Psma binding antibody and use thereof
A technology of antibody molecules and binding fragments, which can be applied to antibody medical components, anti-tumor drugs, drug combinations, etc., and can solve problems affecting in vivo efficiency and T cell exhaustion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Example 1: Generation, identification and production of 10B3 antibodies
[0215] Antibody 10B3 was produced after immunization of female BALB / c mice with irradiated PSMA-transfected Sp2 / 0 Ag14 cells (Sp2 / 0-Ag14 cells were obtained from ATCC, where they were obtained under the trade name CRL-1581 TM commercially available). Four days after the last immunization, splenocytes were fused with transfected Sp2 / 0 cells and cultured in HAT selection medium. Supernatants from growing hybridoma cells were screened for PSMA antibody production by flow cytometry using PSMA-transfected as well as non-transfected Sp2 / 0 cells. Stable monoclonal hybridoma cell lines were obtained after subcloning by limiting dilution. For antibody production, the hybridoma cells were adapted to advanced DMEM medium (Gibco, Thermo Scientific, Waltham, MA, USA02451) supplemented with 1% FCS (Biochrom GmbH, Berlin, Germany) to avoid contamination during purification. Bovine IgG contamination. Antibo...
Embodiment 2
[0216] Example 2: Generation of Humanized 10B3 Antibody
[0217] The 10B3 antibody was obtained by grafting the CDR region to the human variable kappa light chain IGKV3-20*02 (the sequence is deposited in the IMGT / LIGM database under the accession number L37729, see also Ichiyoshi Y., Zhou M., Casali P.A human anti-insulin IgG autoantibody apparently arises through clonal selection from aninsulin-specific 'germ-line' natural antibody template.Analysis by V genesegment reassortment and site-directed mutagenesis'J.Immunol.154(1):226-238(1995)) and variable Heavy chain sequence IGHV3-11*06 (this sequence is deposited in the IMGT / LIGM database under accession number AF064919, see also Watson C.T., et al. Complete haplotype sequence of the human immunoglobulin heavy-chain variable, diversity, and joining genes and characterization of allelic and copy-number variation.Am.J.Hum.Genet.92(4):530-546(2013)) and was humanized in the germline sequence. Note here that IMGT / LIGM-DB is an I...
Embodiment 3
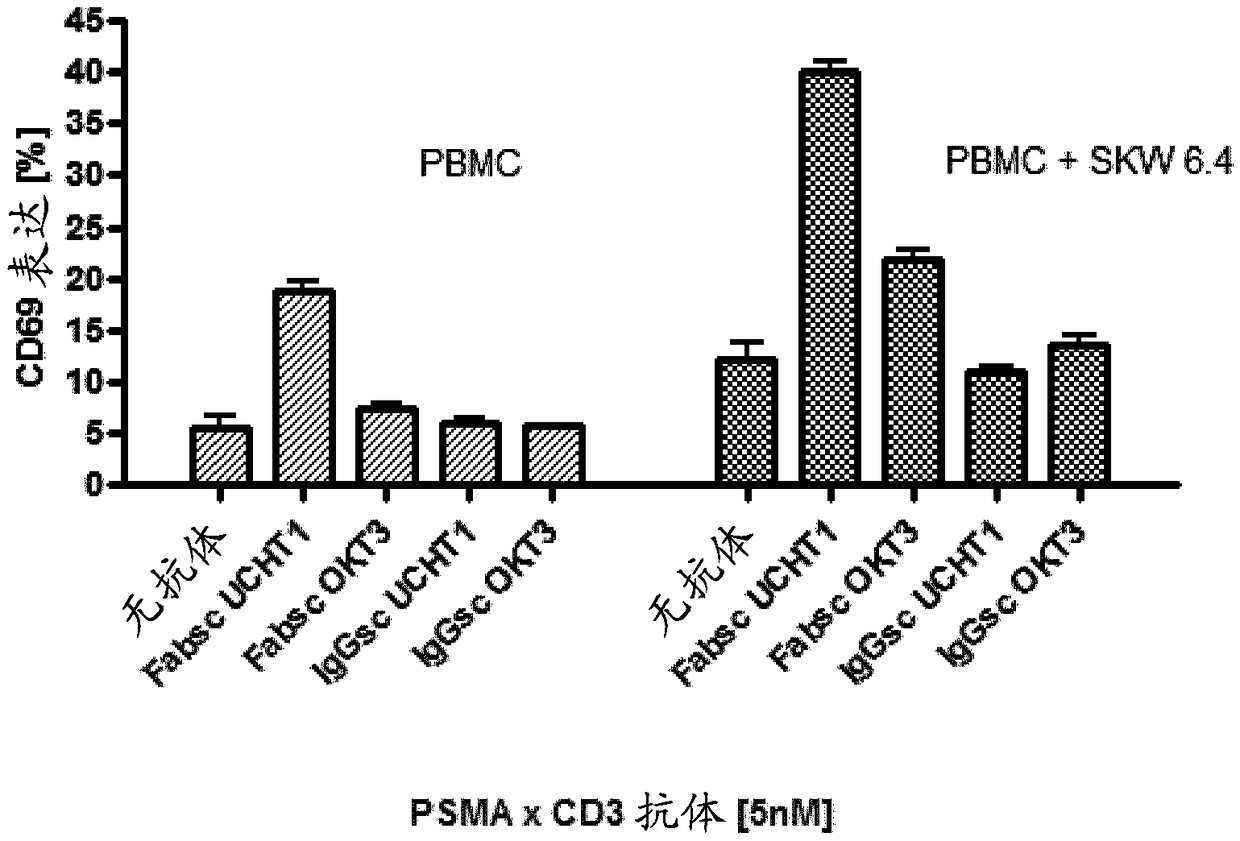
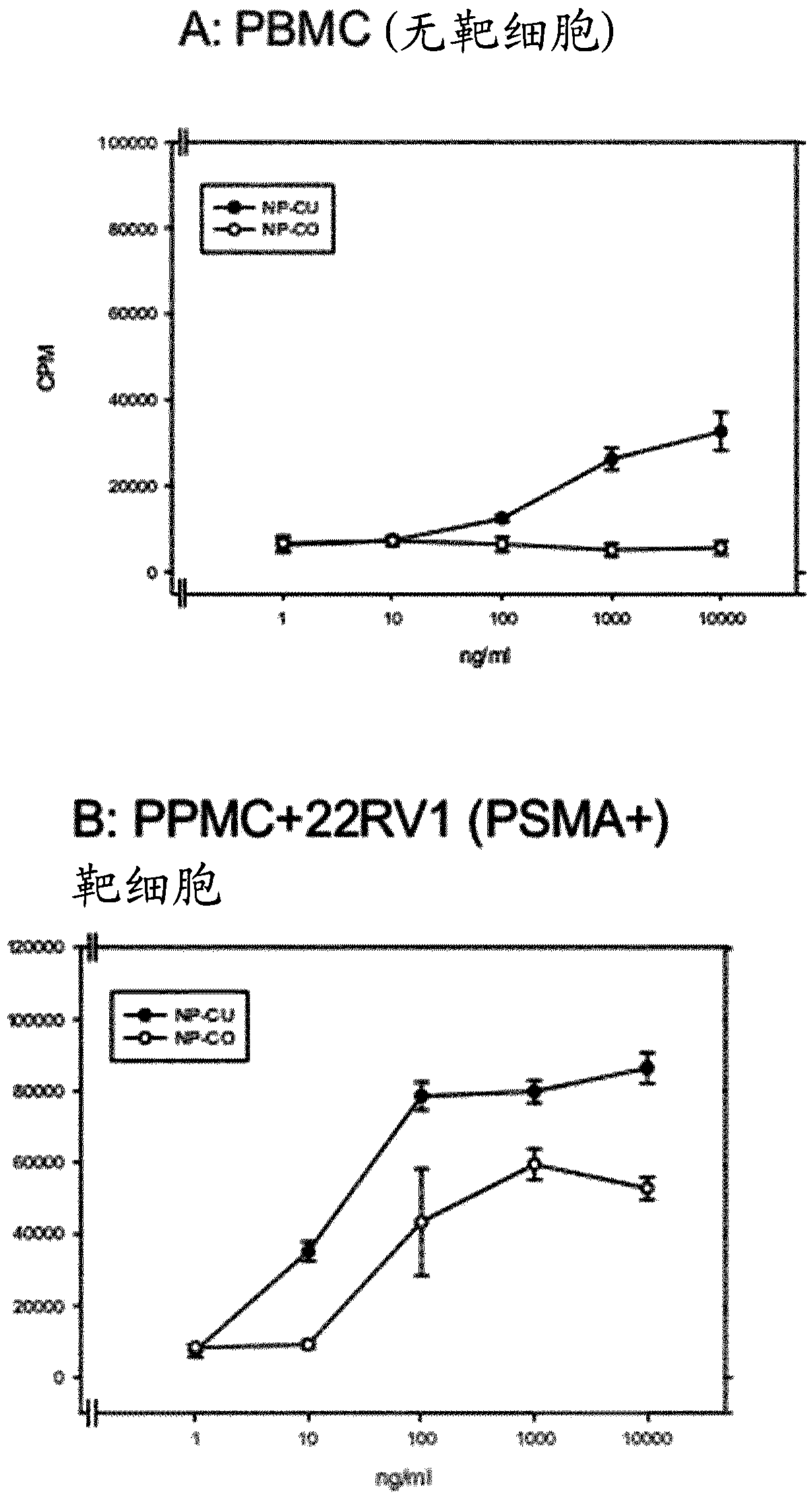
[0219] Example 3: Generation of recombinant antibody molecules and off-target T cell activation by different PSMAxCD3 antibodies
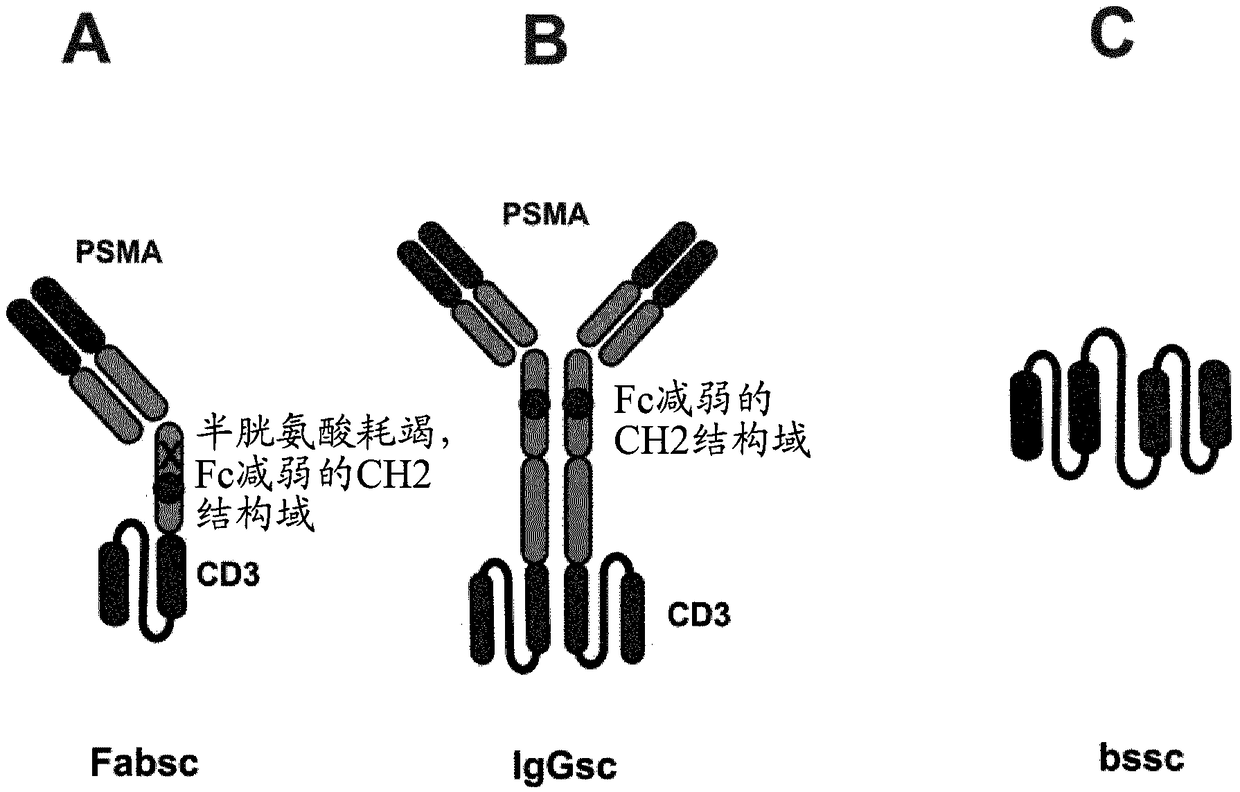
[0220] For the construction of recombinant bispecific antibody molecules, the variable domains of PSMA antibodies J591 and 10B3 were fused with the human constant and variable regions of CD3 antibodies OKT3 or UCHT1 in the following order: VH-CH1-CH2mod-scFv(OKT3 / UCHT1 ) (for the Fabsc format), VH-CH1-CH2mod-CH3-scFv (OKT3 / UCHT1) (for the IgGsc format) (see also figure 1A and 1B). In these antibody molecules, the PMSA binding site exists as a Fab fragment, while the CD3 binding site exists as a scFv fragment (see again figure 1 A and 1B). To eliminate FcR binding, glycosylation sites and disulfide bond formation, the following modifications were introduced into the hinge region and CH2 domain of the Fabsc format (EU-index): C226S; C229S; E233P; L234V; L235A; ΔG236; D265G ; N297Q; A327Q; A330S (see also International Patent Application WO 2013 / 09...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com