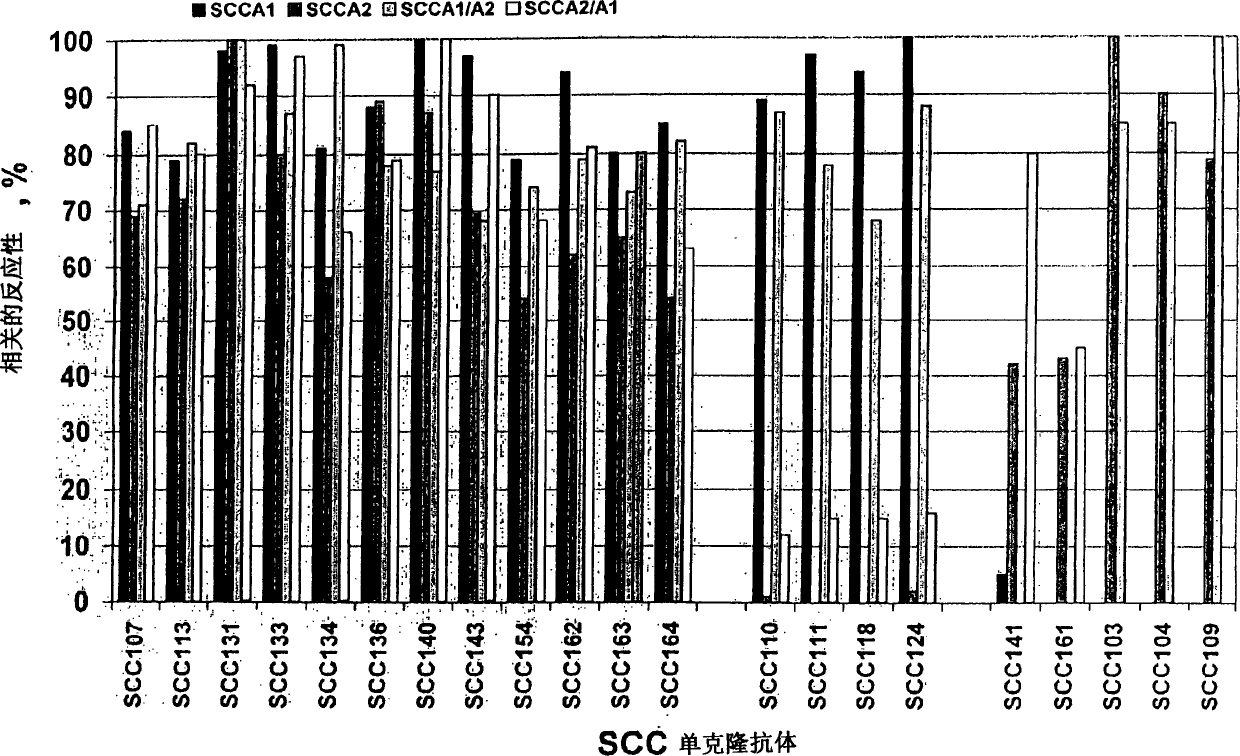
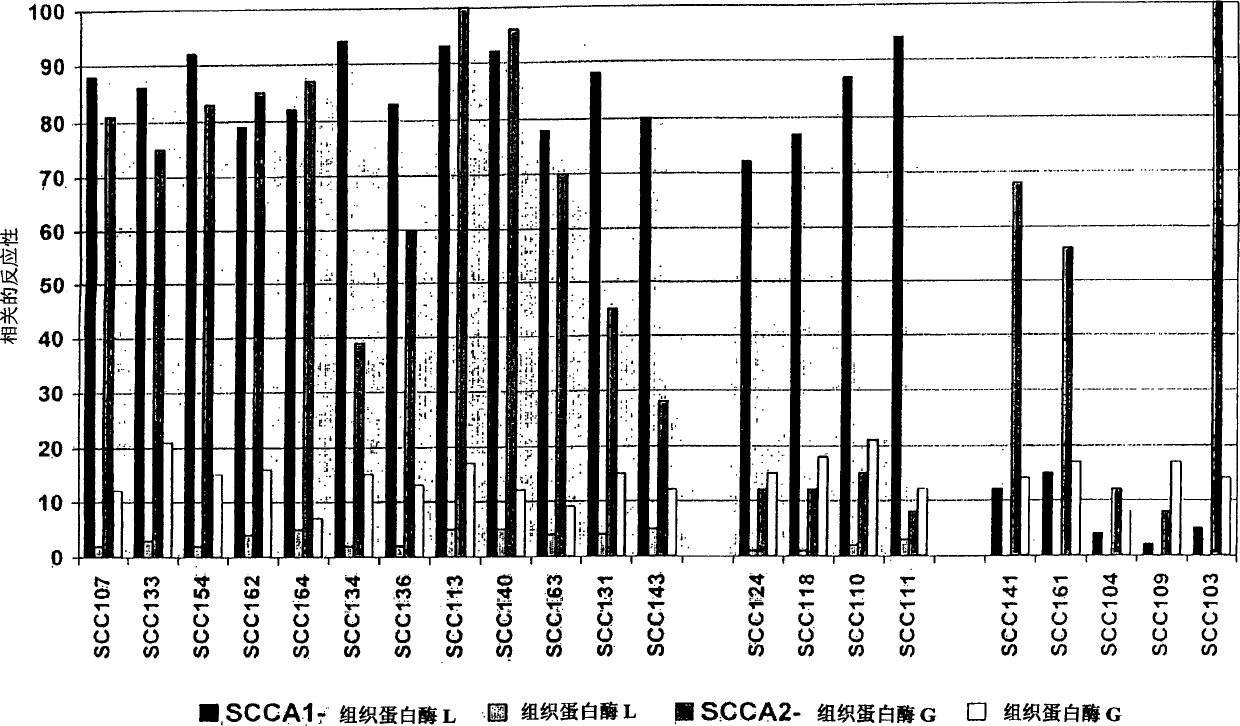
Immunoassays for specific determination of SCCA isoforms
InactiveCN1684981APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenMonoclonal antibody
The present invention relates to monoclonal antibodies capable of distinguishing squamous cell cancer antigens, SCCA, in either free or complex bound forms, preferably antigens SCCA1 and SCCA2, as well as hybridomas recognizing such antibodies, method for diagnosing SCC, as well as diagnostic kits for detecting SCCAs.
Owner:CANAG DIAGNOSTICS
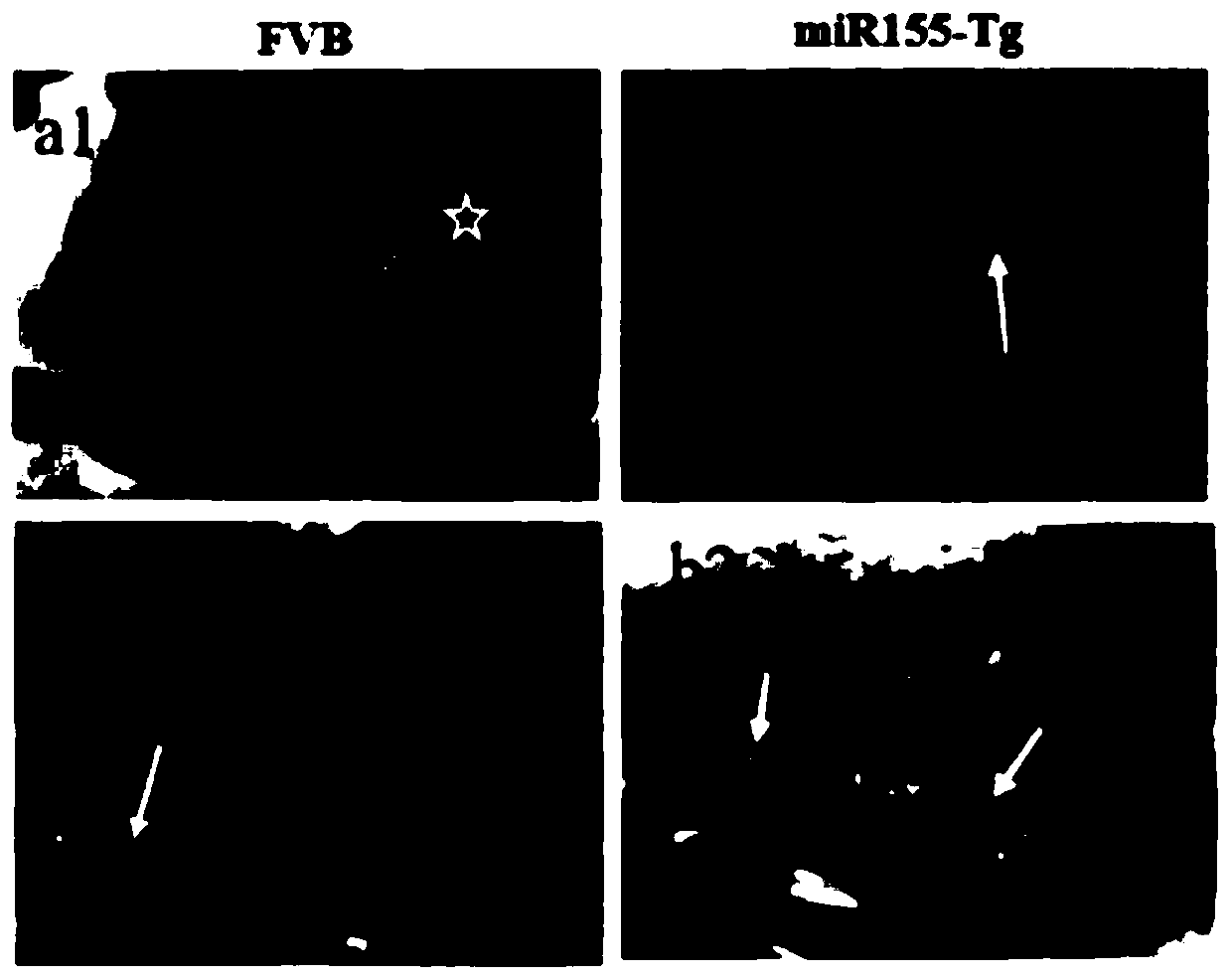
Construction method of miR155 overexpression mouse tongue squamous cell cancer model
InactiveCN110476889ARepresentation stabilityEasy to buildAnimal husbandryAbnormal tissue growthMouse Tongue
The invention discloses a construction method of a miR155 overexpression mouse tongue squamous cell cancer model. According to the method, a miR155 gene is overexpressed first in a mouse body, and then a mouse is fed with a 4NQO solution for more than 10 weeks to obtain the tongue squamous cell cancer model. Compared with an existing TSCC animal model, the model has stable representation and can successfully induce the precancerous lesion of the mouse in a short time, truly simulate the dynamic change process of the occurrence and development of a tongue squamous cell cancer tumor and serve asan ideal model for researching the molecular mechanism of the occurrence and development of tongue squamous cell cancer, screening of molecular targets for diagnosis and treatment and exploring of anew treatment method. In addition, the construction method of the model is simple and feasible, the tumor-forming latent period is short, the modeling accuracy is high, the repeatability is high, themodeling success rate reaches 95.0% or above, and the model has a great application and popularization value in screening medicaments for treating tongue squamous cell cancer.
Owner:STOMATOLOGY AFFILIATED STOMATOLOGY HOSPITAL OF GUANGZHOU MEDICAL UNIV +1
Application of tegaserod maleate in preparation of antitumor drug
InactiveCN111803493AGood treatment and preventionGood effectOrganic active ingredientsAntineoplastic agentsSquamous Cell CancersStage I Esophageal Squamous Cell Carcinoma
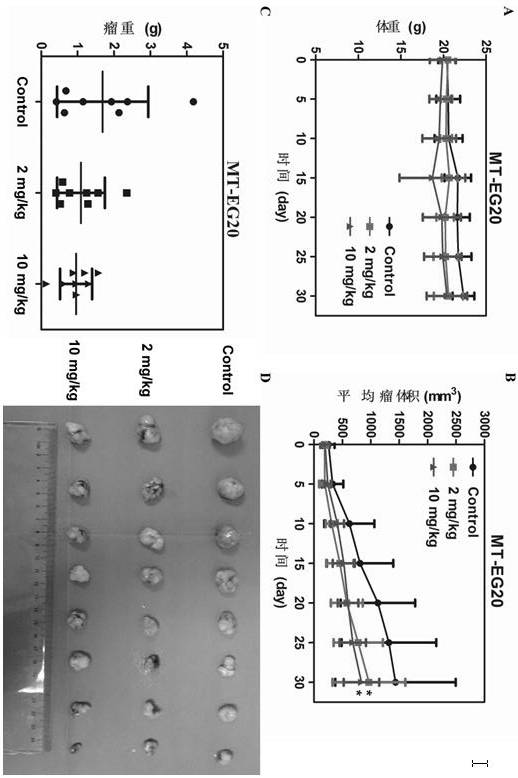
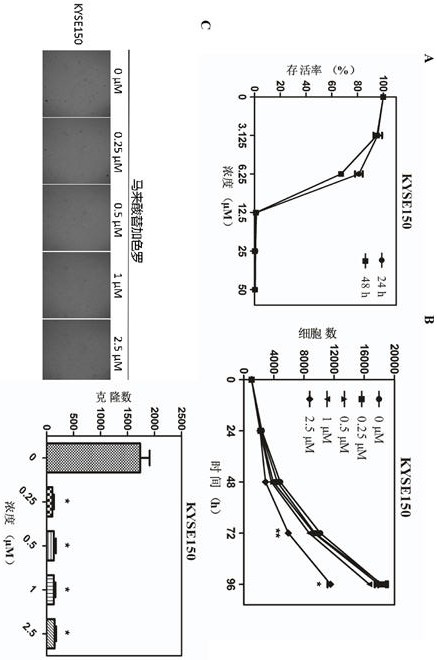
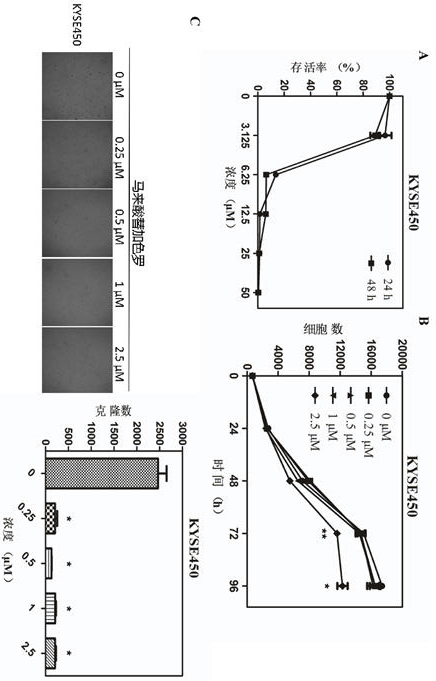
The invention discloses application of tegaserod maleate in preparation of an antitumor drug. The drug tegaserod maleate has a CAS number of 189188-57-6, a chemical name of 2-[(-5 methoxy-1H-indole-3-group) methylene]-N-pentylcarbazide maleate, a molecular formula of C20H27N5O5, and a molecular weight of 417.46. Experiments prove that when the tegaserod maleate is used for esophageal squamous cellcarcinoma cells (KYSE150 cells and KYSE450 cells), the tegaserod maleate can play a role in inhibiting the growth of esophageal squamous cell carcinoma cells, and the suitable concentration of the tegaserod maleate in inhibiting the proliferation of esophageal squamous cell carcinoma cells and the number and size of clone formation is 0.25-2.5 micrometers.
Owner:ZHENGZHOU UNIV
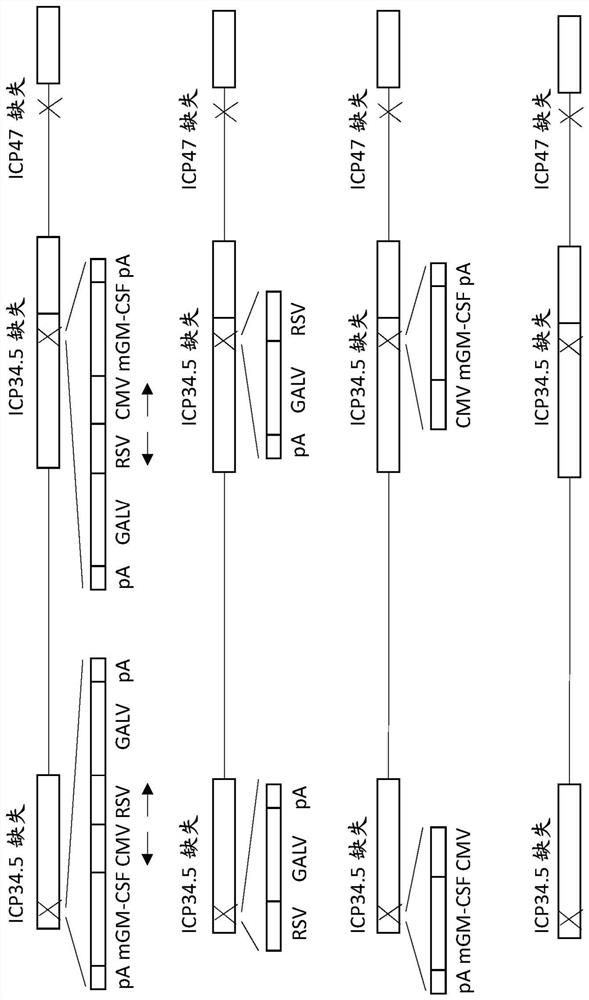
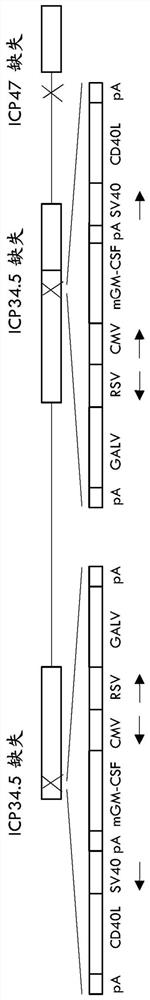
Treatment using oncolytic virus
An oncolytic virus is for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelial carcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patientswith no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / orcancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panelof three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
Diagnosis marker suitable for early-stage esophageal squamous cell cancer diagnosis
The invention discloses a diagnosis marker suitable for an early-stage esophageal squamous cell cancer diagnosis. The diagnosis marker is a composition of two or more than two kinds of materials from the following five kinds of serum metabolic markers including L-tyrosine, L-tryptophan, glycocholic acid, taurocholate and cortisol. When the diagnosis marker provided by the invention is adopted, a diagnosis model can be built. The diagnosis model has the advantages of good effect, high sensitivity and good specificity, is suitable for late-stage and early-stage esophagus cancer diagnoses, and has good clinic use and popularization value.
Owner:SHANDONG RES INST OF TUMOUR PREVENTION TREATMENT
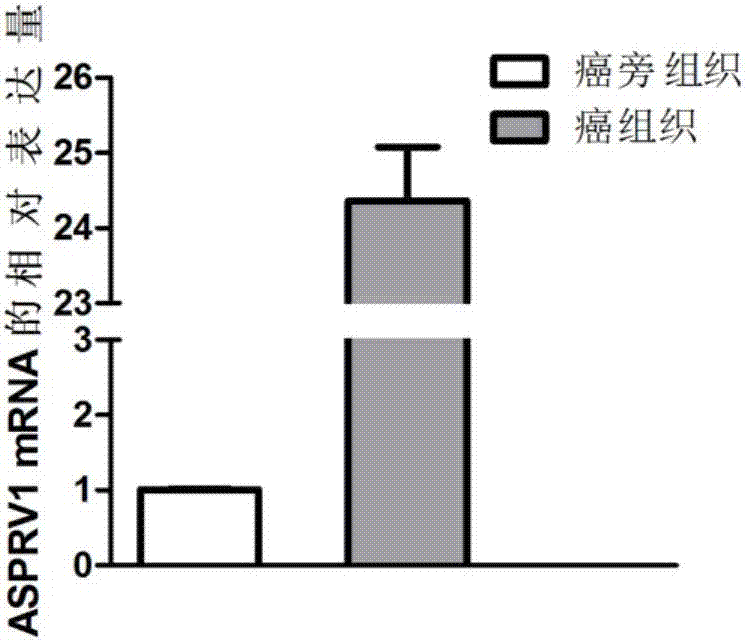
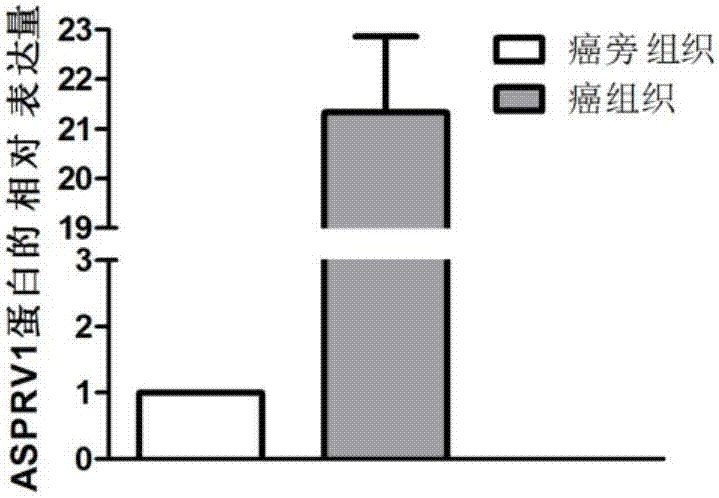
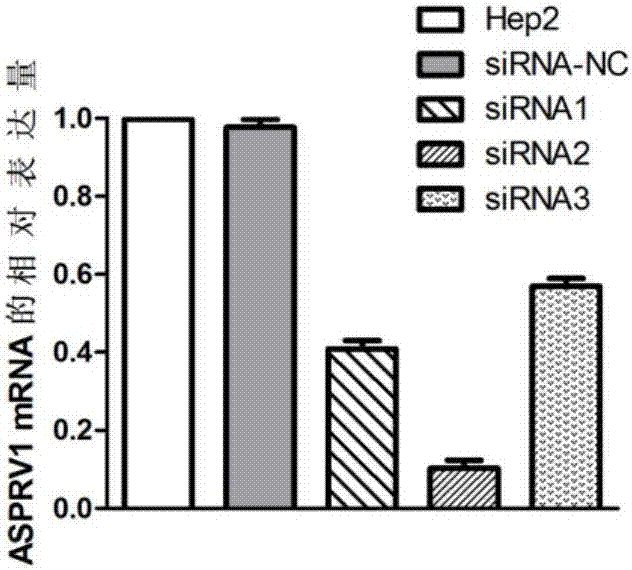
Application of ASPRV1 used as biomarker in diagnosis and treatment of LSCC (laryngeal squamous cell cancer)
ActiveCN107164554AImprove accuracyMicrobiological testing/measurementBiological material analysisSquamous Cell CancersBiomarker (petroleum)
The invention discloses an application of ASPRV1 used as a biomarker in diagnosis and treatment of LSCC (laryngeal squamous cell cancer). Expression up-regulation of ASPRV1 in LSCC tissue is discovered with a high-throughput sequencing technology, significant overexpression of the gene and protein expressed by the gene is further verified through experiments, and the ASPRV1 can be used as the diagnosis marker to be applied to clinical diagnosis of the LSCC. In-vitro cell experiments further prove that change of the expression level of the ASPRV1 can play a role in inhibiting proliferation, migration and invasion of cells, and the ASPRV1 can be used as a potential target to be applied to clinical treatment of the LSCC.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
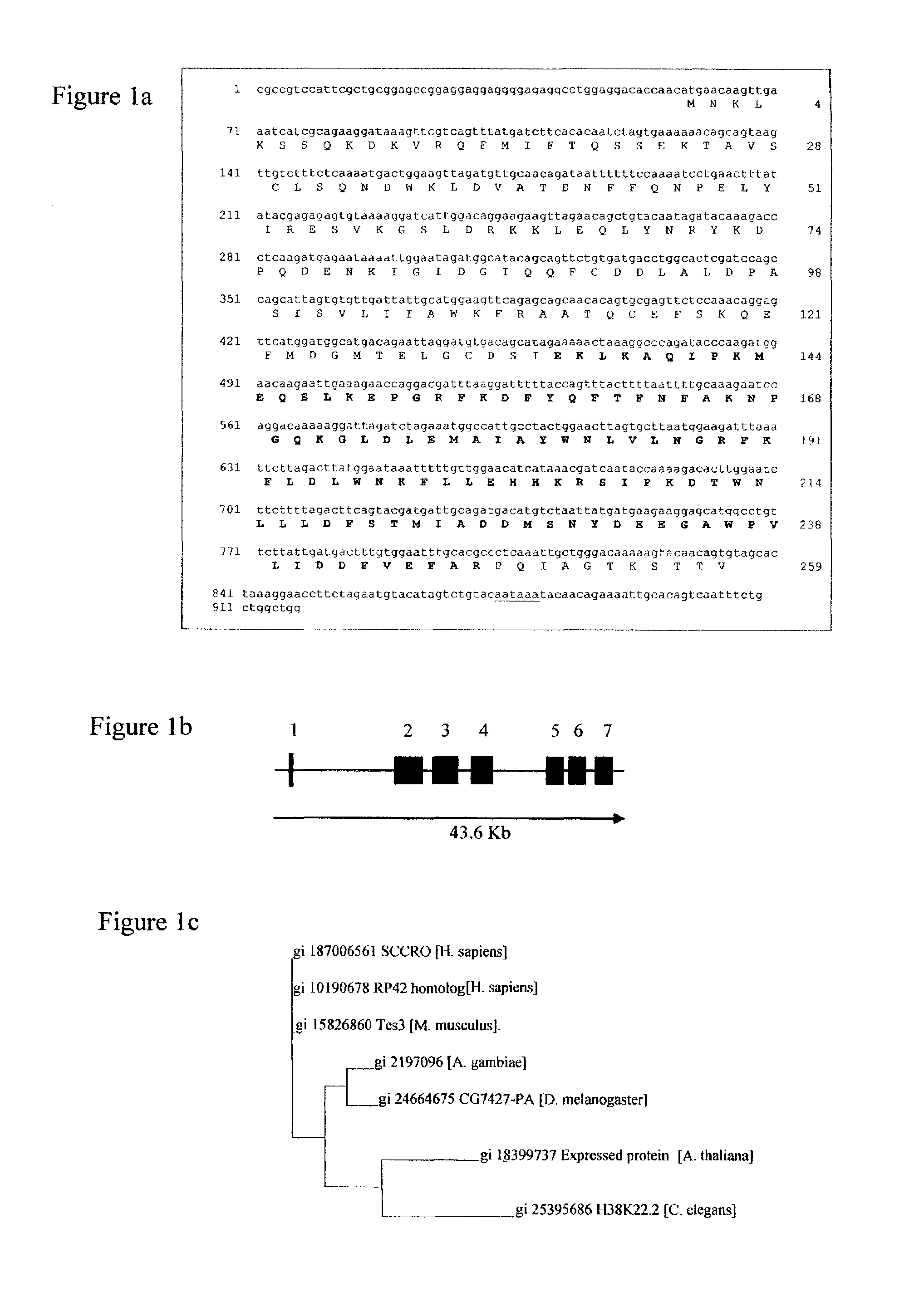
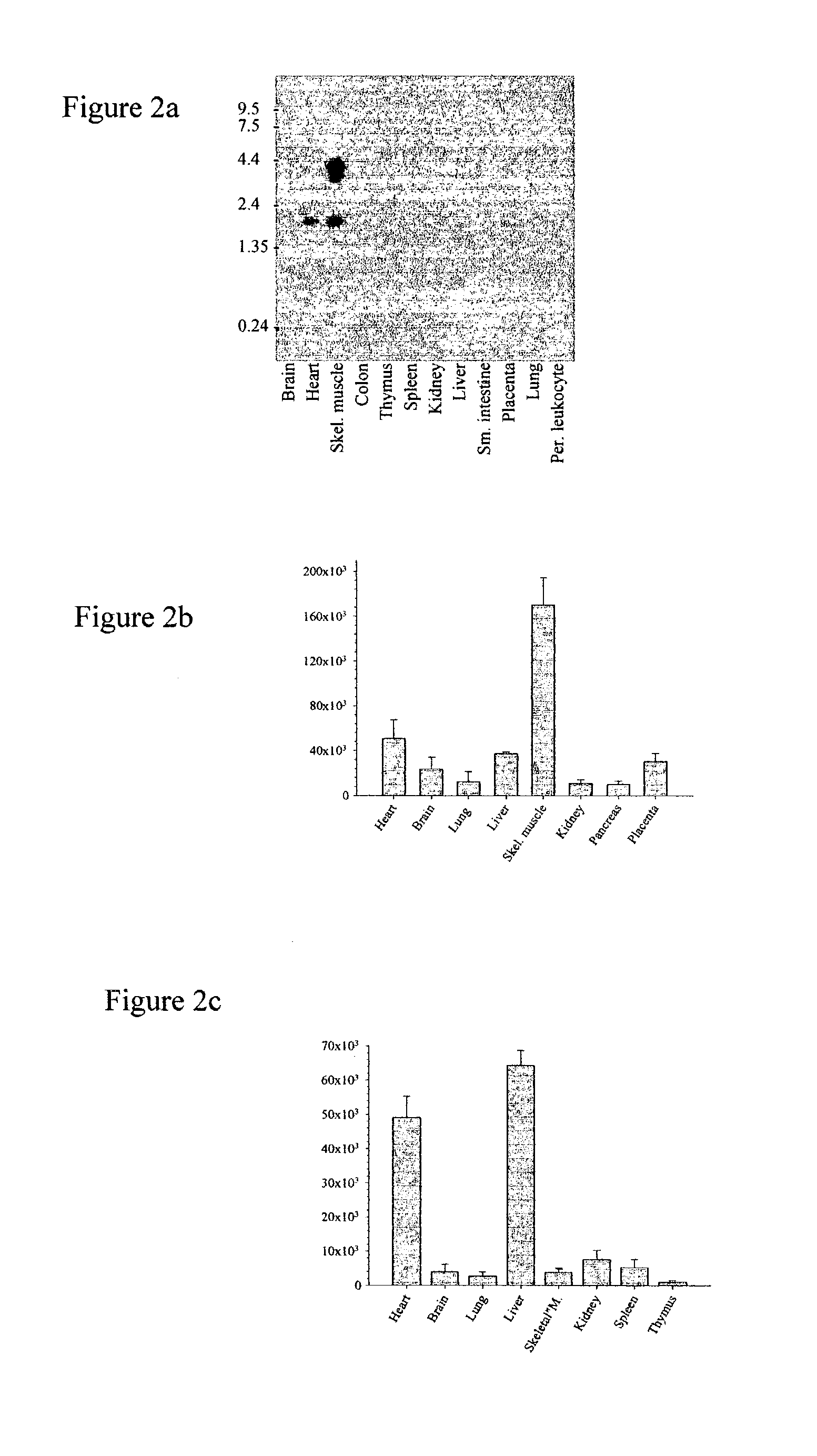
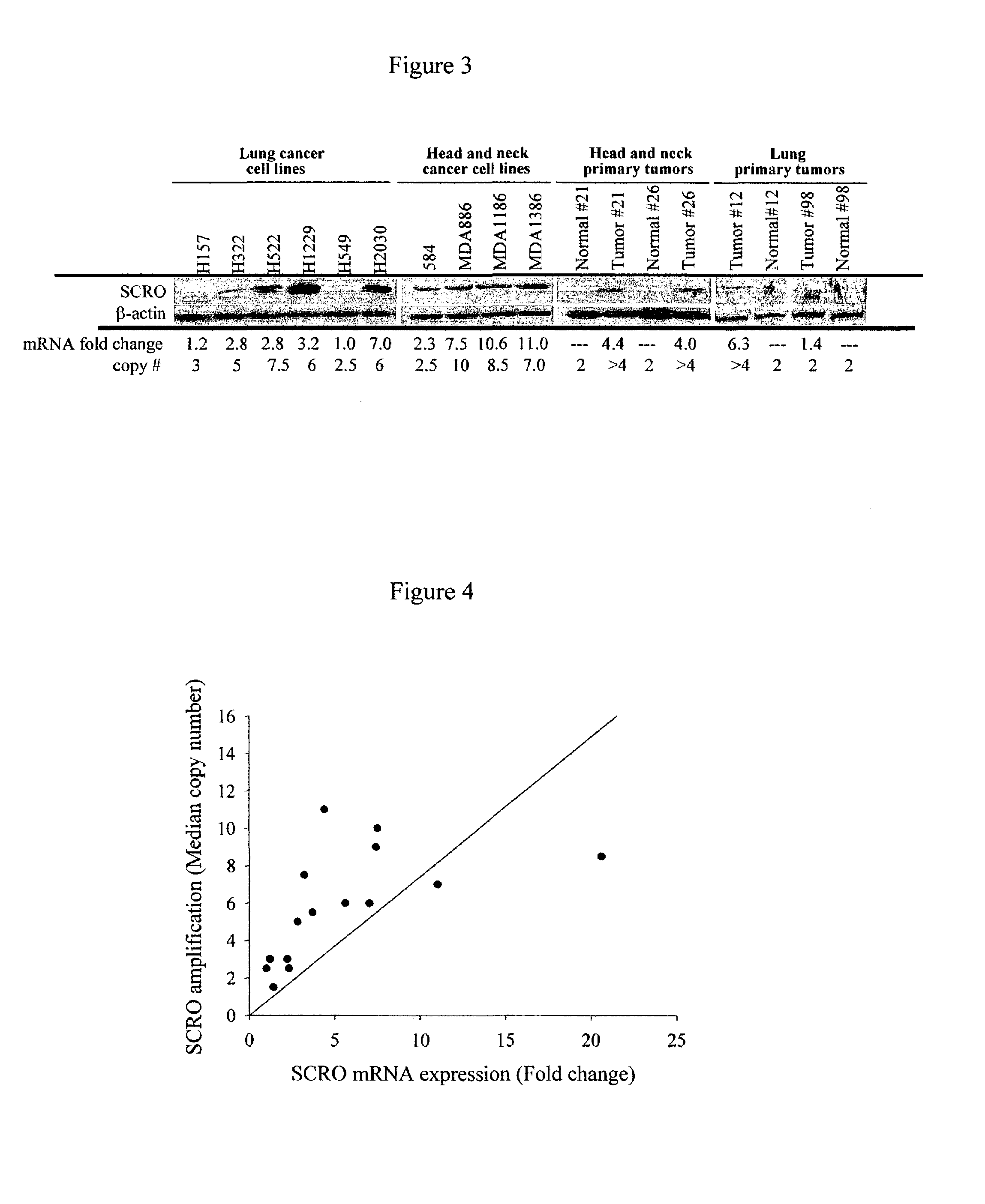
Carcinoma-related genes and polypeptides and methods of use thereof
InactiveUS7348418B2Sugar derivativesMicrobiological testing/measurementSquamous CarcinomasNovel gene
Novel nucleic acids and polypeptides encoded thereby are provided that are highly duplicated and overexpressed in squamous cell carcinomas of a variety of tissues. Antibodies specific for binding the novel polypeptides are also provided. The invention further discloses several assays for gene duplication and overexpression of the novel gene and excessive production of the novel polypeptide in a sample. These assays permit assessing copy number in a sample from a subject, and contribute to the diagnosis, prognosis and development of therapeutic strategy for a pathology such as squamous cell carcinoma in a subject.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Treatment using oncolytic virus
PendingUS20210252135A1Direct effectImprove immunityPeptide/protein ingredientsViral antigen ingredientsAppendiceal carcinomaSquamous Carcinomas
An oncolytic virus for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelialcarcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patients with no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / or cancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus: is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panel of three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
Biomarkers for diagnosis of lung cancer
PendingCN110662844AMicrobiological testing/measurementMass spectrometric analysisAdenocarcinomaSquamous Carcinomas
The present invention relates to biomarkers of lung cancer, particularly to markers that enable distinguishing between subtypes of non-small cell lung cancer (NSCLC), particularly between adenocarcinoma (AC) and squamous cell carcinoma (SCC). In particular, the present invention relates to means and methods for diagnosing, assessing the level of severity and selecting methods of treating NSCLC.
Owner:THE NAT INST FOR BIOTECH IN THE NEGEV LTD
Psma binding antibody and use thereof
ActiveCN108699157AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody medical ingredientsAntiendomysial antibodiesSquamous Carcinomas
The present invention provides a novel PSMA binding antibody termed 10B3 and pharmaceutical and diagnostic uses of the antibody 10B3. The PSMA antibody 10B3 does not cross-compete with the state of the art PMSA binding antibody J591 and has a reduced induction of antigen shift compared to J591 and a unique reactivity with squamous cell carcinoma (SCC) cells of different origin.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS +1
Prognosis and treatment of squamous cell carcinomas
DNA methylation profiles predictive of head and neck squamous cell carcinoma (HNSCC) patient prognosis, as well as therapeutic protein and adoptive cell compositions useful in the treatment of HNSCC.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Method for treating cancerous and pre-cancerous skin
The present disclosure provides a method for treating clinical or pre-clinical skin damage in a skin field of a subject, wherein the skin field has been allocated a skin cancerization field index (SCFI) score of at least 1 as determined by a process comprising the steps of: (i) assessing the number of keratoses in the skin field; (ii) assessing the thickness of the thickest keratosis in the skin field; and (iii) assessing the proportion of the field affected by clinical or subclinical skin damage. Based on the assessments made in (i), (ii) and (iii) the subject is optionally treated by at least one of (a) freezing one or more lesions, (b) shaving, curetting or surgically removing one or more lesions, (c) applying a topical treatment for actinic keratosis, basal cell carcinoma or squamous cell carcinoma, and (d) radiation therapy.
Owner:GENESISCARE VENTURES PTY LTD
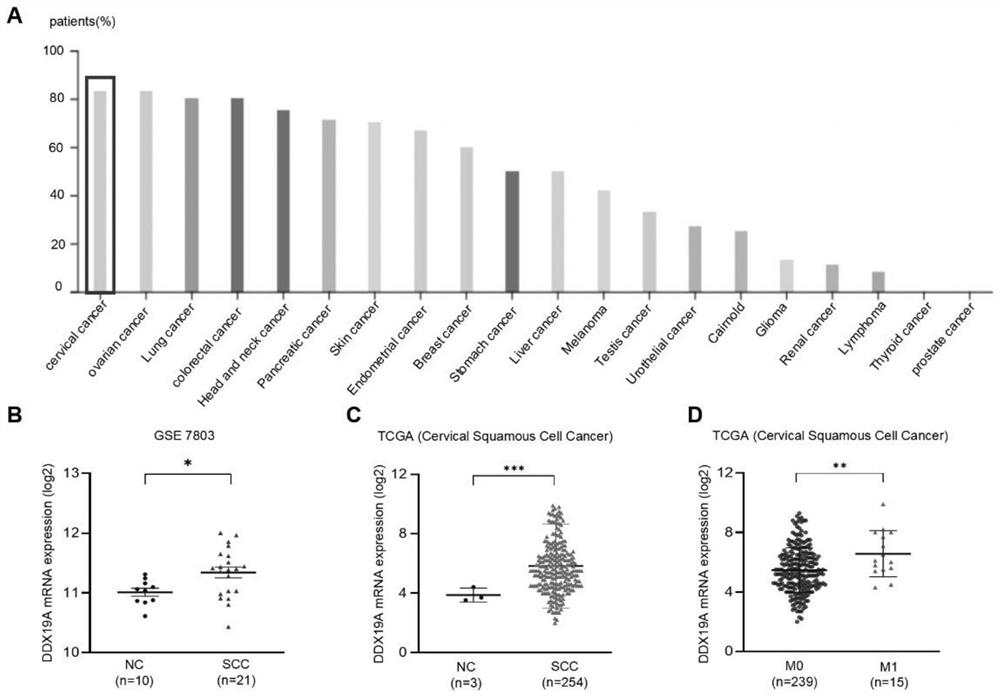
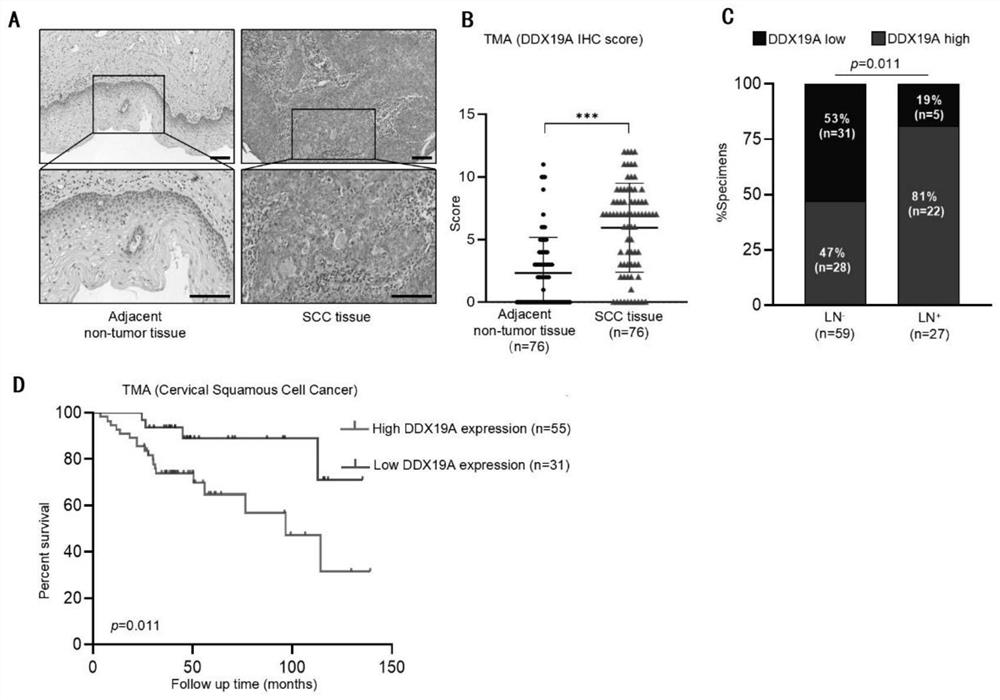
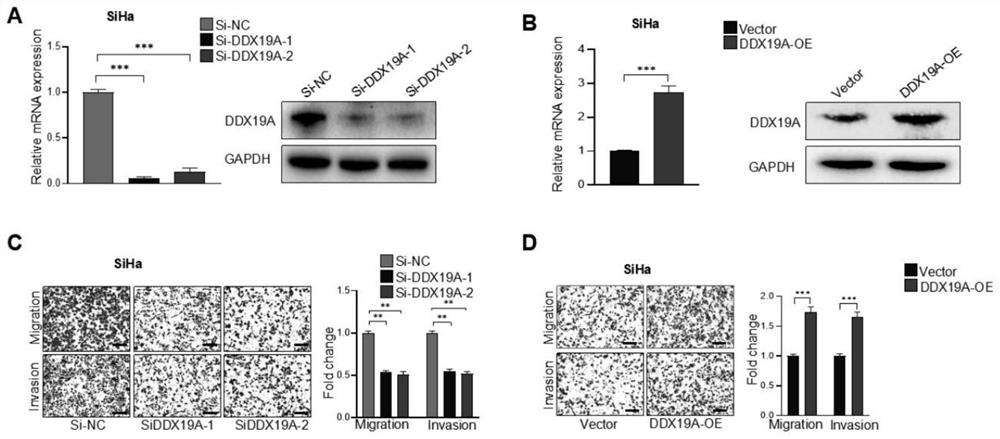
Application to promotion of cervical squamous cell carcinoma metastasis based on DDX19A
ActiveCN112813162AEasy transferPromote migrationMicrobiological testing/measurementAntineoplastic agentsCell invasionSquamous Carcinomas
The invention belongs to the technical field of medical products, and discloses an application to promotion of squamous cell carcinoma metastasis based on DDX19A. In function, DDX19A is found to promote in-vitro migration and invasion of cervical squamous cell carcinoma cells and in-vivo lung metastasis. In terms of mechanism, overexpression of the DDX19A increases NOX1 expression, increases production of reactive oxygen species (ROS), and induces migration and invasion of the cervical squamous cell carcinoma cells. Rescue experiments show that overexpression of NOX1 can retrieve and knock down cervical cancer squamous cell carcinoma cell invasion and migration and ROS generation caused by DDX19A, and the inventor further discovers that an ROS inhibitor N-acetylcysteine can inhibit DDX19A-induced cervical squamous cell carcinoma cell invasion and migration. Therefore, the DDX19A is proved to promote metastasis of the cervical squamous cell carcinoma by inducing NOX1 to mediate ROS production. Blocking of a DDX19A / NOX1 / ROS axis can be a potential therapeutic target for patients with cervical squamous cell carcinoma.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
Methods for identification, assessment, prevention, and therapy of cancer
InactiveUS20050202448A1Reduced expression levelSugar derivativesMicrobiological testing/measurementAdenocarcinomaSquamous Carcinomas
The present invention relates to the newly identified cancer therapeutic targets and biomarkers. These targets / biomarkers are overexpressed in carcinomas generally, and more specifically to adenocarcinoma and squamous cell carcinoma. The invention provides methods of diagnosis, characterization, and therapy of carcinoma based on the degree of overexpression of the targets / biomarkers.
Owner:DIGIGENOMICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com