Immunoassays for specific determination of SCCA isoforms
An immunoassay, SCCA2 technology, applied in the direction of anti-animal/human immunoglobulin, immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as incomplete understanding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
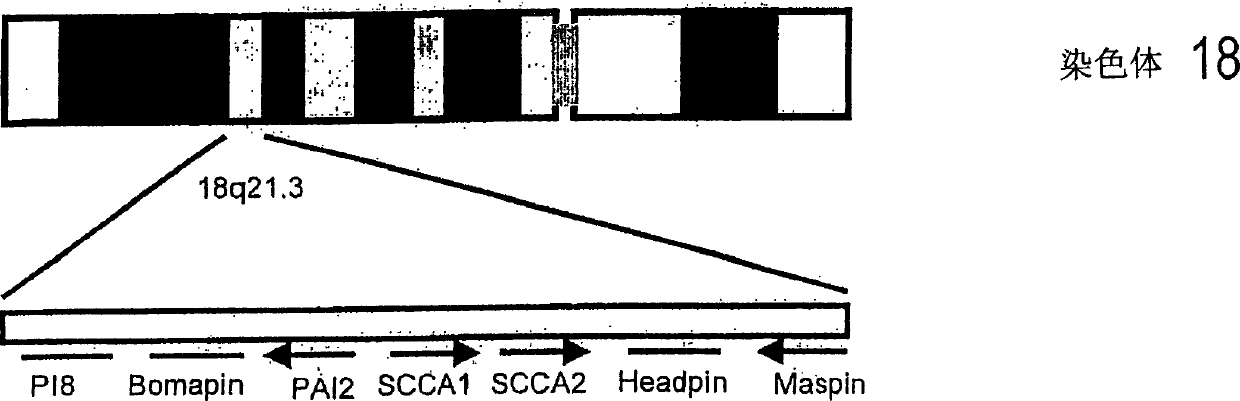
[0014] Preparation of recombinant SCCA
[0015] 1.1 Clone SCCA
[0016] The mRNAs from cell line Caski (cervix), C-4I (cervix), A549 (lung) and RPMI2650 (pharynx) were prepared using QuickPrep Micro mRNA Purification Kit (Pharmacia Company), cDNA was prepared using the First-Strand cDNA Synthesis Kit (Pharmacia). In 100μl containing 10mM Tris-HCl pH 8.85, 25mM KCl, 5mM (NH 4 ) 2 SO 4 , 2mM MgSO 4 (Boehringer Company), 0.2mM dNTP (Pharmacia Company), 10μM SCCA 1-7F (the DNA sequences of all primers are listed in Table 1), 10μM SCCA 391-397B, 2μl cDNA and 2.5U Pwo-polymerase (Boehringer Company ) in the reaction solution, the 1218bp DNA fragment covering the SCCA coding sequence was amplified by PCR reaction. After denaturing the samples at 96°C for 5 minutes, a total of 30 cycles were performed, each cycle consisting of denaturation at 96°C for 15 seconds, annealing at 60°C for 15 seconds, and stretching at 72°C for 30 seconds. Finally, stretch at 72°C for 10 minutes to ...
Embodiment 2
[0033] Establishment of hybridomas and monoclonal antibodies
[0034] 2.1 Immunization and initial selection of anti-SCCA hybridomas
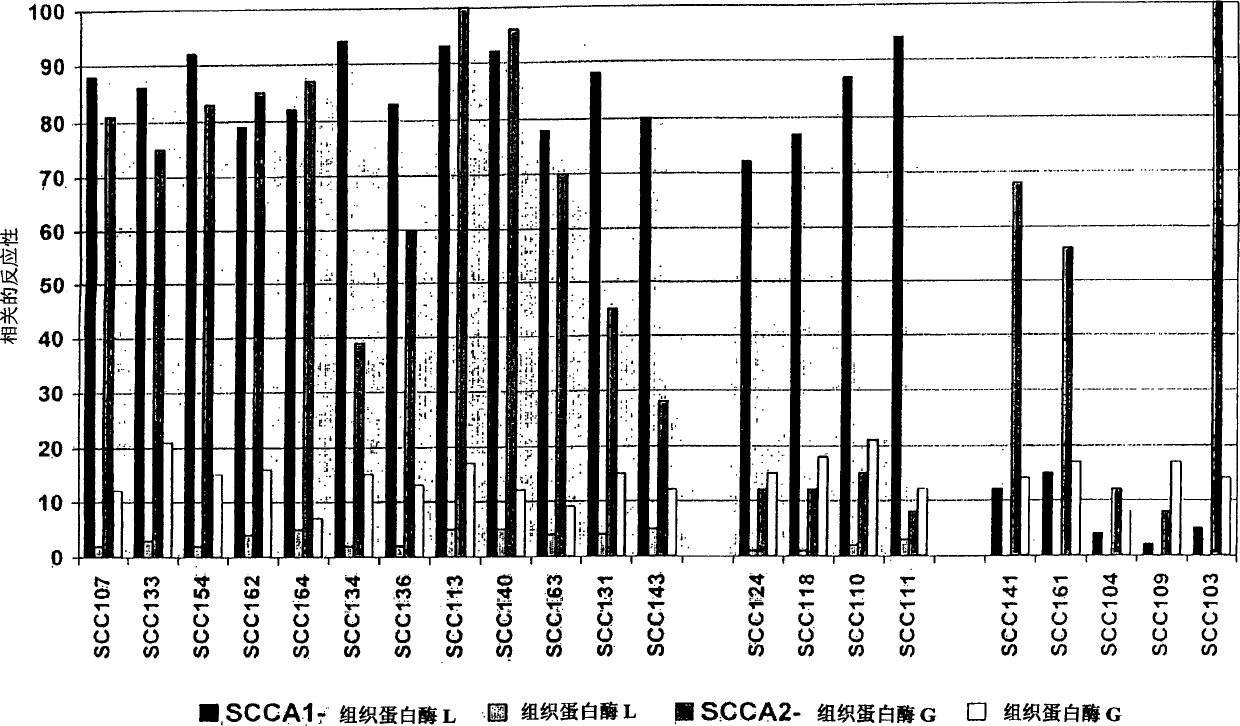
[0035] Polyclonal antisera reactive with the SCC antigen were obtained by immunizing rabbits subcutaneously with the recombinant SCC antigen and collecting immune sera according to standard procedures. The titer of the polyclonal antisera was checked by measuring the reactivity of the antisera with biotinylated SCCA2 and SCCA1 immobilized in streptavidin plates (Labsystems Oy, Helsinki, Finland). Recombinant SCCA2 and SCCA1 were biotinylated with biotin-N-succinimidyl hexanoate according to standard procedures.
[0036] Monoclonal antibodies reactive with SCCA1 and SCCA2 were obtained by immunizing Balb / c mice intraperitoneally with 10-50 μg of recombinant SCCA in Ribi adjuvant. After immunization, after receiving 2-4 booster doses within 60-90 days, the splenocytes of the immunized mice were fused with the P3×63Ag 8 myeloma cells.
[0037] ...
Embodiment 3
[0131] Establishment of Immunoassay
[0132] Using established monoclonal antibodies and recombinant proteins, it is likely to be possible to develop immunoassays for the separate determination of total SCCA and "free" SCCA, and for the separate determination of total SCCA1 and "free" SCCA1. Assays and Assays for Individual Determination of Total SCCA2 and "Free" SCCA2.
[0133] 3.1 Immunoassay for the determination of total SCCA
[0134] 3.1.1 Immunoassay for the determination of "Total SCCA"
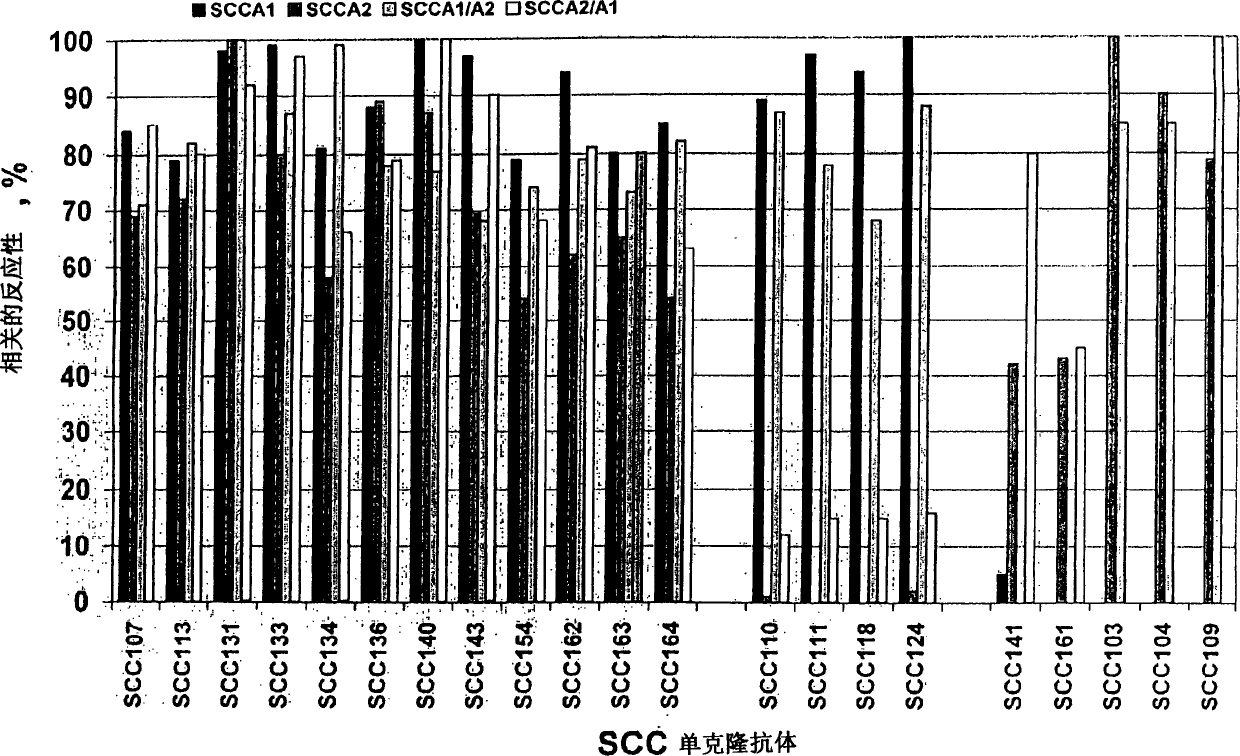
[0135] SCCA-specific assays were designed using antibodies from A1a (Table 1) in combination with antibodies from group A2 or A3a, i.e. "free" SCCA1, "free" SCCA2, complexed SCCA1 and complexed The sum of SCCA2.
[0136] A preferred combination is antibody SCC113 as capture antibody and SCC107 as detection antibody.
[0137] SCC113 monoclonal antibody was biotinylated with biotin NHRS hexanoate (Sigma Chemical Company, USA) using standard procedures and used as capture antibody. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com