Patents
Literature
833 results about "Esophageal tube" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
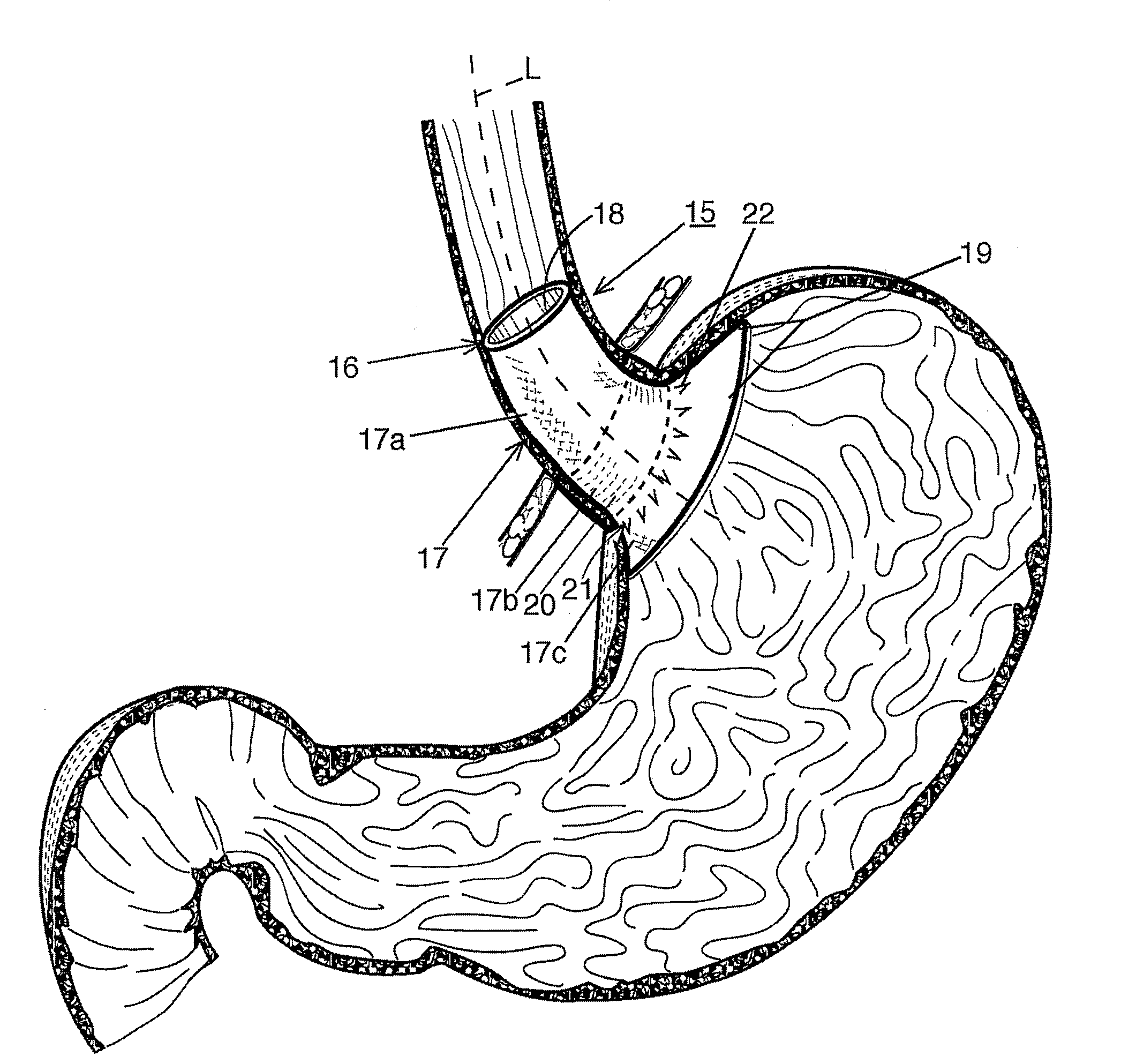
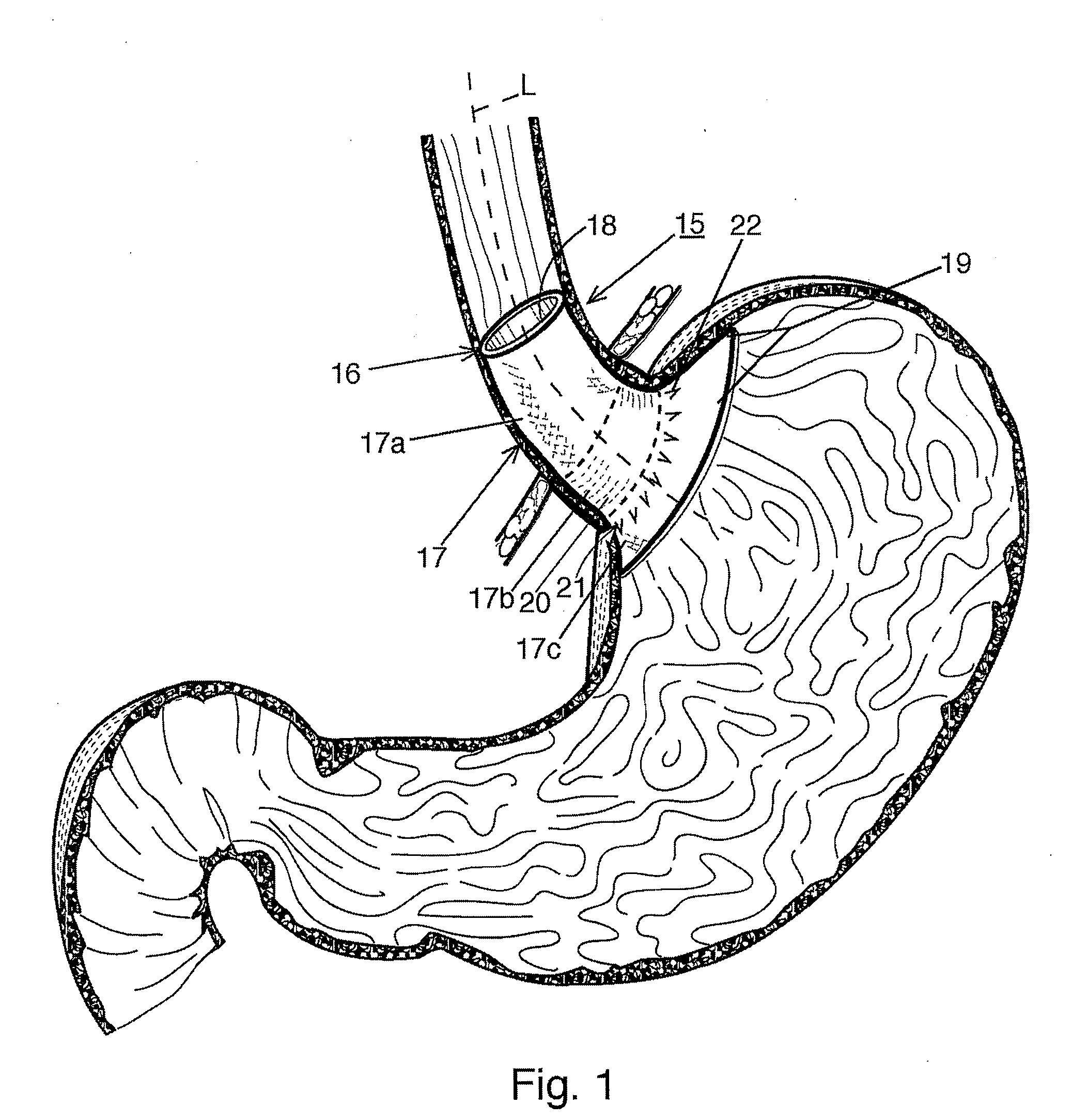
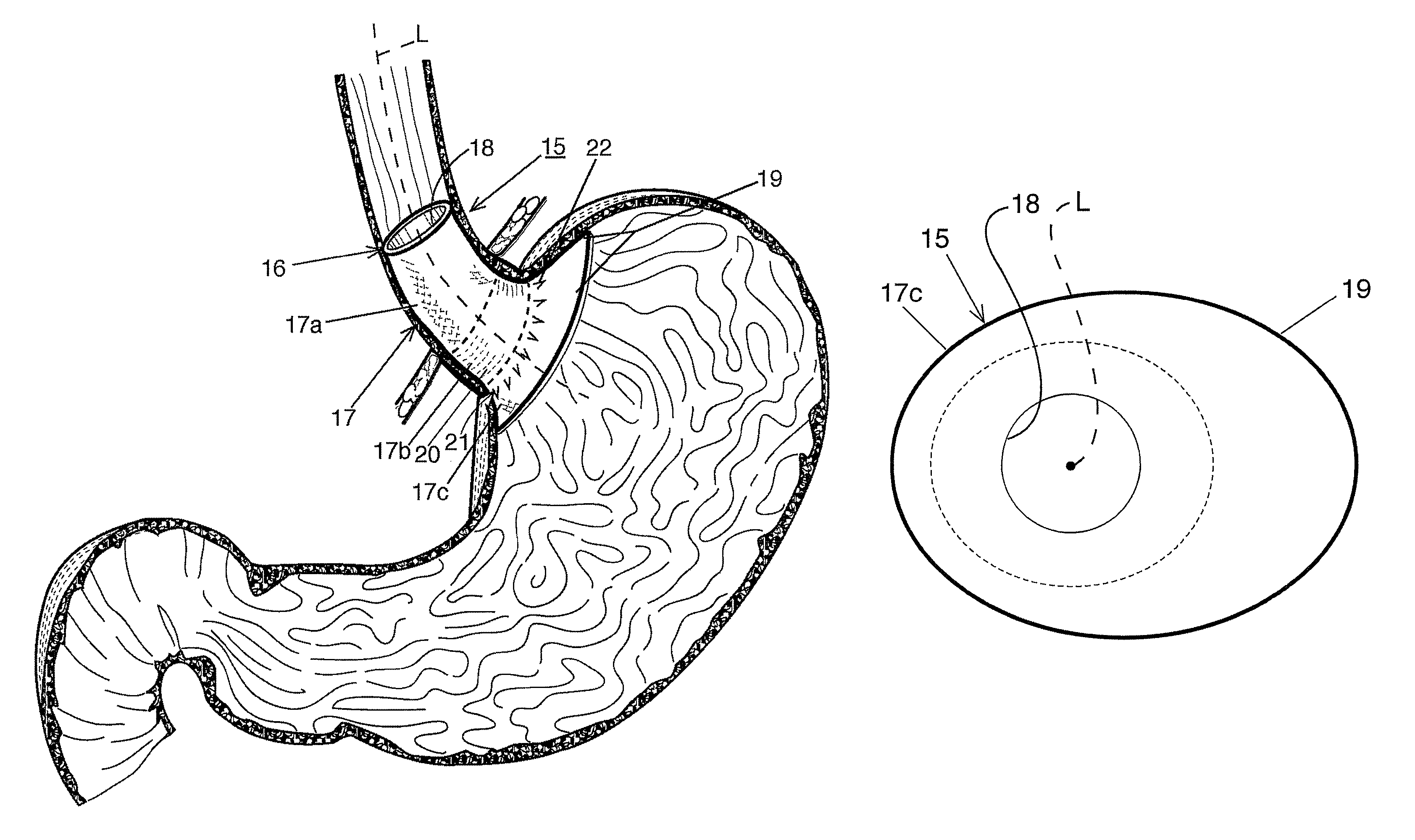
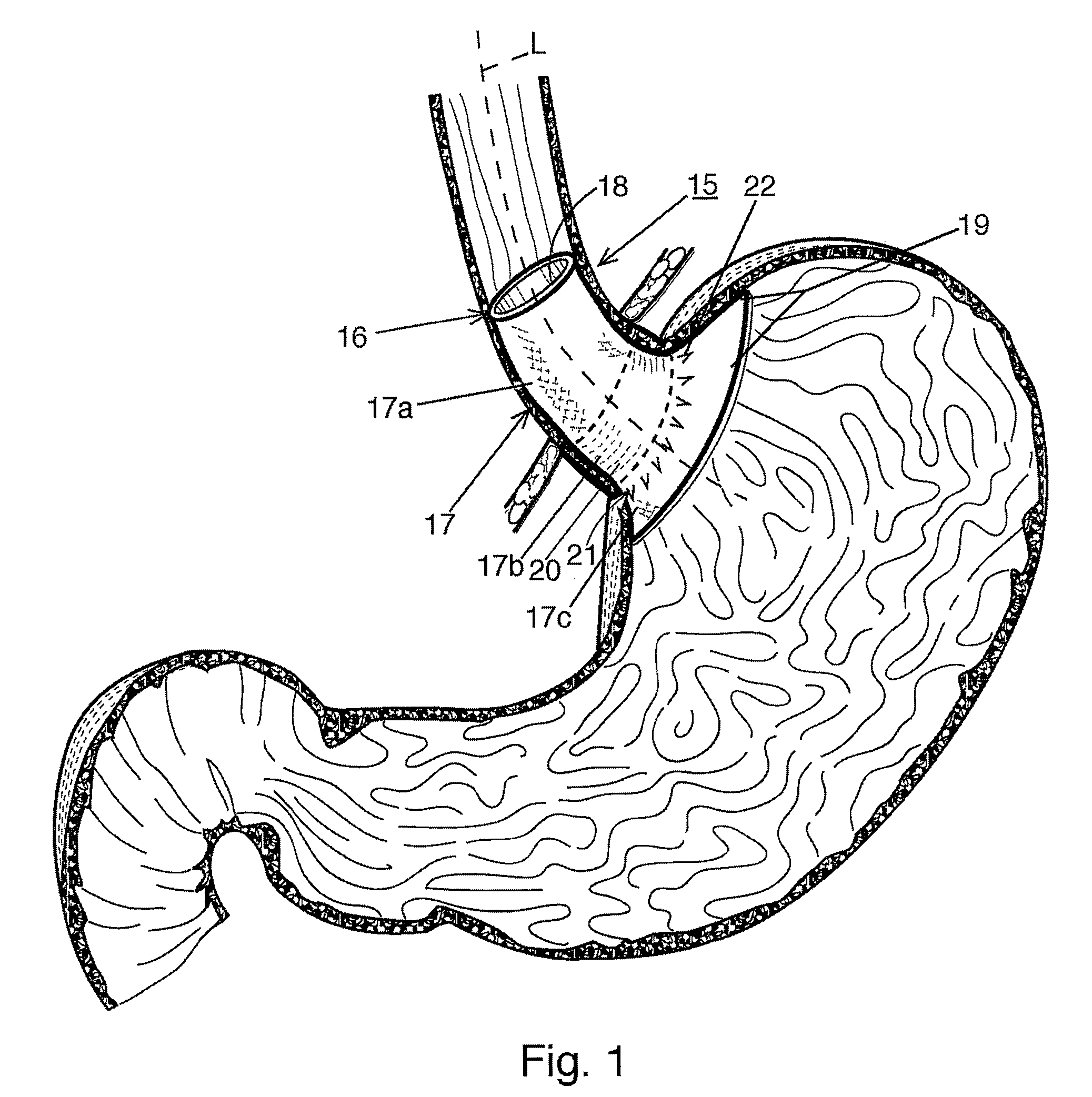
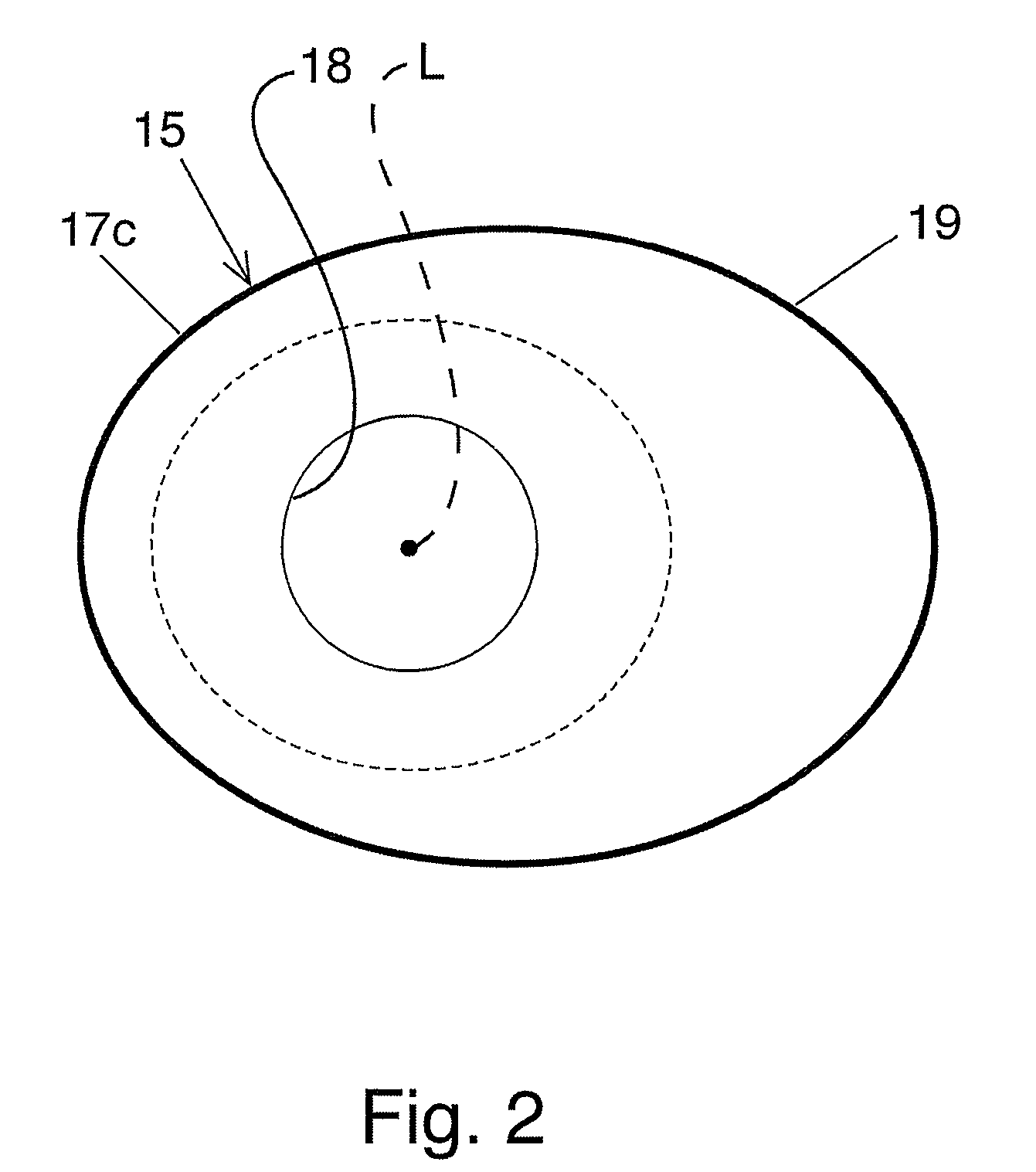
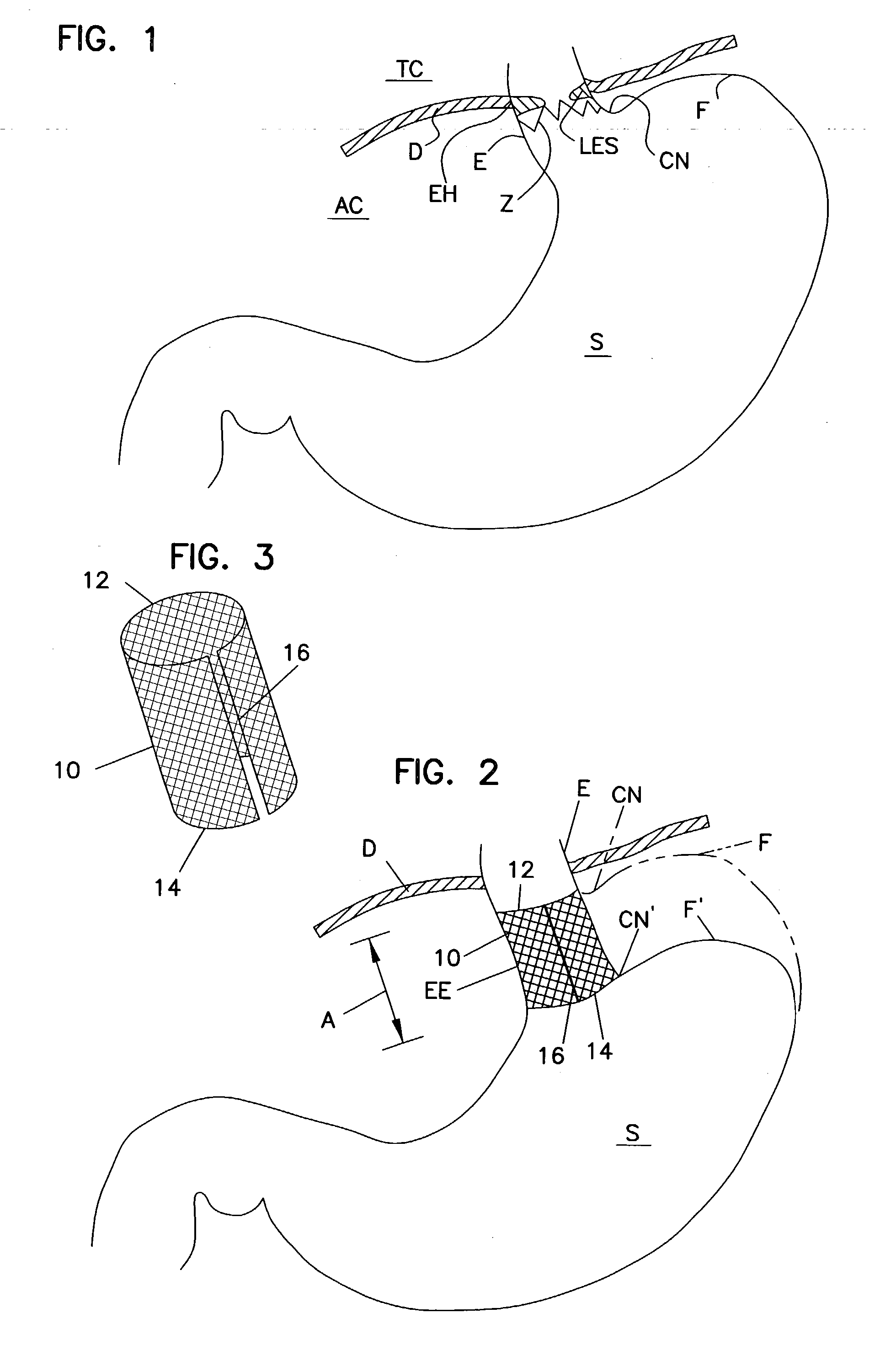
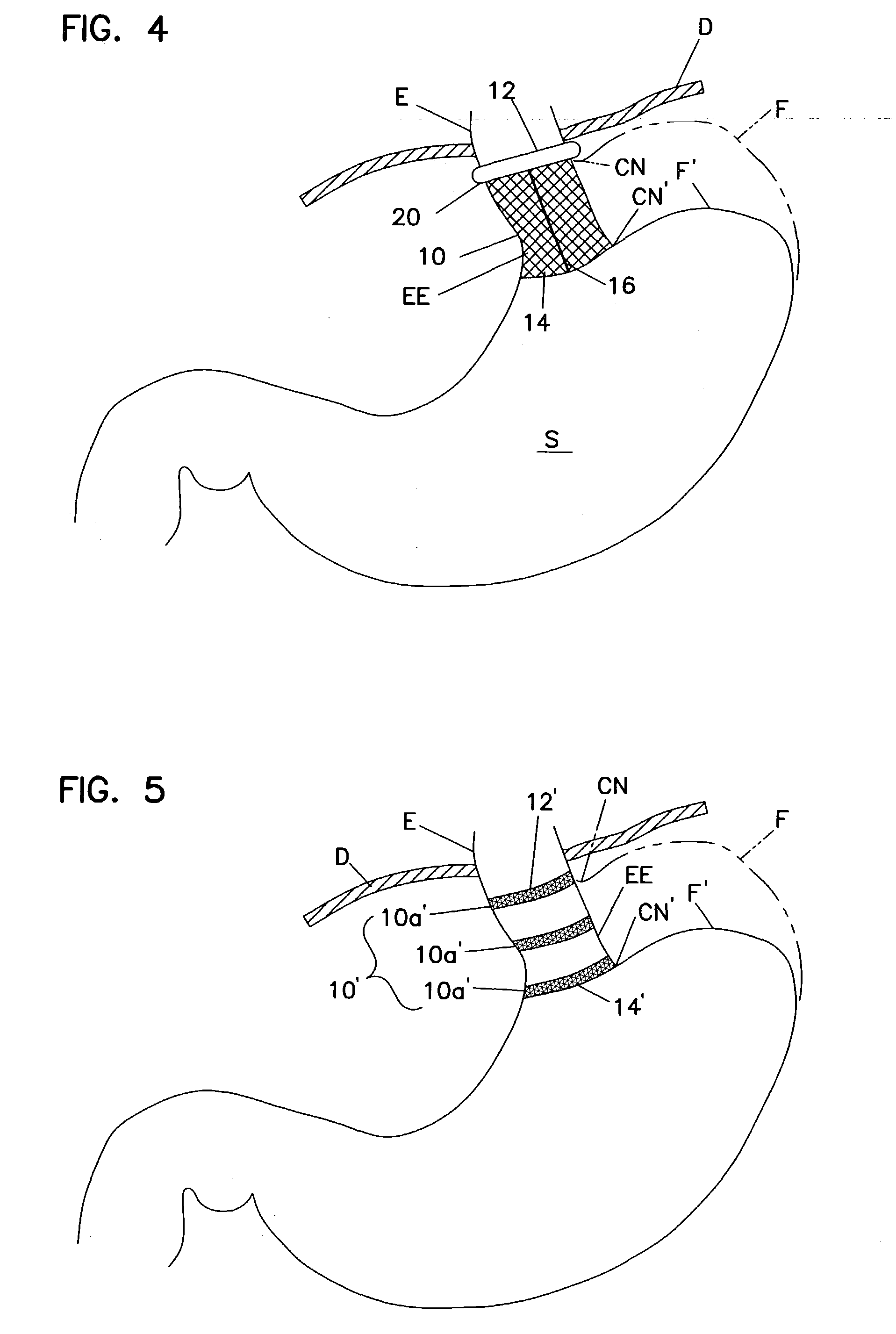
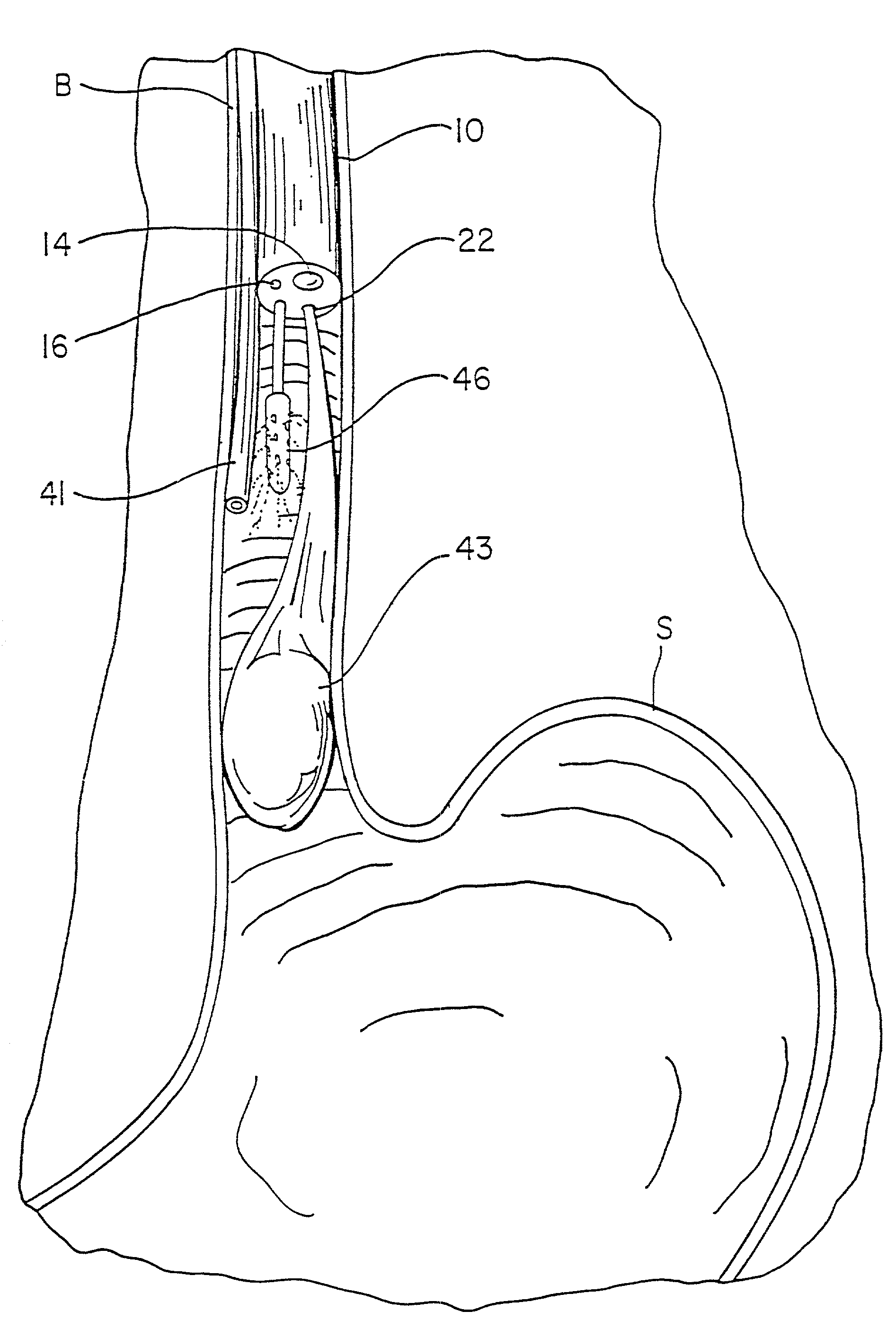
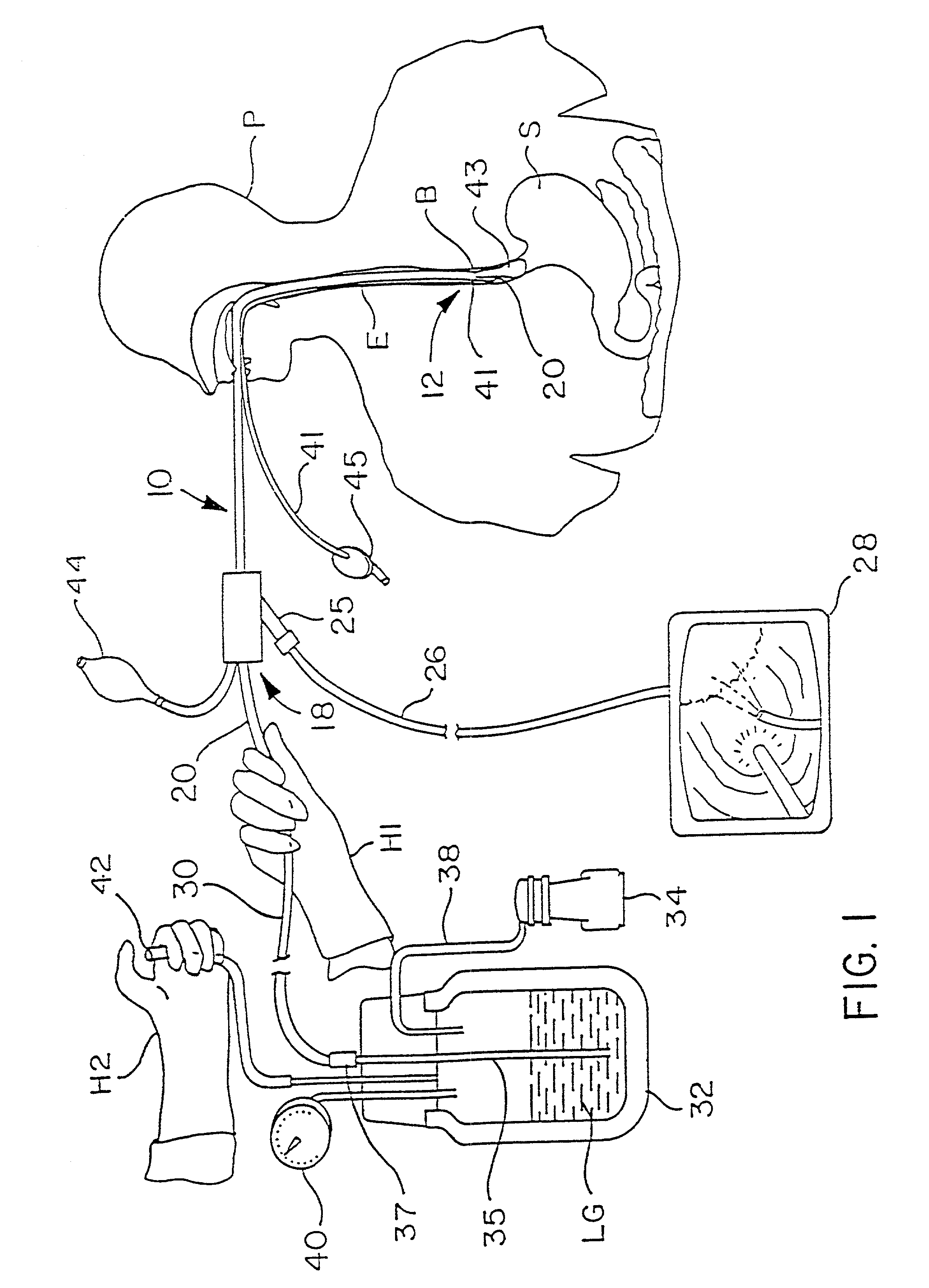
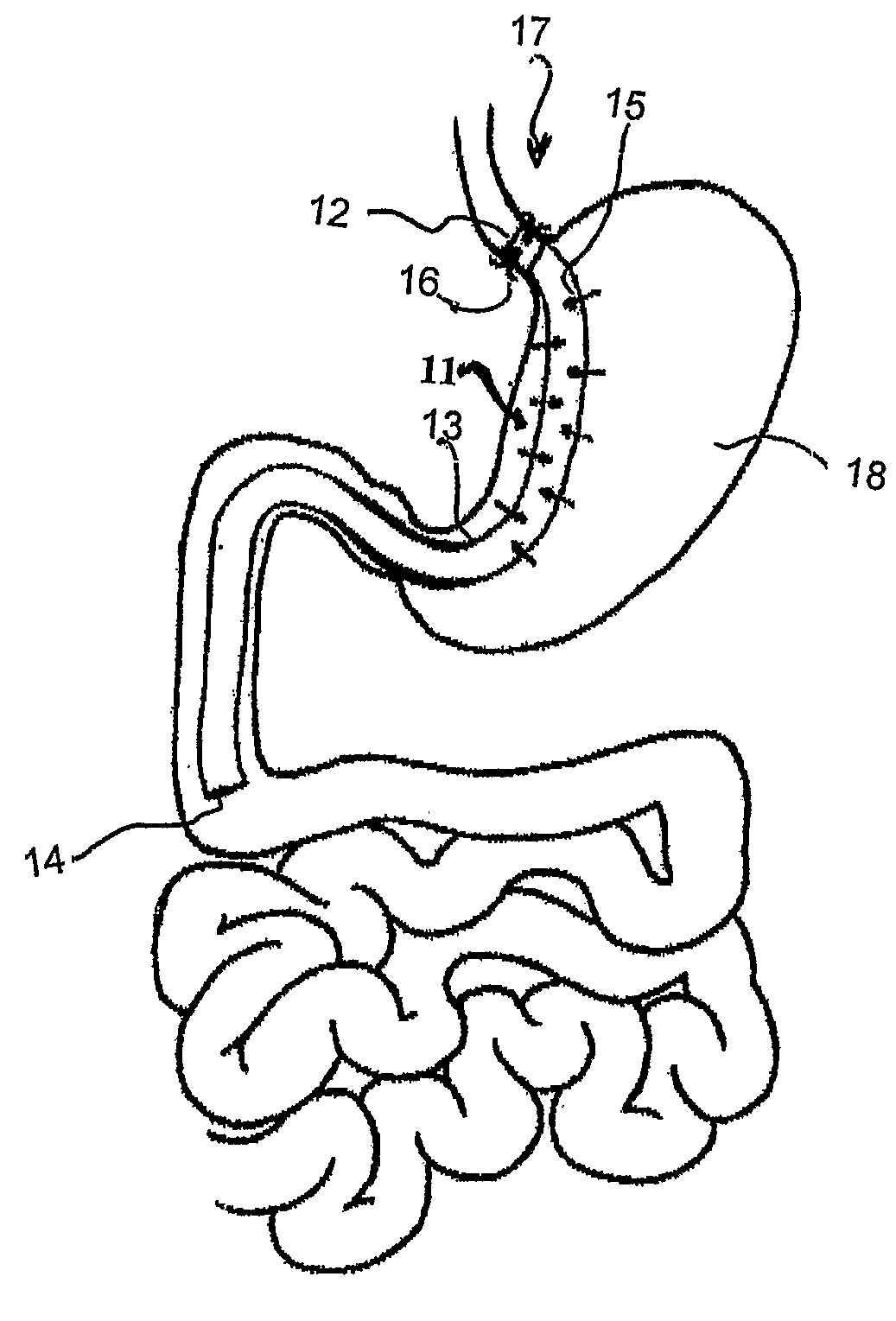
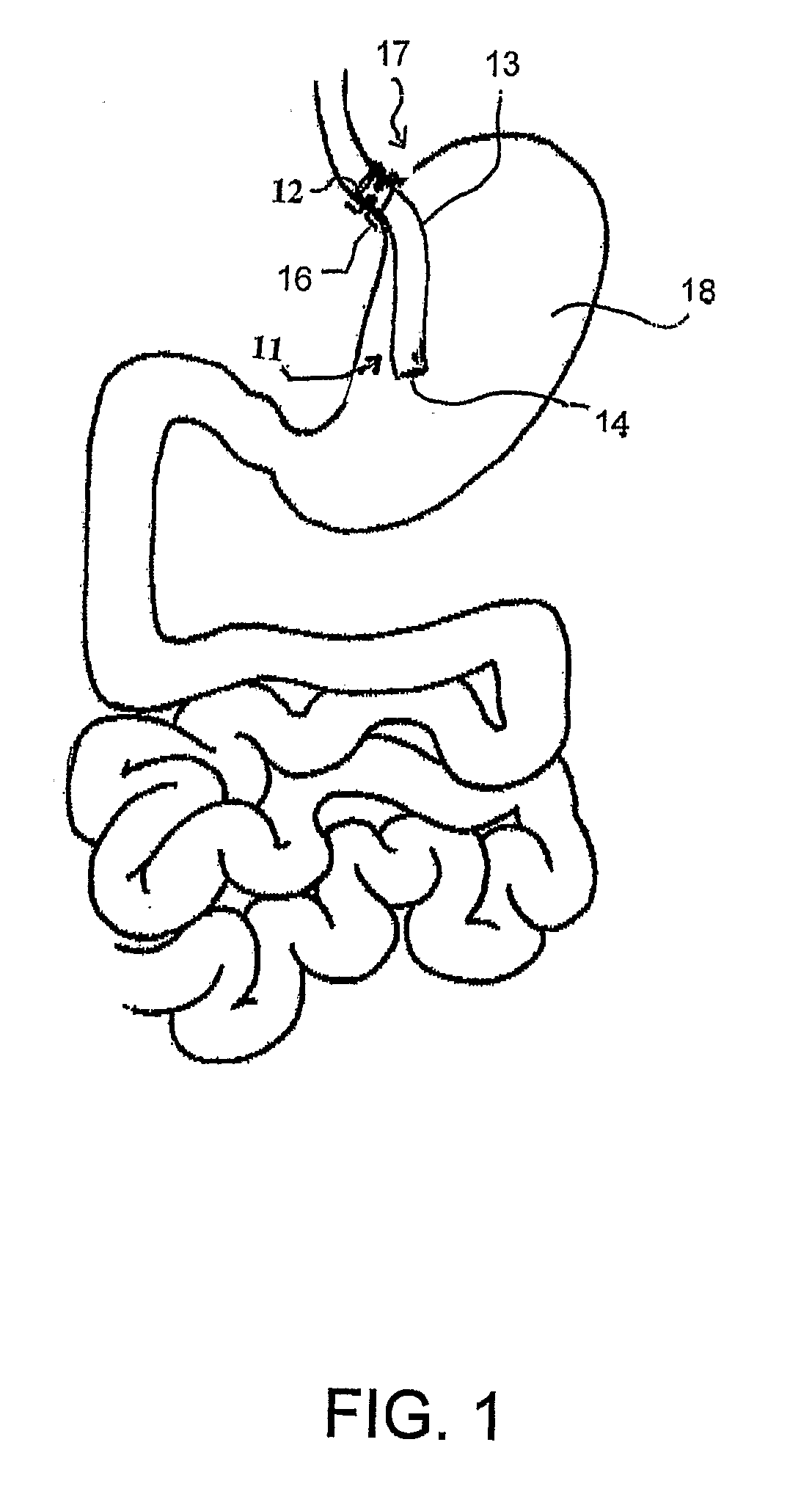
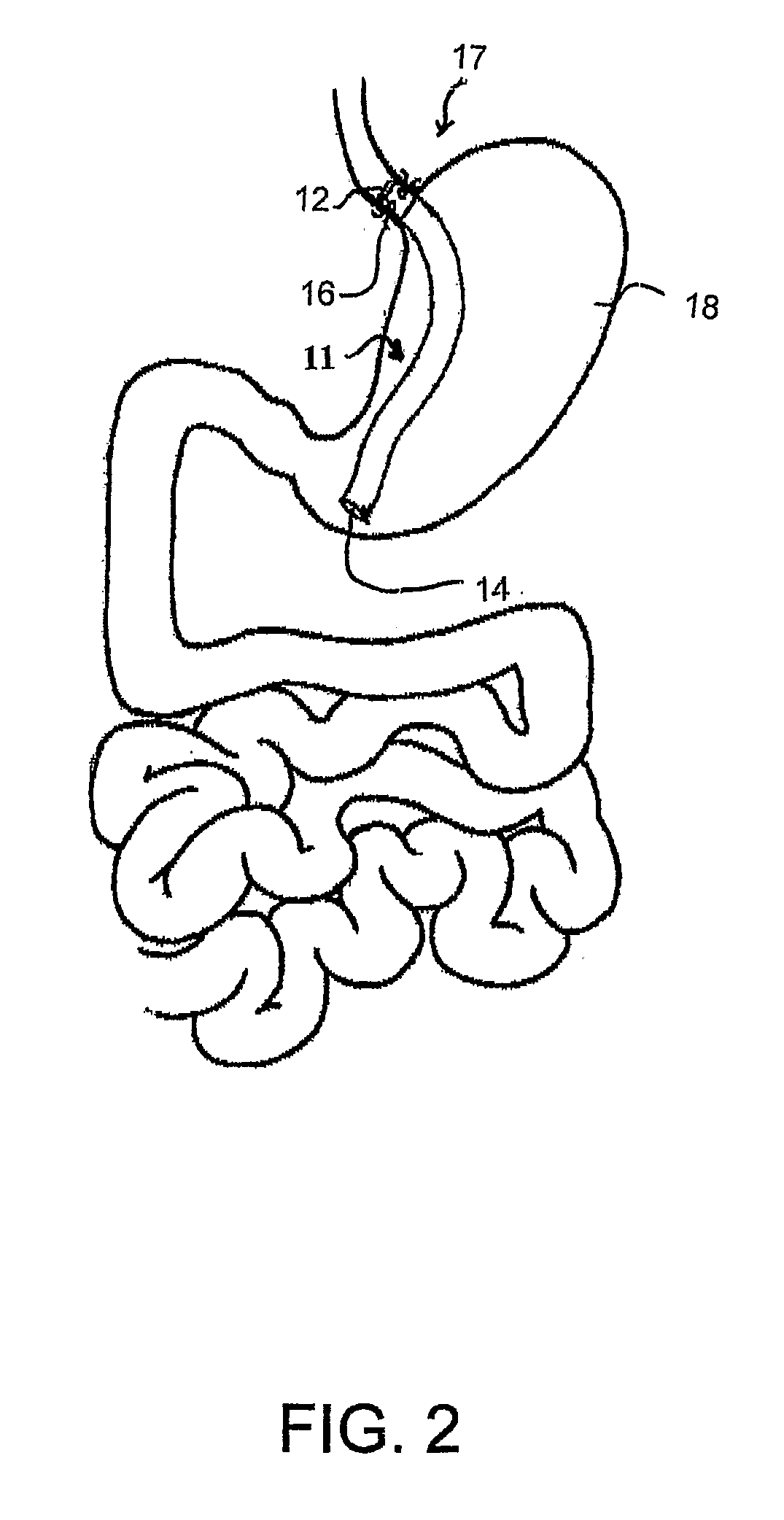
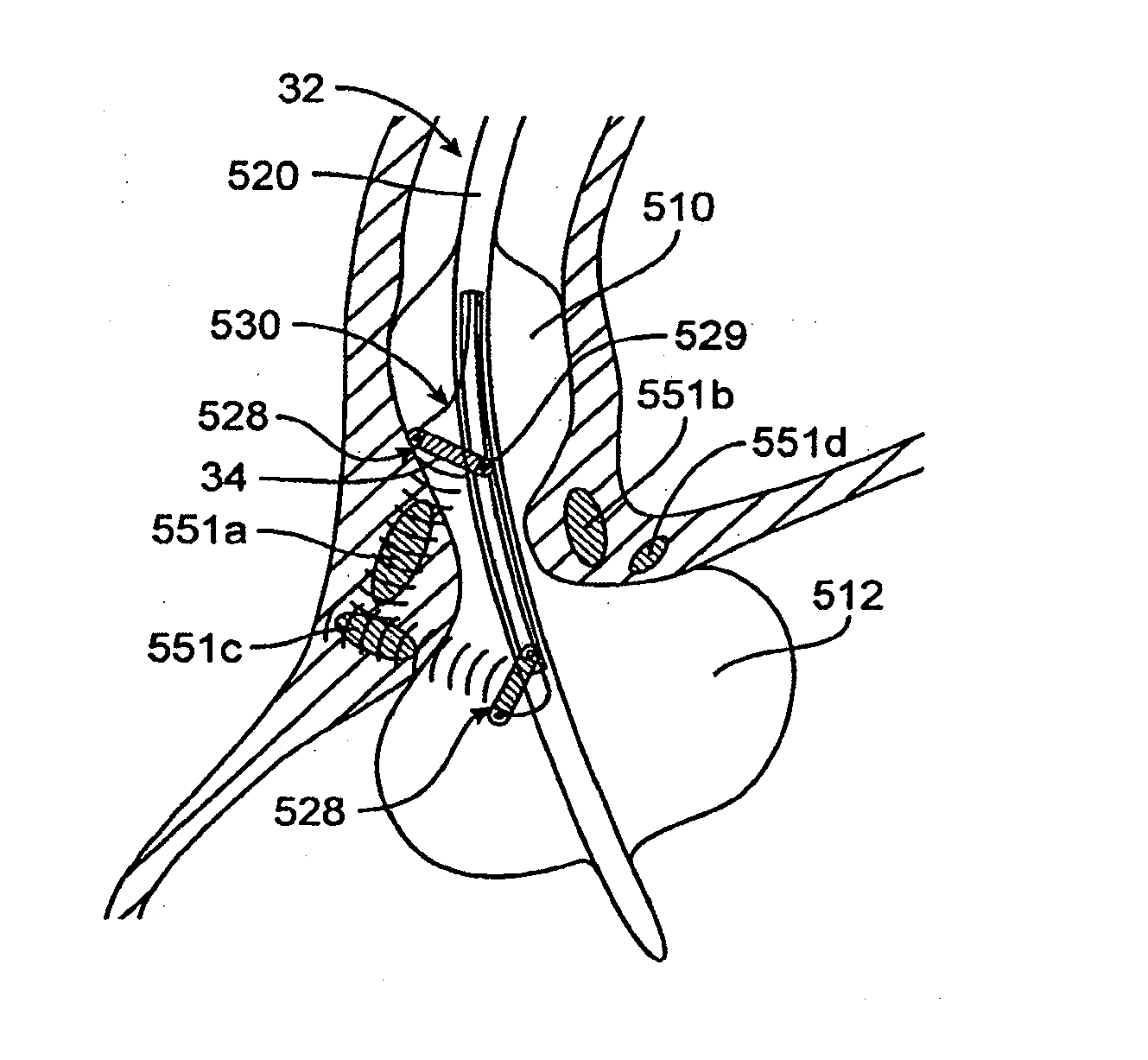
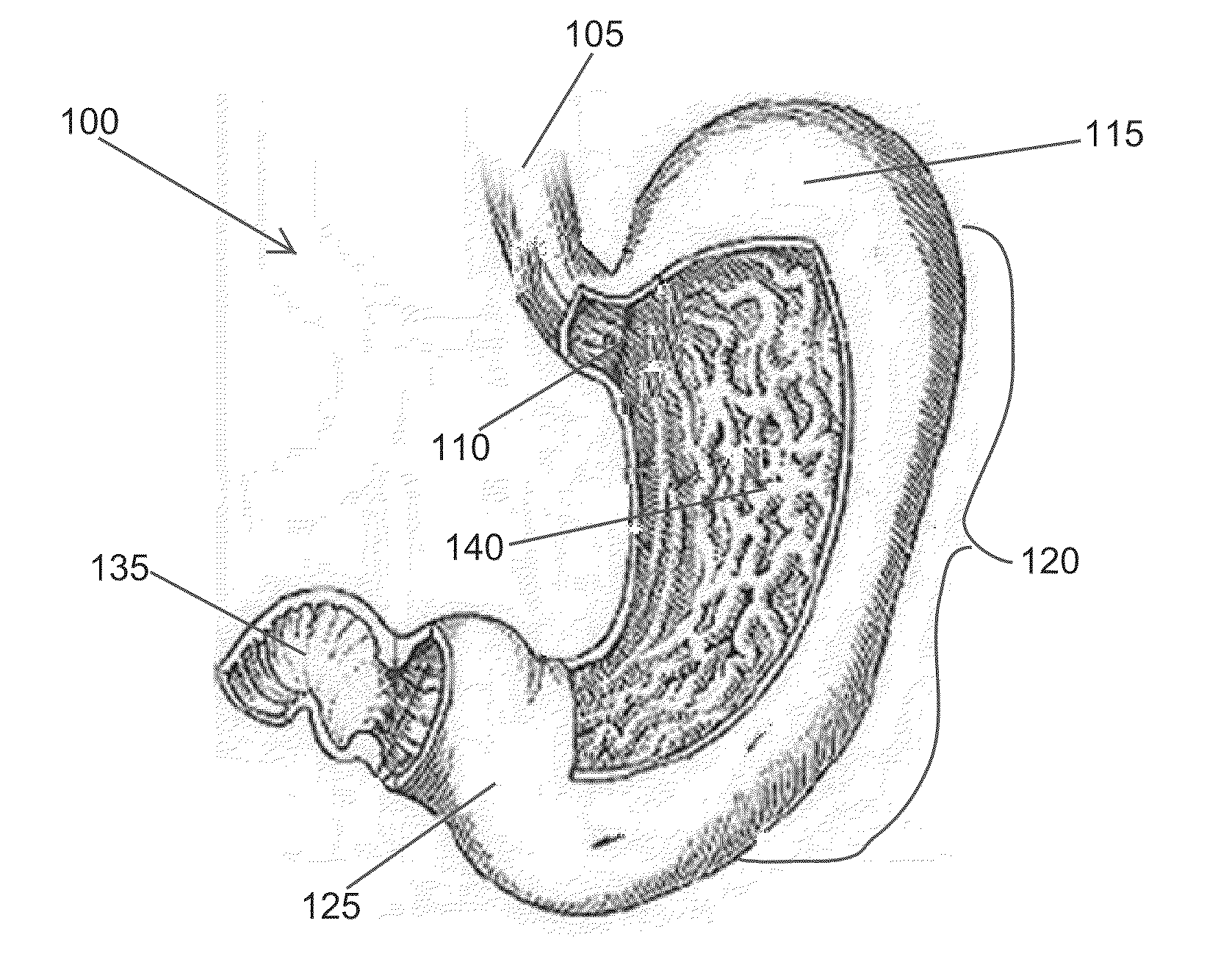
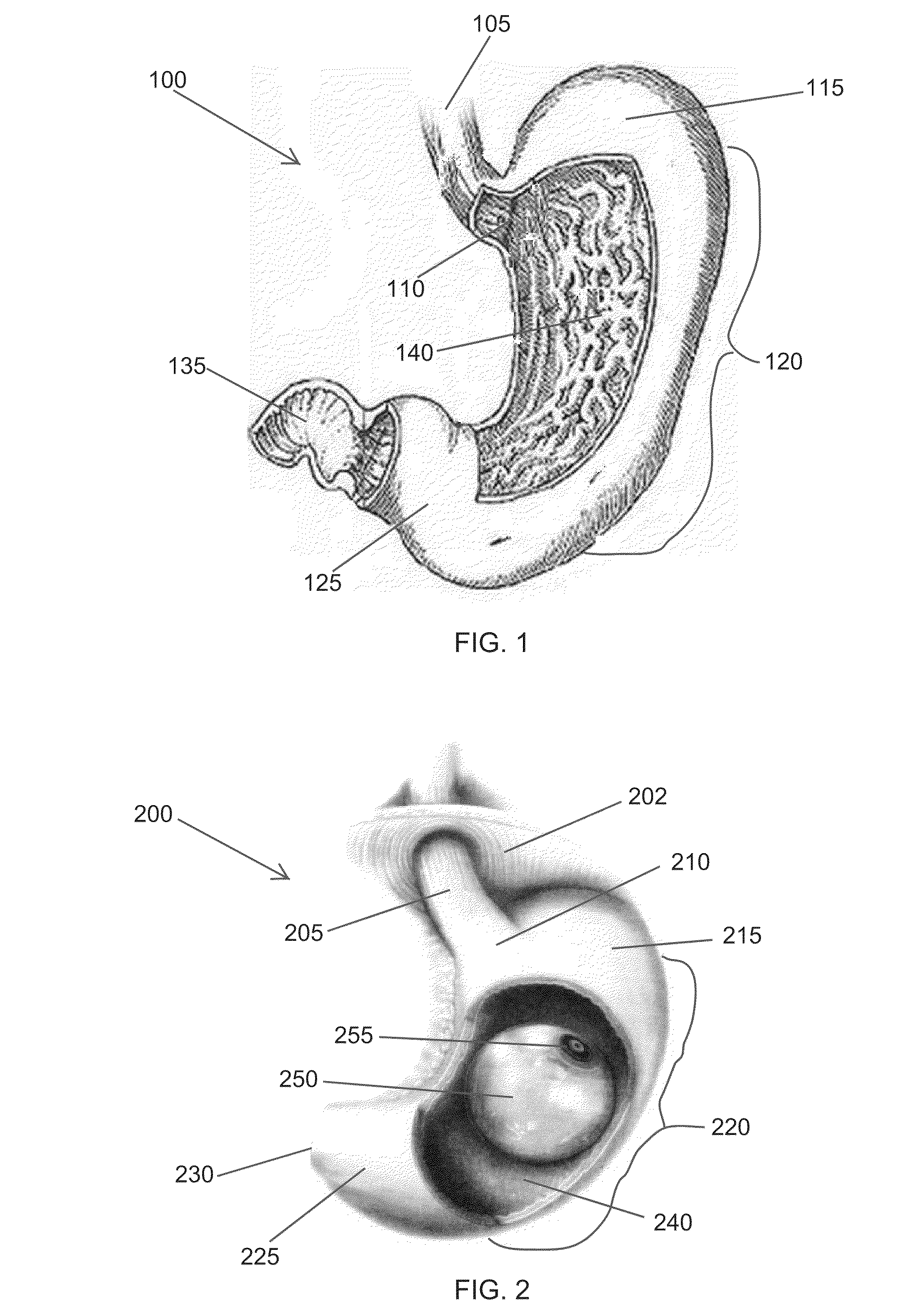
Fundoplication apparatus and method
An endoscopic device for the partial fundoplication for the treatment of GERD, comprises: a) a distal bending portion and a flexible portion suitable to be positioned in extended shape within the esophagus of a subject; b) a positioning assembly comprising two separate elements, one of which is located on said distal bending portion, and the other on said flexible portion; c) a stapling assembly comprising a staple ejecting device, wherein said staple ejecting device is located on either said bending portion or on said flexible portion, said staple ejecting devices being in working positioned relationship when said two separate elements of said positioning assembly are aligned; and d) circuitry for determining when said two separate elements of said positioning assembly are aligned.
Owner:MEDIGUS LTD
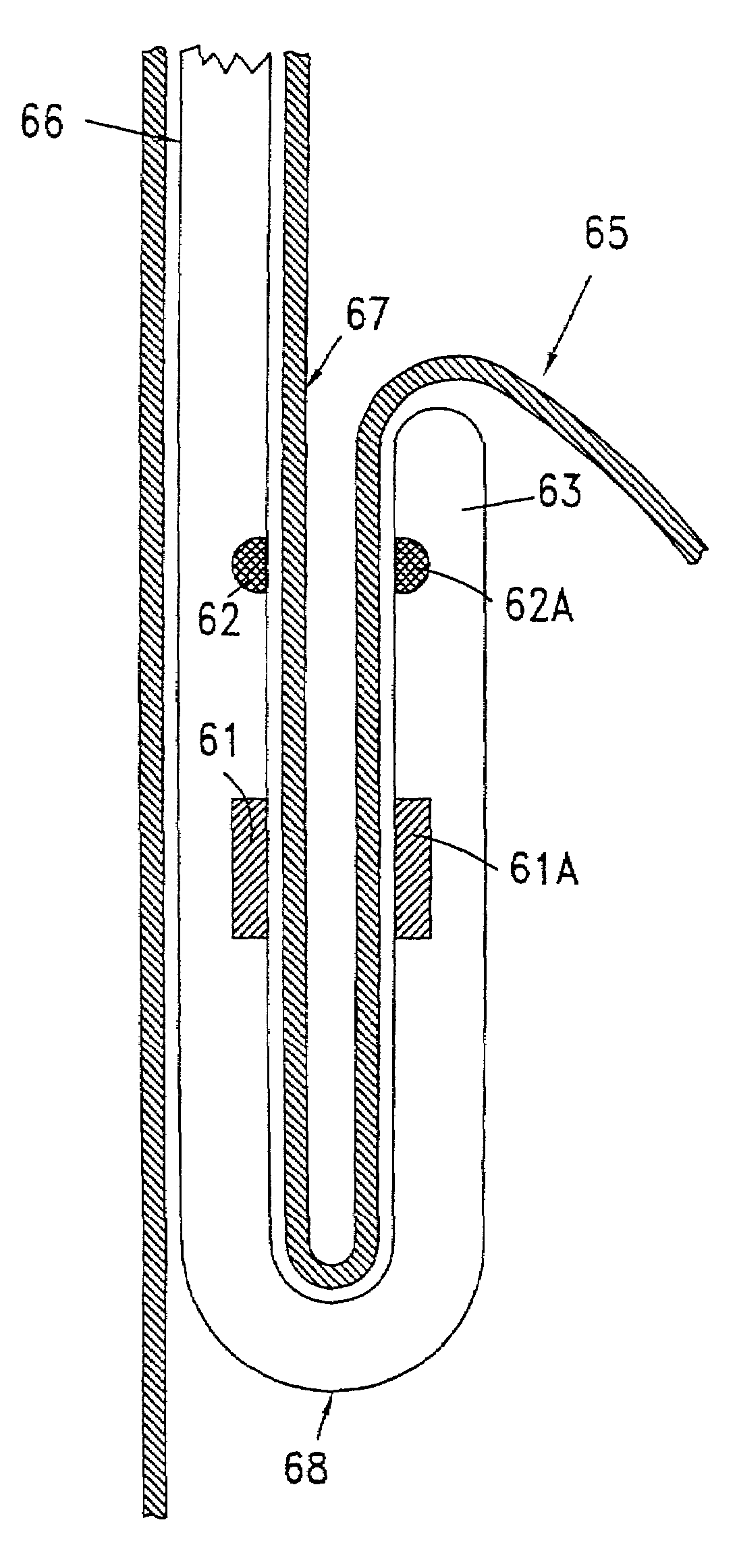
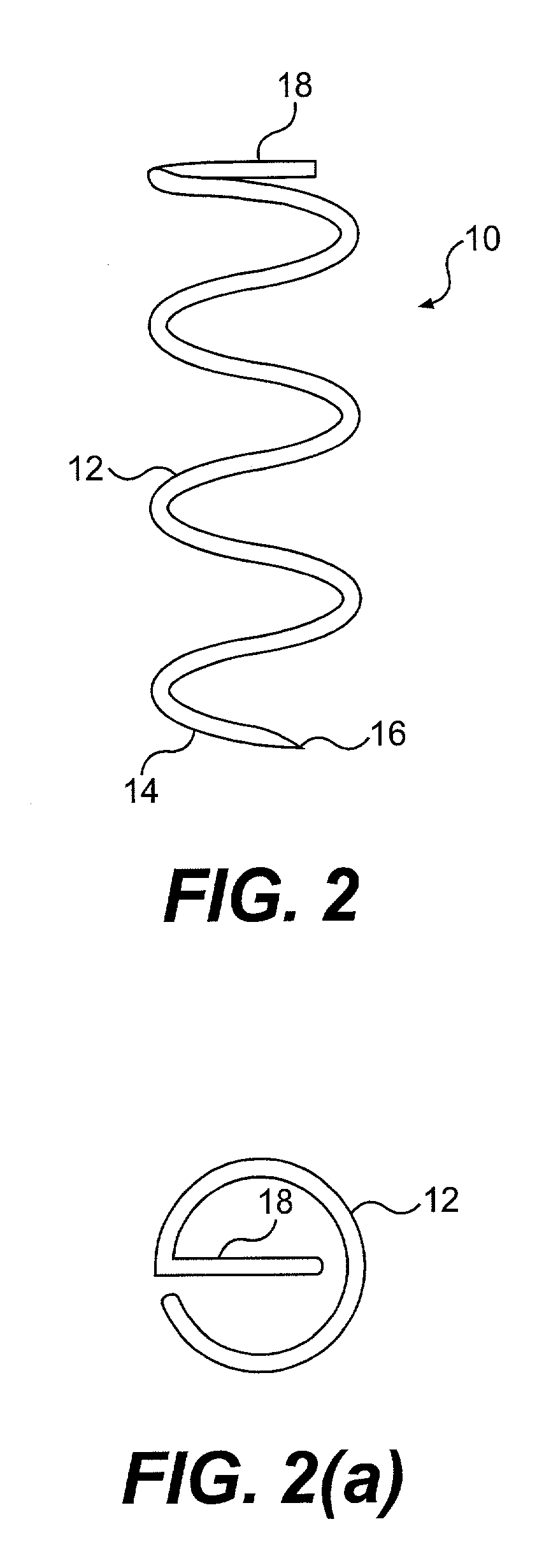
Tissue fastening devices and related insertion tools and methods
The invention in certain aspects relates to a one-piece coil-shaped surgical fastener for fastening tissue segments, especially suitable for fastening segments of the lower esophogeal sphincter and fundus in an endoscopic procedure for the treatment of GERD. The invention also relates to related methods and devices for insertion of such a fastener, especially along a juncture of the surfaces of such tissue segments.
Owner:BOSTON SCI SCIMED INC
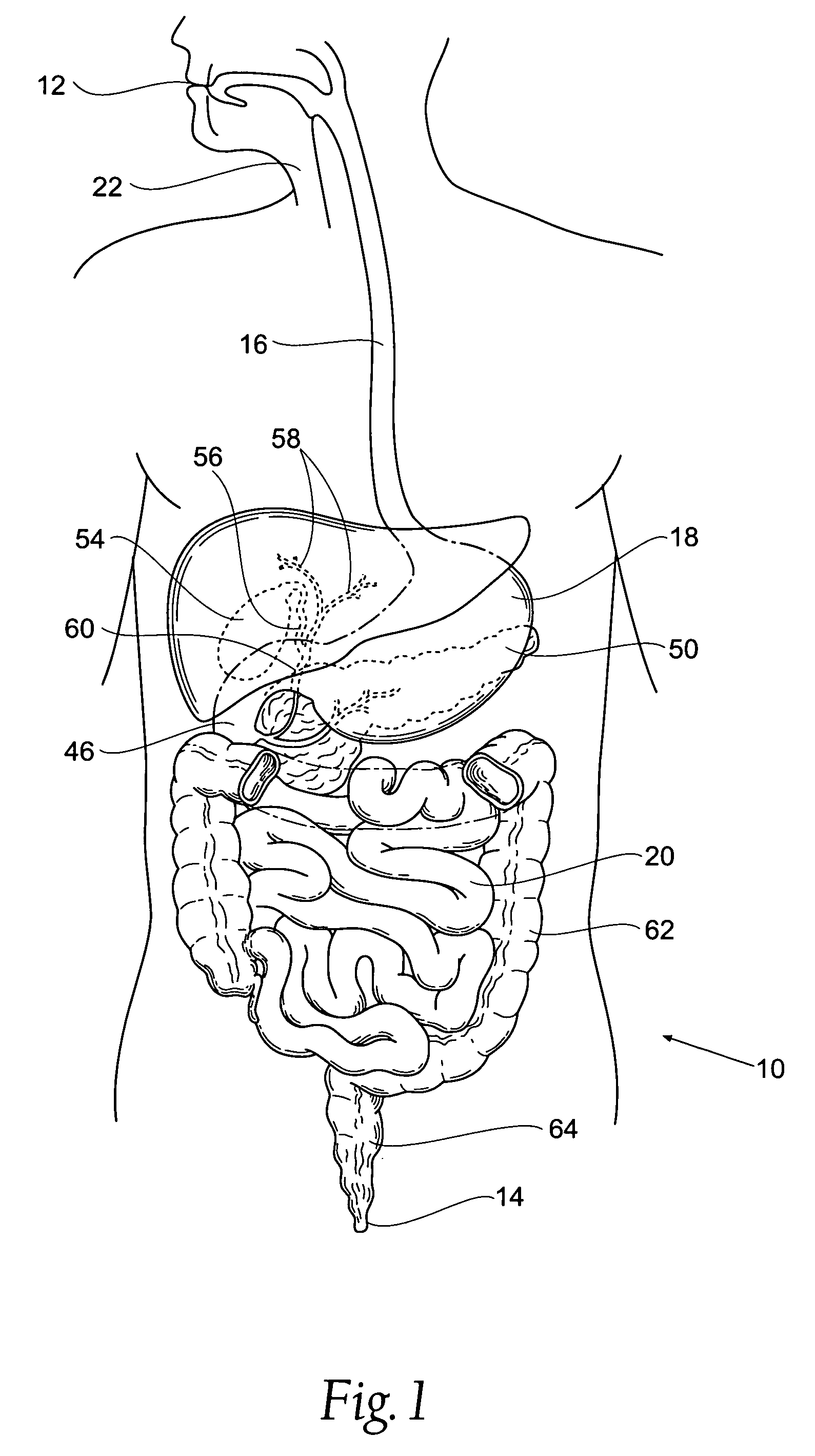
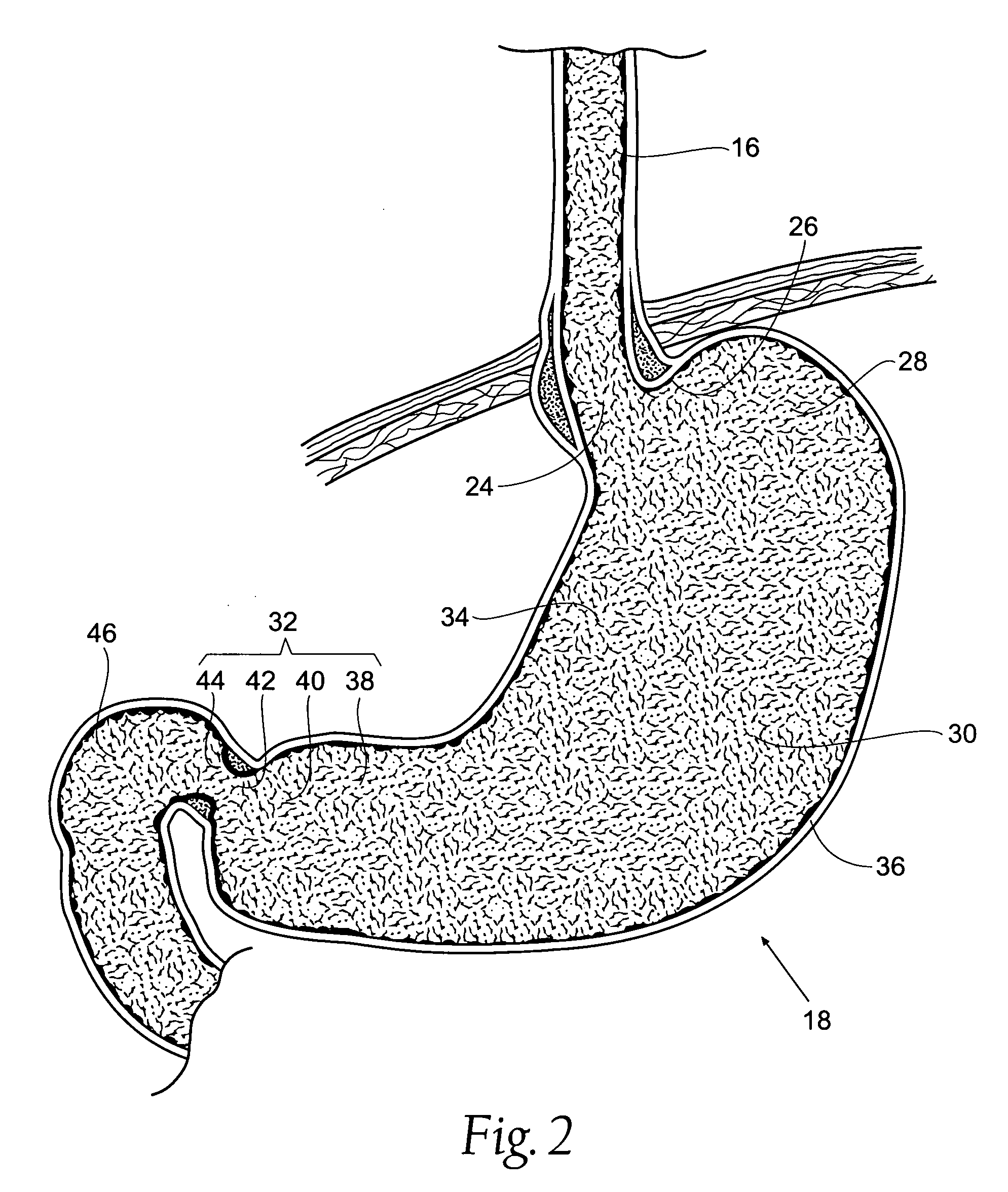
Bariatric device and method
A bariatric device includes a body having a wall defining a lumen, the wall configured to generally conform to the shape and size of at least one chosen from i) the abdominal portion of the esophagus, ii) the esophageal-gastric junction, and iii) the proximal cardiac portion of the stomach, with the wall adapted to exert pressure on the at least one chosen from i) the abdominal portion of the esophagus, ii) the esophageal-gastric junction, and iii) the proximal cardiac portion of the stomach, thereby influencing a neurohormonal feedback mechanism of the patient to cause at least partial satiety by augmenting fullness caused by food and simulating fullness in the absence of food.
Owner:BFKW
Device and method for esophageal cooling
InactiveUS20070055328A1Equally distributedDiagnosticsSurgical instrument detailsOesophageal injuryPreventing injury
The present invention includes a device and a method for preventing injury of the esophagus during thermal ablation of the left atrium. The device has an esophageal probe with a balloon tip for insertion into the esophagus of a patient. During usage, coolant passes into the esophageal probe and then fills its balloon. The coolant, when circulating through the balloon and an external cooling machine, protects the esophageal tissue in contact with the esophageal probe from thermal damage during ablation of the posterior wall of the left atrium of the heart, or other procedure.
Owner:MAYSE MARTIN L +1
Bariatric device and method
ActiveUS7846174B2Reduce distractionsReduce pressureSurgeryMedical devicesEsophago-esophagealNeurohormones
A bariatric device includes a body having a wall defining a lumen, the wall configured to generally conform to the shape and size of at least one chosen from i) the abdominal portion of the esophagus, ii) the esophageal-gastric junction, and iii) the proximal cardiac portion of the stomach, with the wall adapted to exert pressure on the at least one chosen from i) the abdominal portion of the esophagus, ii) the esophageal-gastric junction, and iii) the proximal cardiac portion of the stomach, thereby influencing a neurohormonal feedback mechanism of the patient to cause at least partial satiety by augmenting fullness caused by food and simulating fullness in the absence of food.
Owner:BFKW
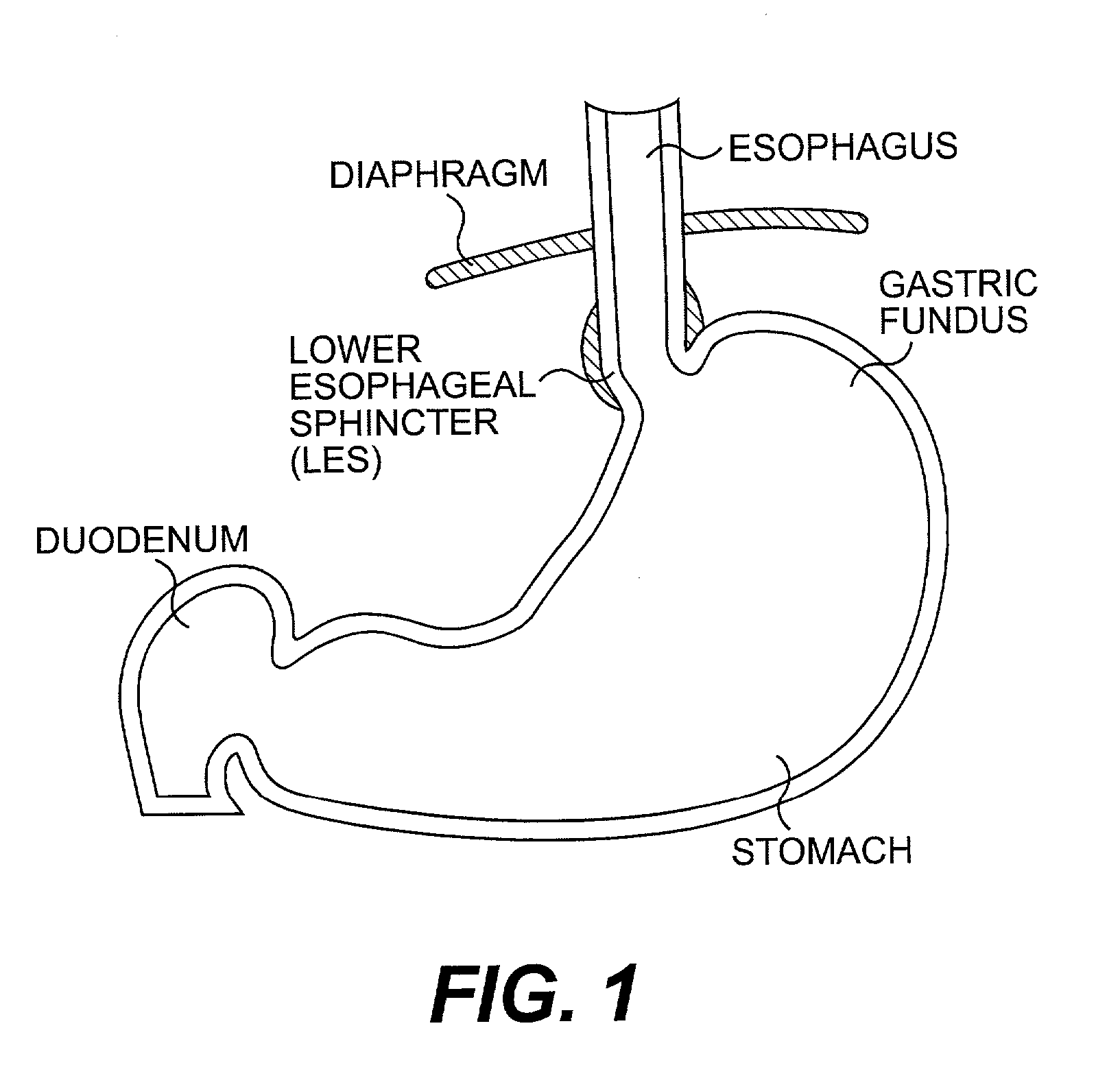
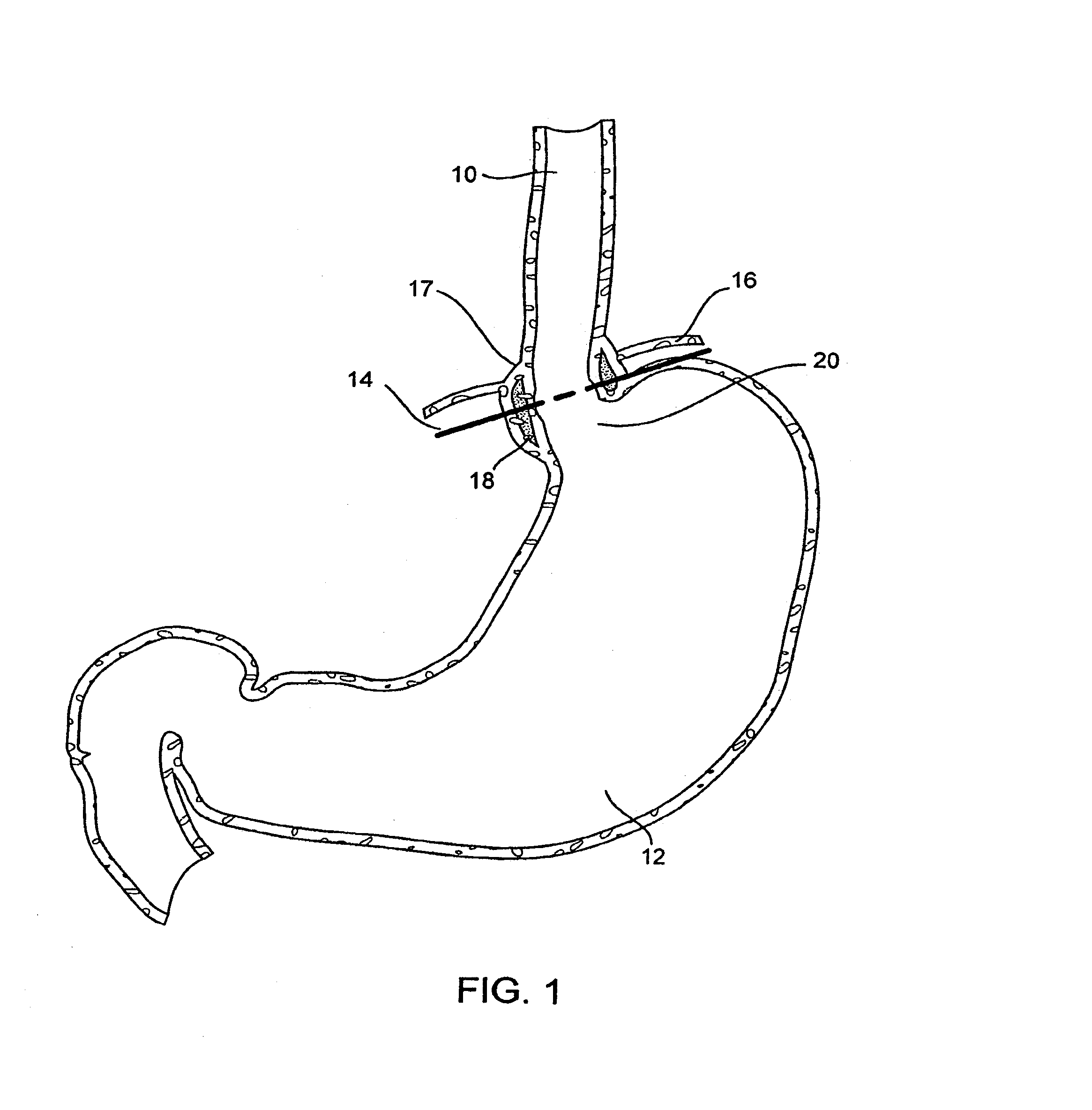
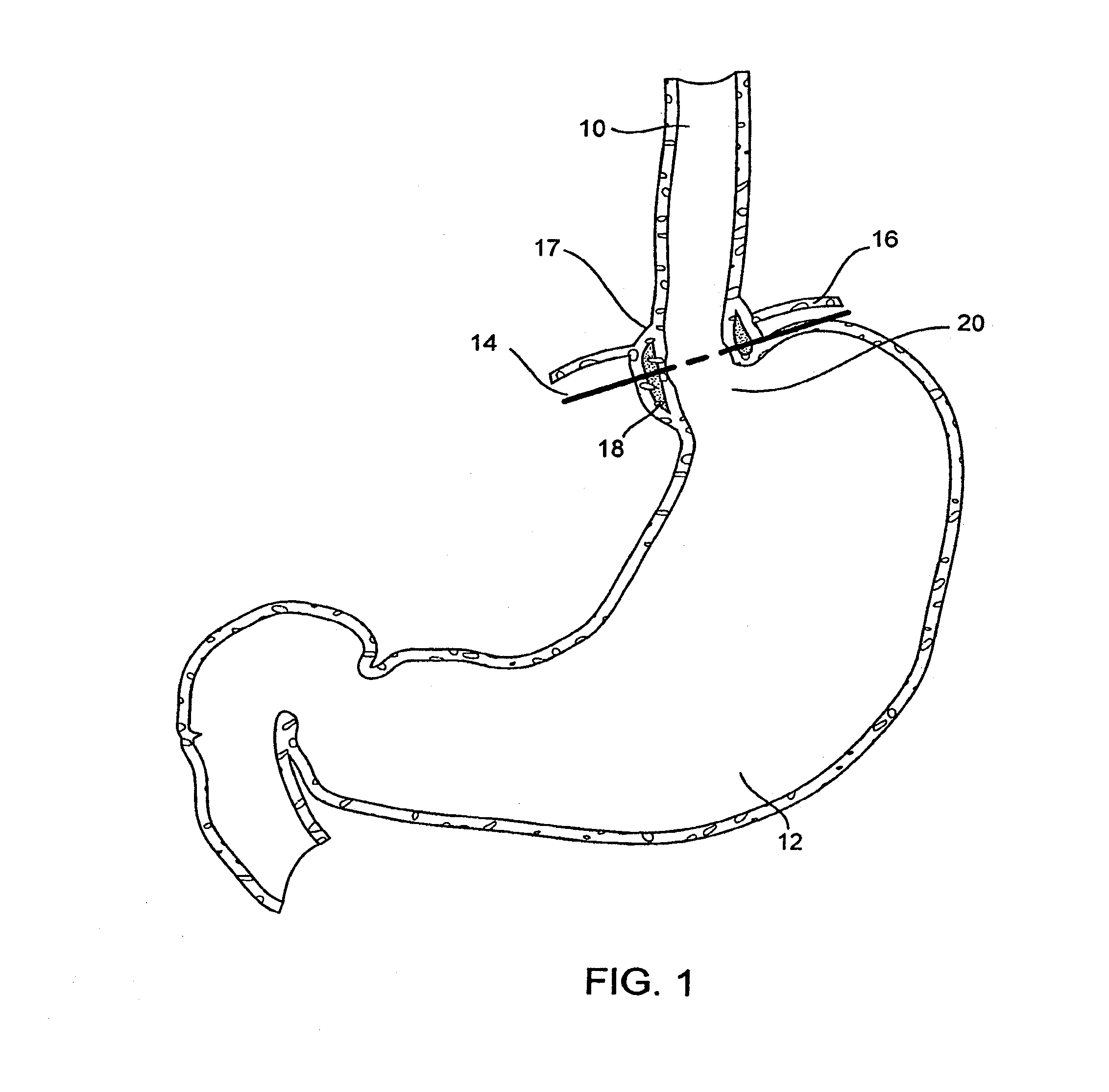
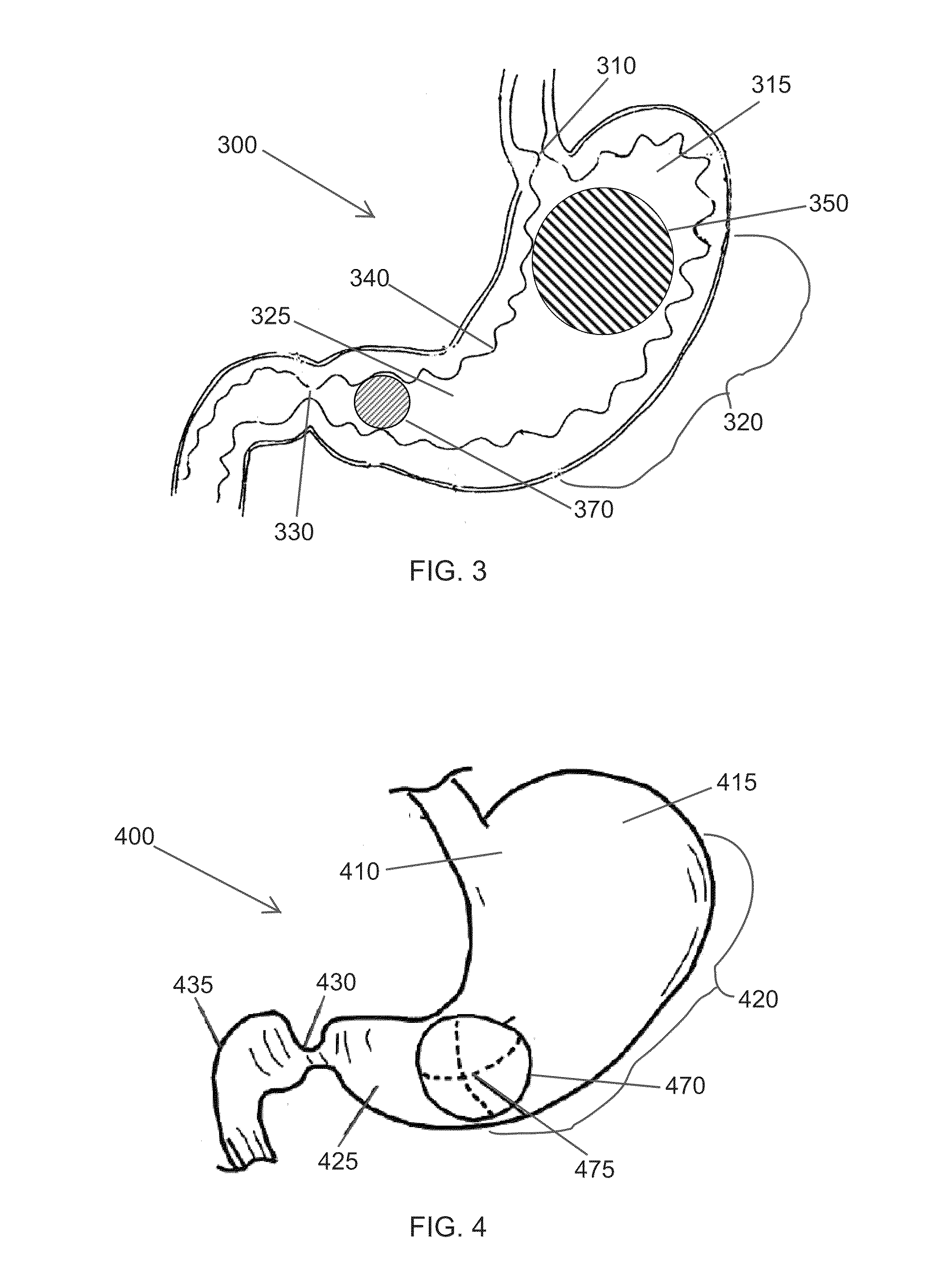
Gastro-esophageal reflux disease (GERD) treatment method and apparatus
The method of the invention includes accessing a juncture of an esophagus and a stomach of the patient on a distal side of a diaphragm. The esophagus and a fundus of the stomach intersect at a cardiac notch located at an original cardiac notch position. A reducing element is placed at the junction with the reducing element selected to reposition the cardiac notch to a position more distal to a lower esophageal sphincter of the patient.
Owner:RESHAPE LIFESCIENCES INC
Method and apparatus for cryogenic spray ablation of gastrointestinal mucosa
InactiveUS7025762B2Readily and inexpensivelyOvercomes drawbackSurgical furnitureDiagnosticsCryoablationSphincter of Oddi
A method and apparatus to treat Barrett's tissue, a pre-cancerous condition, by removing the epithelium above the lower esophageal sphincter through cryo-ablation. An endoscope with fiber optics is used to view the operation, and a catheter for supplying liquid nitrogen is passed through the lumen of the endoscope. Liquid nitrogen at low pressure is sprayed directly onto the Barrett's tissue through the catheter while the physician views the operation through the fiberoptics of the endoscope and controls the spray via a valve. Freezing is indicated by whiteness and shows that the epithelium has been cryoablated. The apparatus can also be used to treat various other gastrointestinal tract lesions. The catheter is insulated to withstand extremely cold temperatures without becoming stiff and without affecting the inherent flexibility and maneuverability of the endoscope.
Owner:CRYMED TECH
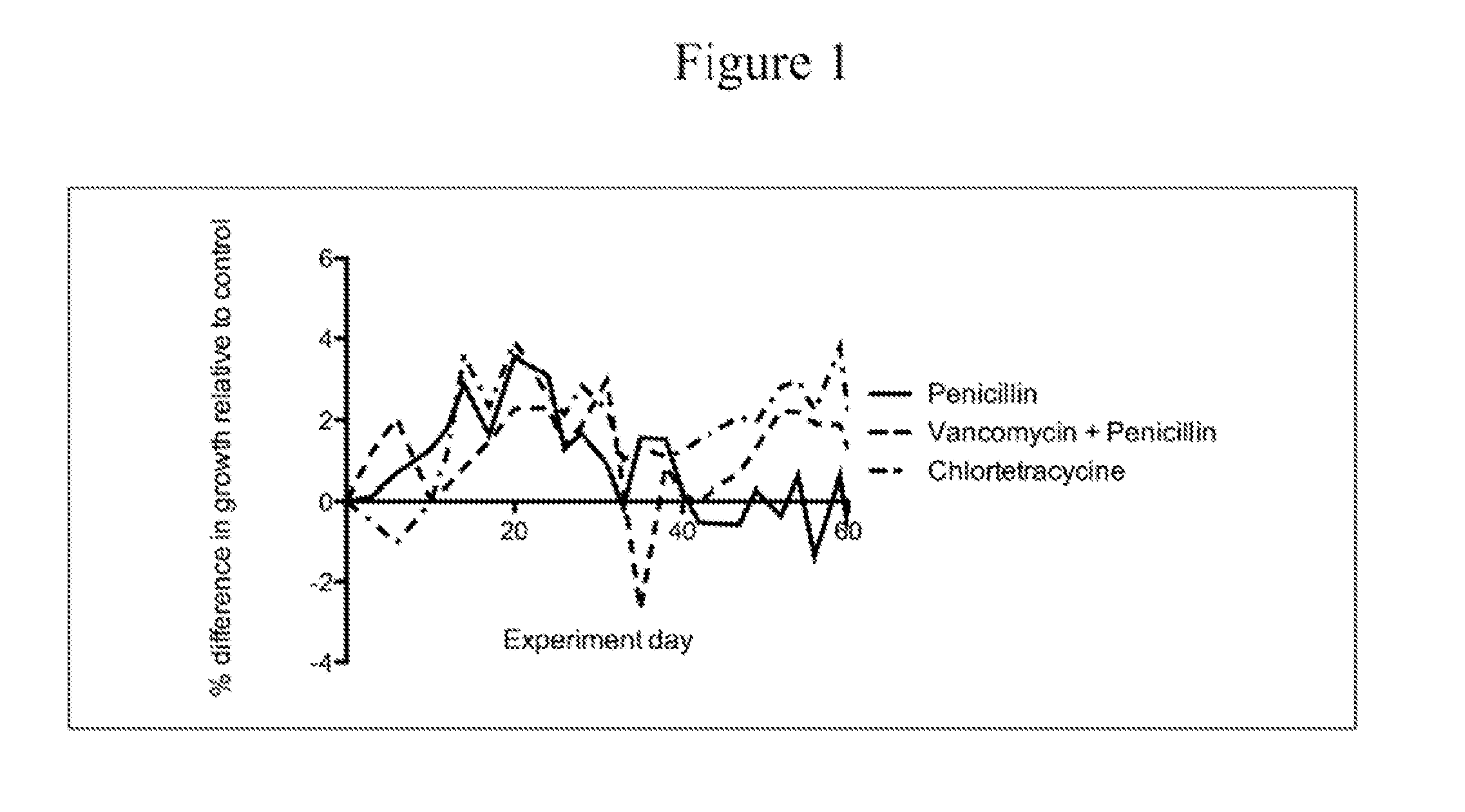
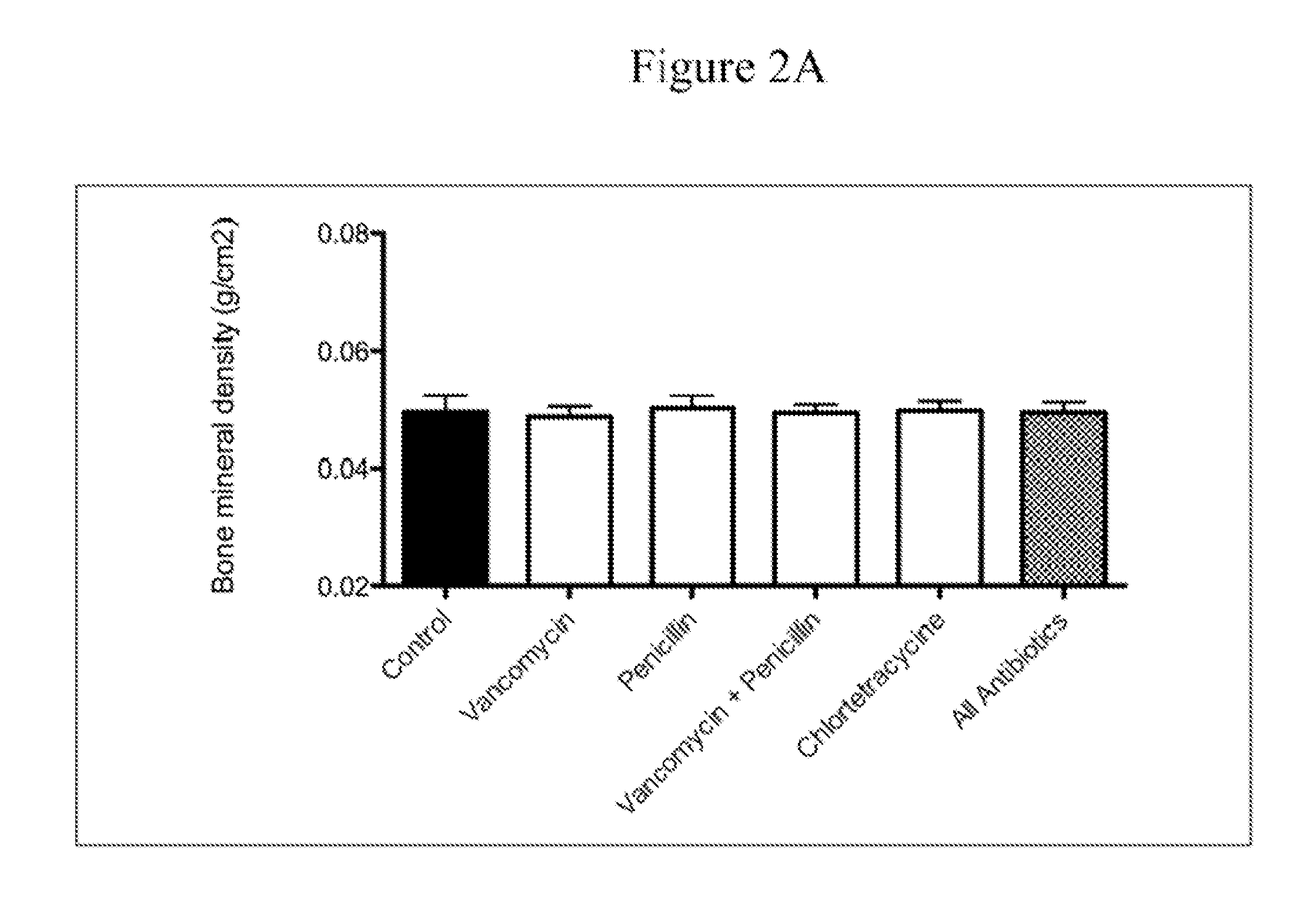
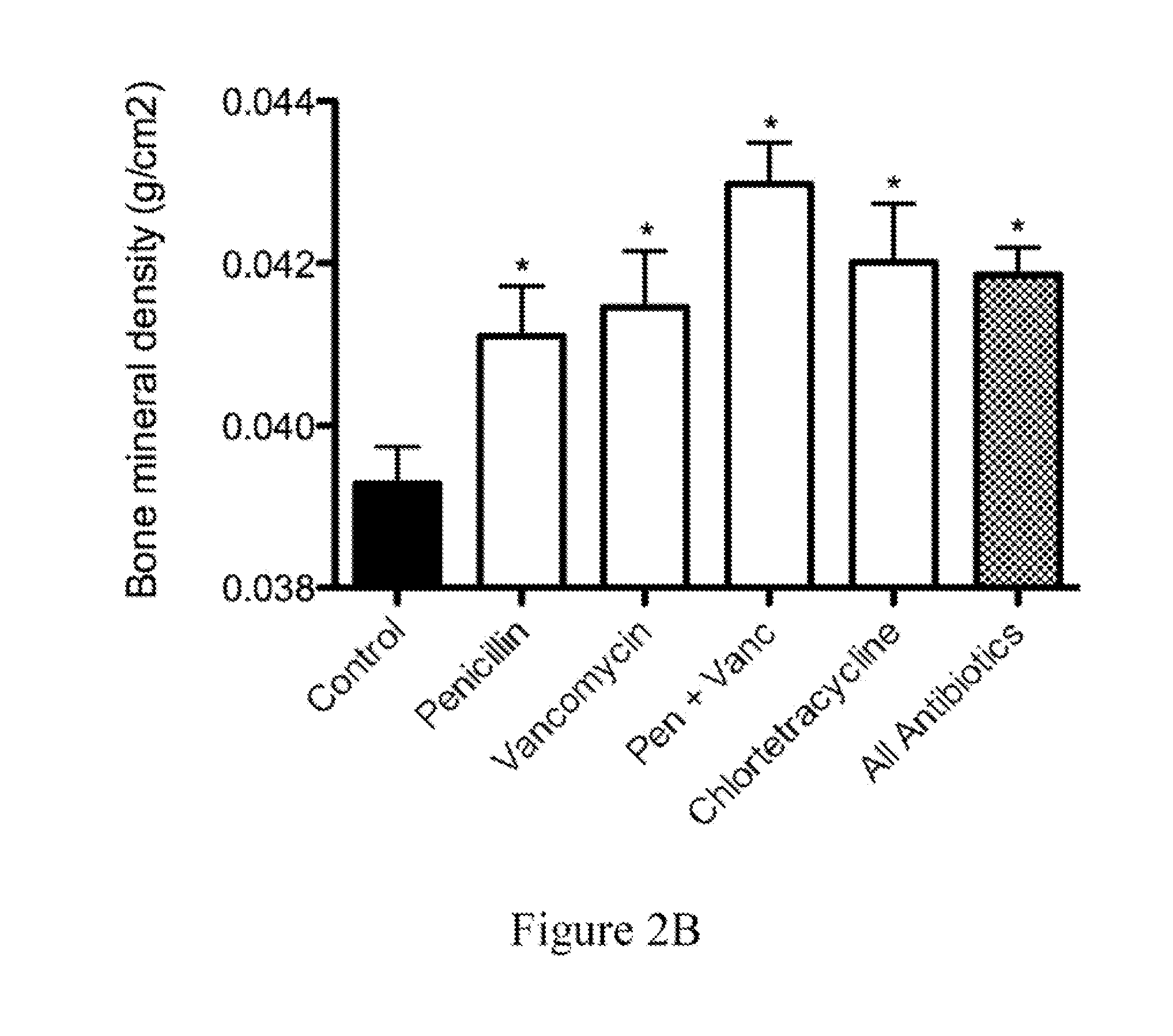
Compositions and methods for characterizing and restoring gastrointestinal, skin, and nasal microbiota
ActiveUS20100074872A1Growth inhibitionFacilitate calorie uptakeBiocideMetabolism disorderBacteroidesDisease
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
Medical Device and Method For Controlling Obesity
InactiveUS20080249533A1Slow down passage of foodEat more slowlyEar treatmentOesophagiPyloric orificeDiaphragm muscle
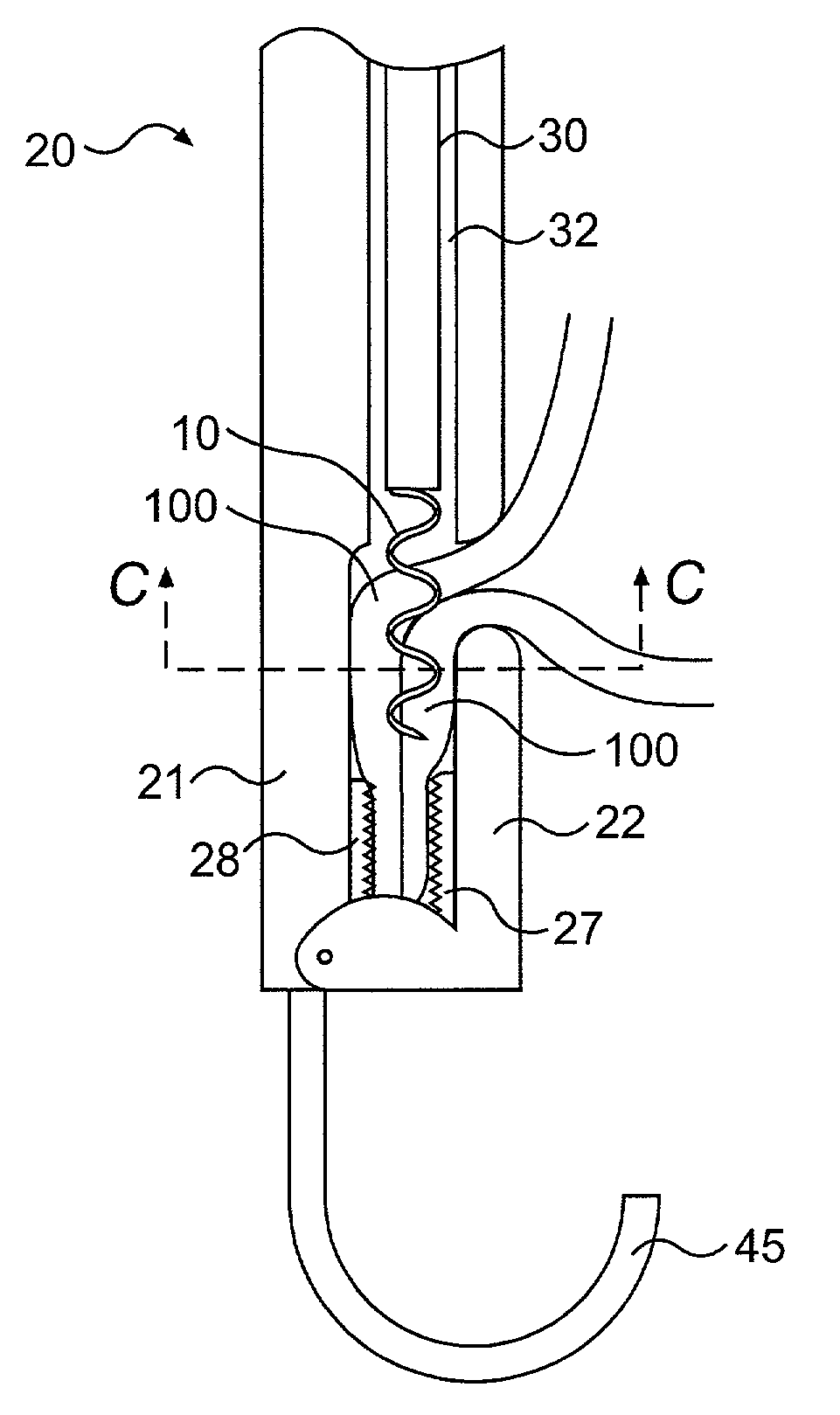
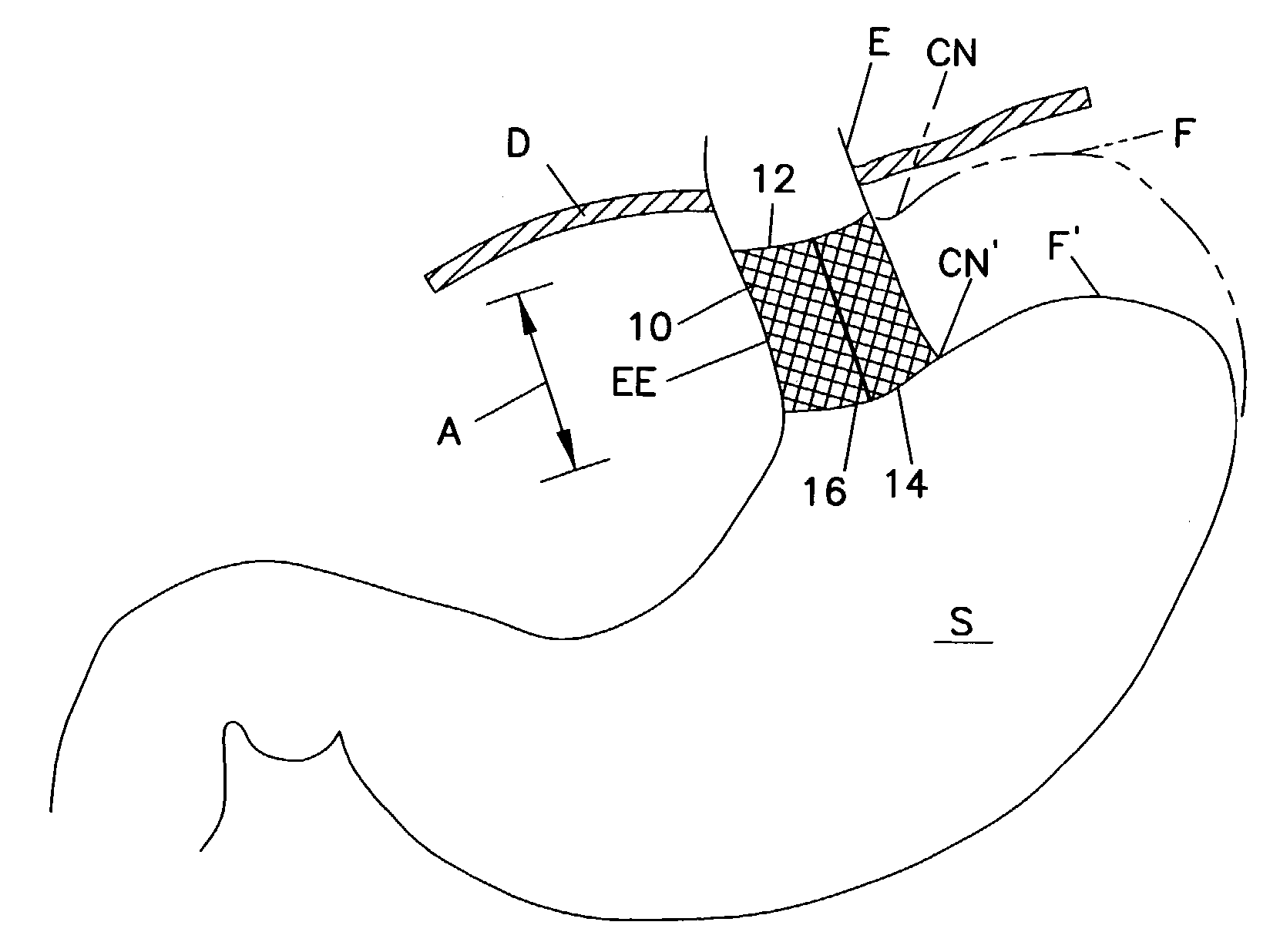
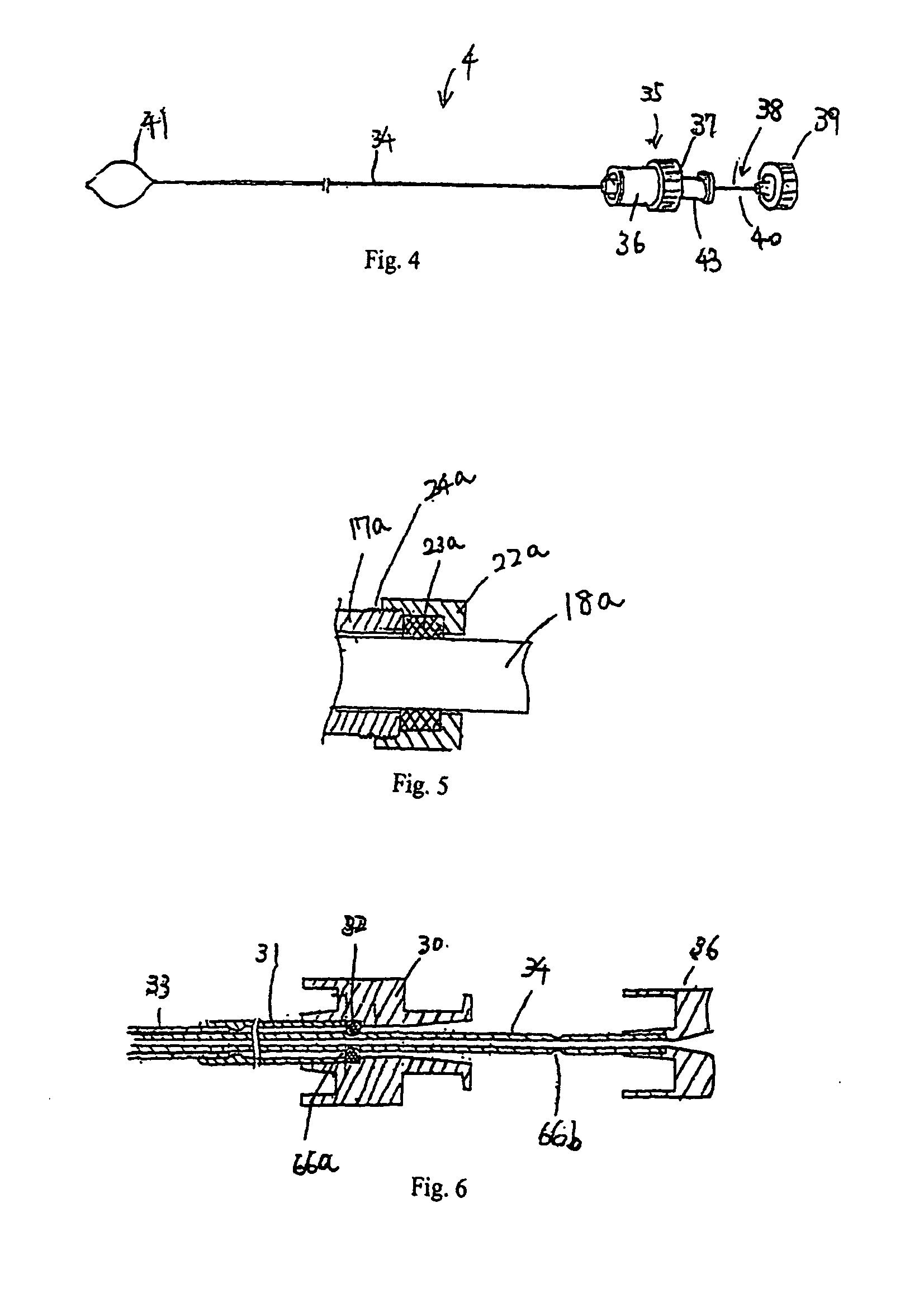
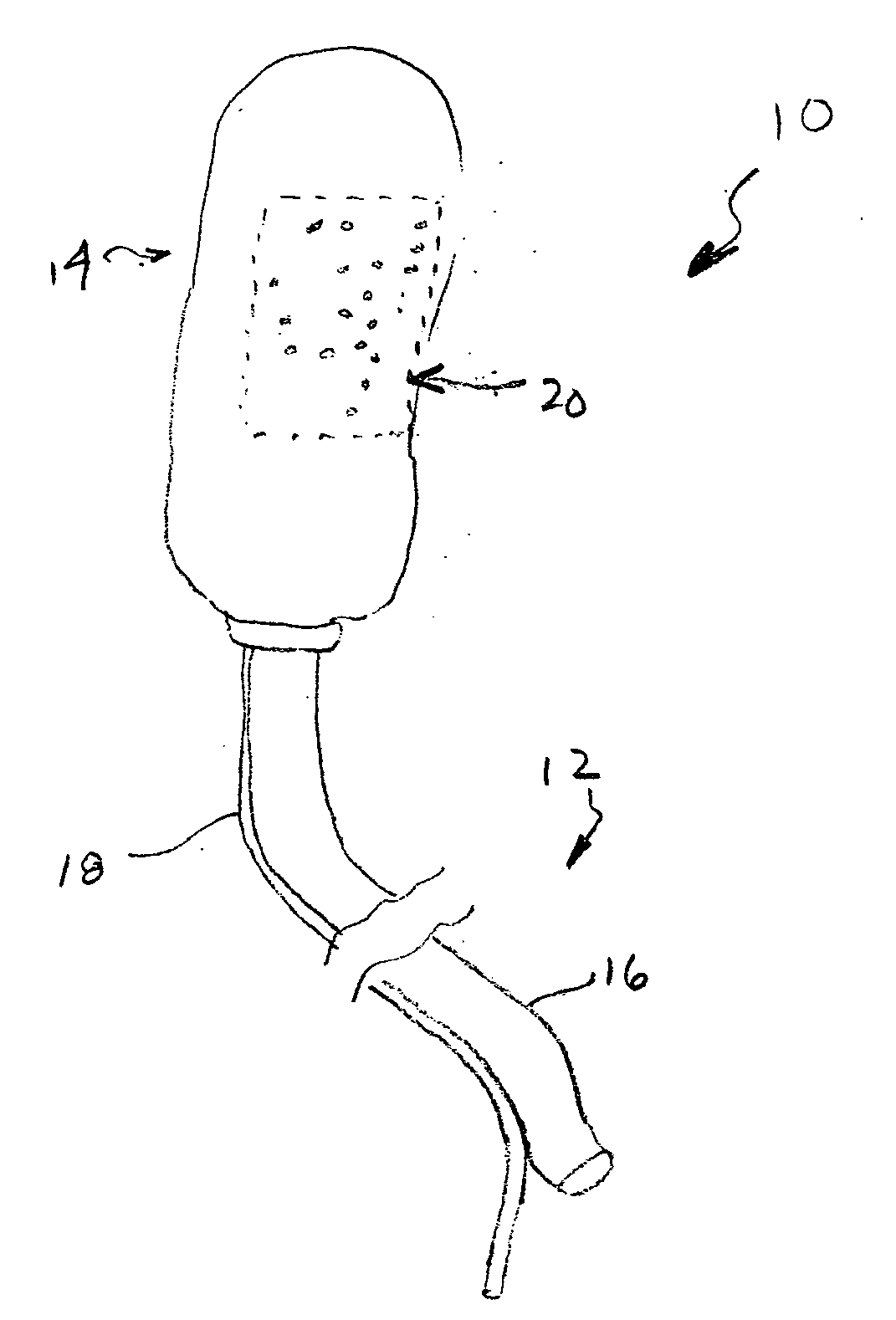
A method of, and device for, slowing the passage of food through a digestive tract of a patient and thereby treating obesity. The device is an obesity tube comprising (A) an upper ring of a size corresponding to a point under a patient's esophagus and above the patient's diaphragm muscle, and (B) a lower tube having a length and a distal opening. The method comprises stapling the upper ring under the patient's esophagus, above the patient's diaphragm muscle, and placing the lower tube distal to the upper ring. The length of the lower tube depends on whether the tube is to terminate distally in the stomach or terminate past the pylorus, in which case a section can be provided which is thick enough to resist collapsing under pylorus pressure. The lower tube can be entirely or partially non-permeable or semi-permeable. Semi-permeable tubes or sections thereof have walls which permit the passage of gastric hydrochloric acid but not food.
Owner:KS BIOMEDIX LTD
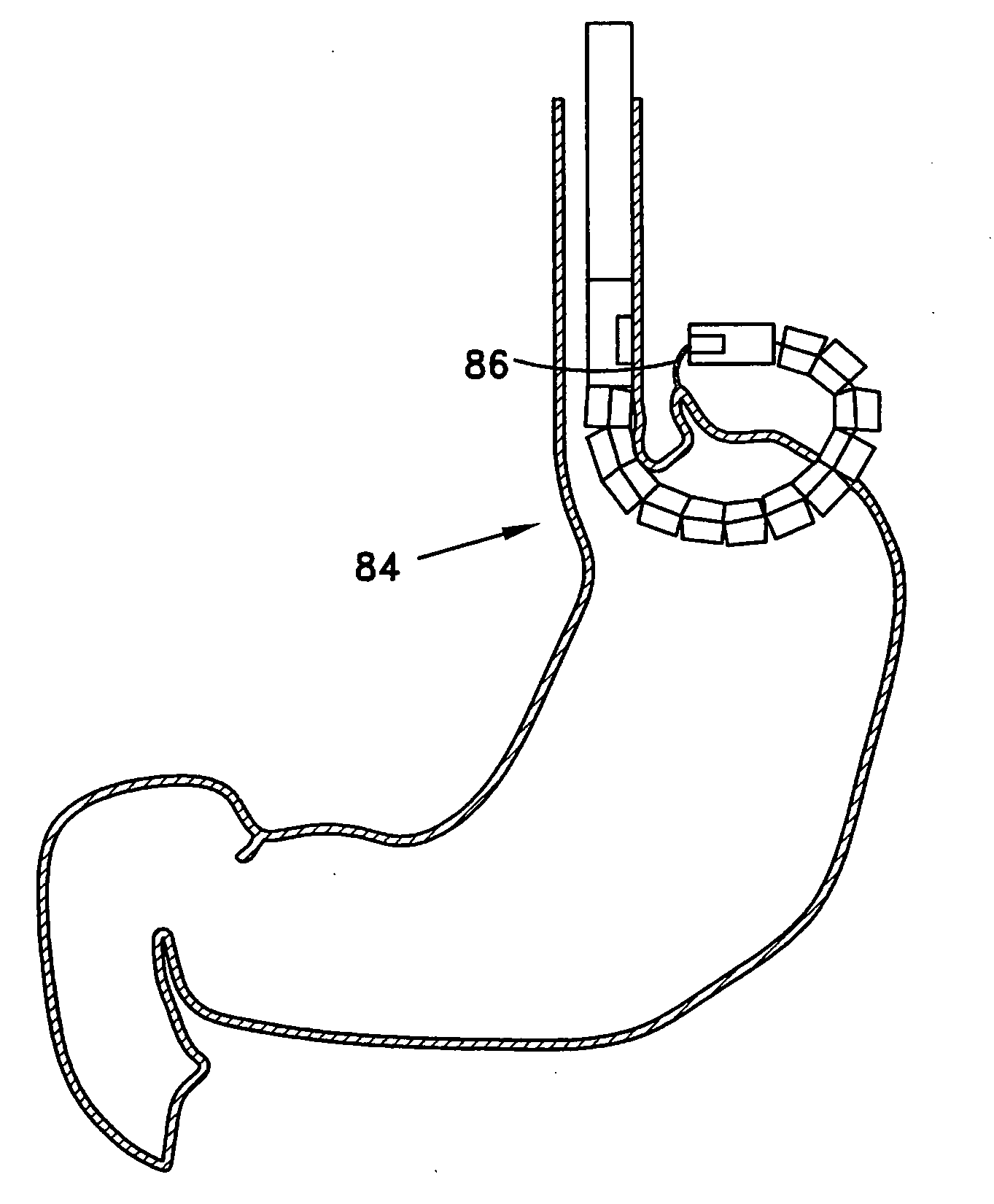
Transgastric method for carrying out a partial fundoplication
The invention is a transgastric method for the endoscopic partial fundoplication for the treatment of gastroesophageal reflux disease (GERD). The method makes use of an articulated endoscope, which is introduced through the mouth and esophagus into the stomach of the patient. A cutting tool is introduced through the working channel of the endoscope to cut a hole in the wall of the stomach. The endoscope is pushed through the hole and a grabbing tool is introduced through the working channel and used to grab the tissue at a location on the outer surface of the stomach and move the grabbed tissue close to the esophagus. The grabbed tissue is then stapled to the esophagus using a stapling device that is an integral part of the endoscope. If desired, after stapling the endoscope can be rotated one or more times and the procedure repeated. After the selected portions of the stomach wall have been attached to the esophagus, the endoscope is withdrawn from the body of the patient and the hole in the stomach wall is closed, preferably by means of a dedicated endoscopic stapling device especially designed for the task of closing holes in tissue.
Owner:MEDIGUS LTD
Endoscopic instruments
InactiveUS7156857B2Prevent gastroesophageal reflux effectivelyEasy to operateSuture equipmentsSurgical needlesEndoscopyEsophageal reflux disease
Owner:OLYMPUS CORP +1
Devices and methods for promoting the formation of blood clots in esophageal varices
InactiveUS20070167971A1Inhibit injectionPromote formationBalloon catheterSurgeryThrombusSilicon dioxide
A device for promoting the clotting of blood in body cavities includes a flexible body portion; an expandable member located on the flexible body portion; and a blood clotting material attached to the expandable member. When used, insertion of at least a portion of the blood clotting material into the body cavity causes at least a portion of the blood clotting material to contact blood emanating from a bleed site. Methods of providing therapies to tube-shaped organs include the steps of providing suitable devices having expansion capabilities, positioning the devices at the appropriate bleed sites, and expanding the devices to cause blood clotting materials to contact the bleed sites. Materials that may be used as the blood clotting material include zeolites, molecular sieve materials, diatomaceous earth, clay, silica-based materials, oxidized cellulose, carboxymethyl cellulose, bioactive glass, biological hemostats, chitosan, and combinations of the foregoing.
Owner:TELEFLEX LIFE SCI LTD
Composition and vaccine for treating lung cancer
InactiveUS20160168227A1High in proteinEffectively stimulating the (adaptive) immune systemOrganic active ingredientsTumor rejection antigen precursorsAntigenDisease
The present invention relates to a composition comprising at least one mRNA encoding a combination of antigens capable of eliciting an (adaptive) immune response in a mammal, wherein the antigens are selected from the group consisting of 5T4 (Trophoblast glycoprotein, TPBG), Survivin (Baculoviral TAP repeat-containing protein 5; BIRC5), NY-ESO-1 (New York esophageal squamous cell carcinoma 1, CTAG1B), MAGE-C1 (Melanoma antigen family C1), MAGE-C2 (Melanoma antigen family C2), and MUC1 (Mucin 1). The invention furthermore relates to a vaccine comprising at least one mRNA encoding such a combination of antigens, and to the use of said composition (for the preparation of a vaccine) and / or of the vaccine for eliciting an (adaptive) immune response for the treatment of lung cancer, preferably of non-small cell lung cancer (NSCLC), and diseases or disorders related thereto. Finally, the invention relates to kits, particularly to kits of parts, containing the composition and / or the vaccine.
Owner:CUREVAC AG
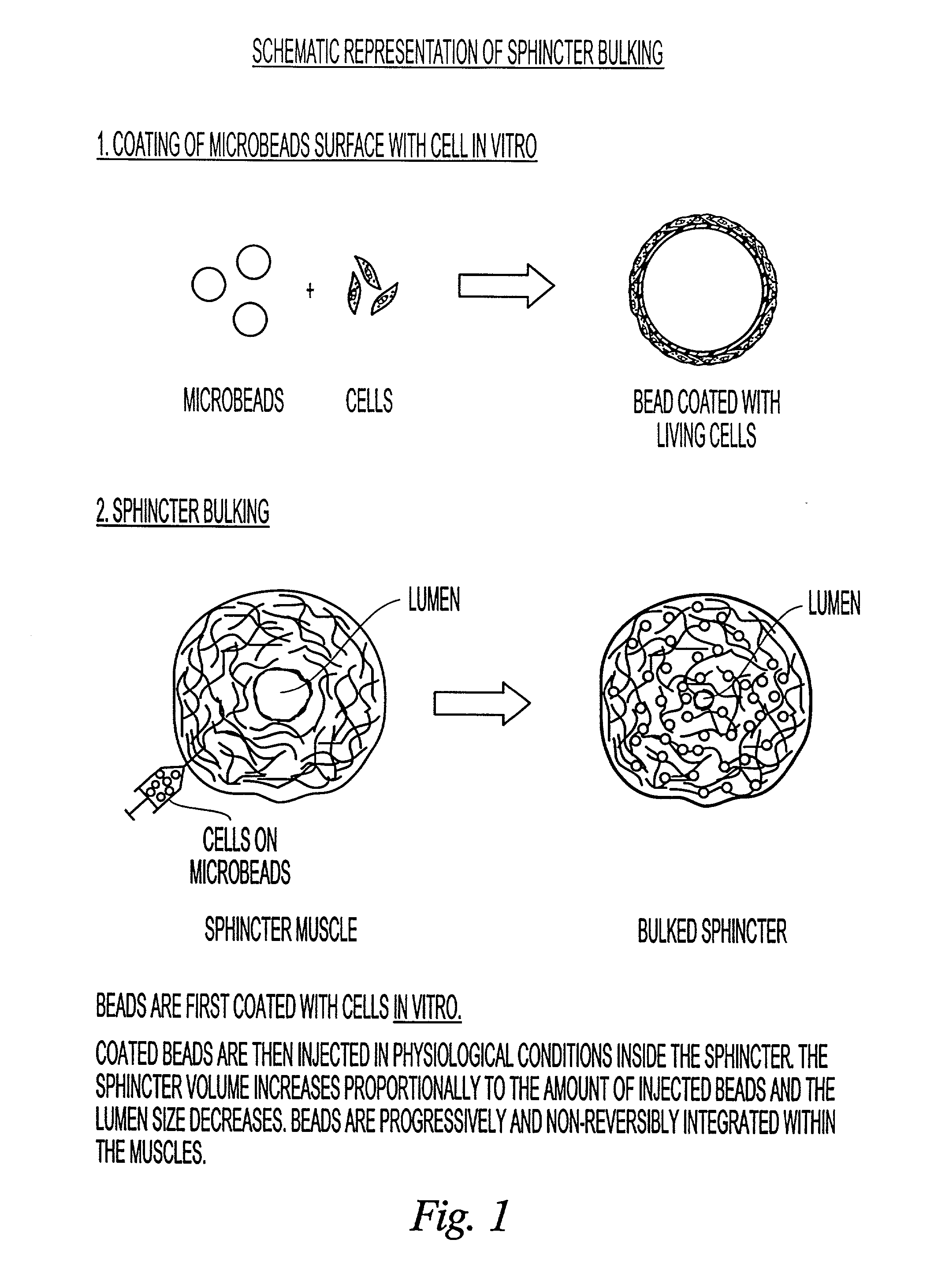
Implantable particles for tissue bulking and the treatment of gastroesophageal reflux disease, urinary incontinence, and skin wrinkles
InactiveUS20020068089A1Faster and safe and more comfortable recoveryPowder deliveryBiocideCell adhesionBiocompatibility
Owner:BIOSPHERE MEDICAL INC
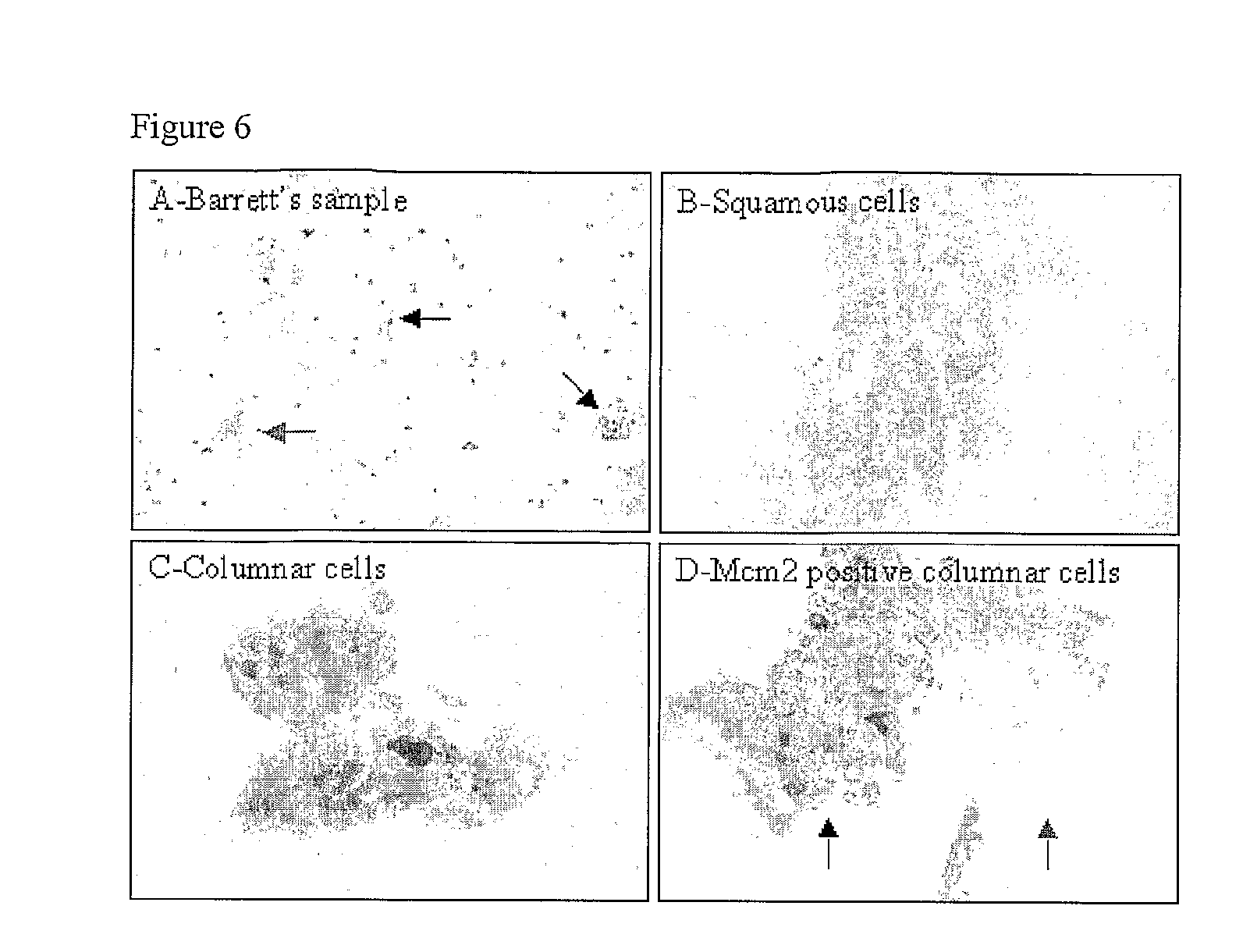
Diagnostic Kits and Methods for Oesophageal Abnormalities
InactiveUS20090286237A1Great proportionIncrease valueMicrobiological testing/measurementSurgical needlesEsophago-esophagealOesophagram
The invention relates to kits and methods for aiding the diagnosis of Barrett's oesophagus or Barrett's associated dysplasia. Preferred is a method comprising assaying cells from the surface of a subject's oesophagus for a non-squamous cellular marker, wherein detection of such a marker indicates increased likelihood of the presence of Barrett's or Barrett's associated dysplasia, preferably wherein said sample of cells is not directed to a particular site within the oesophagus. The invention also encompasses a method comprising sampling the cellular surface of the oesophagus of said subject. The invention also relates to a kit comprising a swallowable device comprising abrasive material capable of collecting cells from the surface of the oesophagus, together with printed instructions for its use in detection of Barrett's oesophagus or Barrett's associated dysplasia. Preferably said device comprises a capsule sponge.
Owner:MEDICAL RESEARCH COUNCIL +1
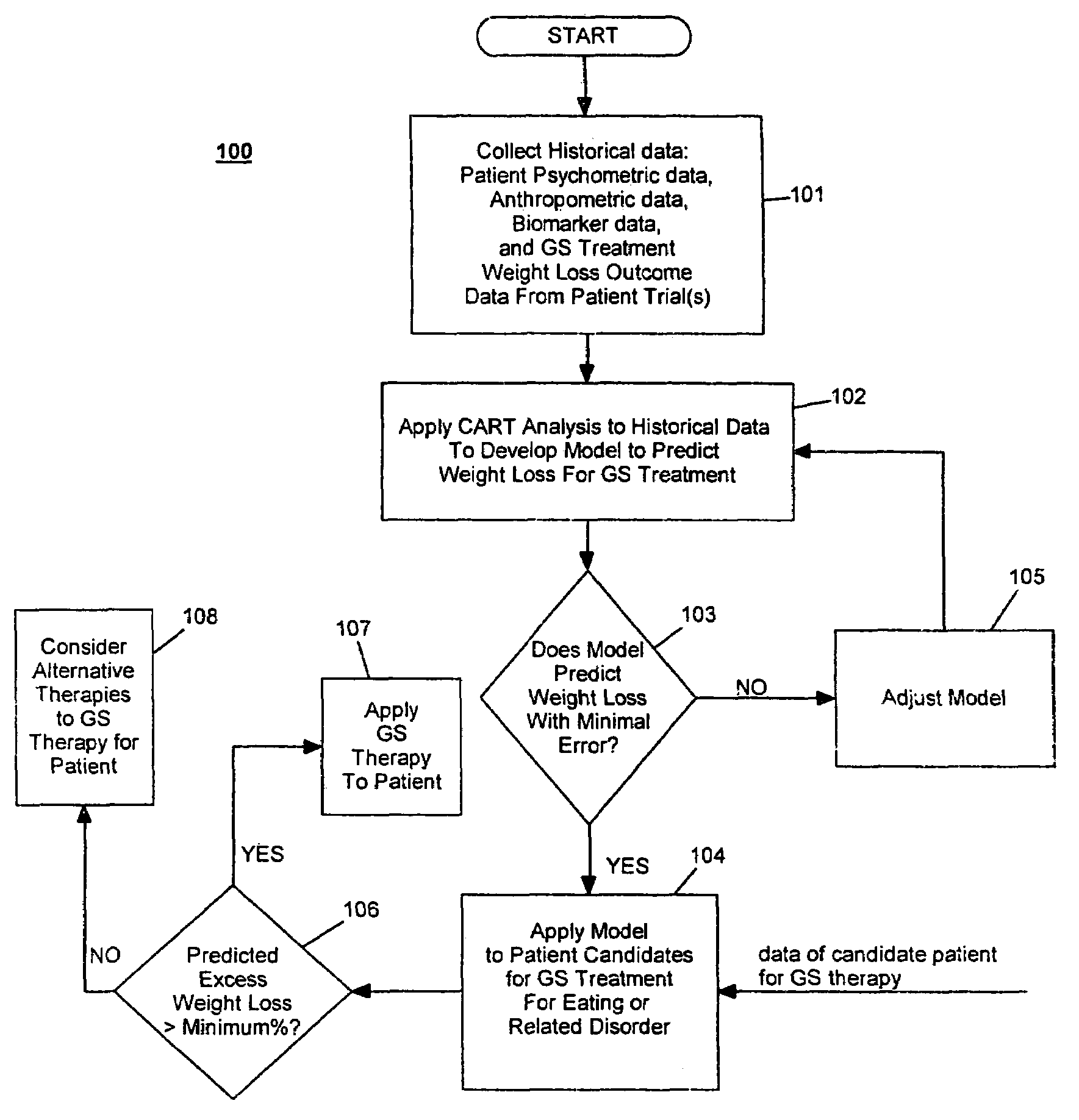
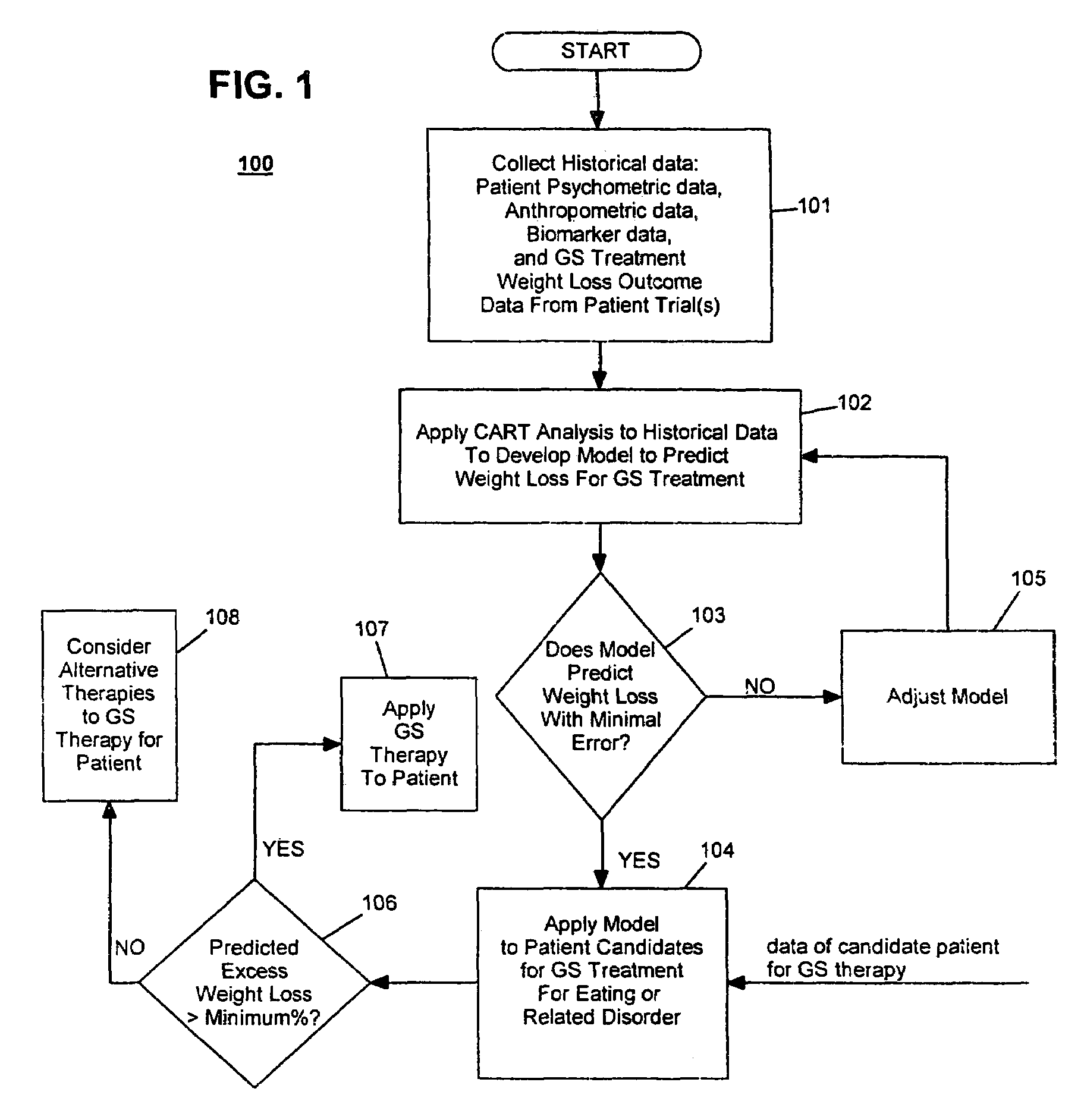
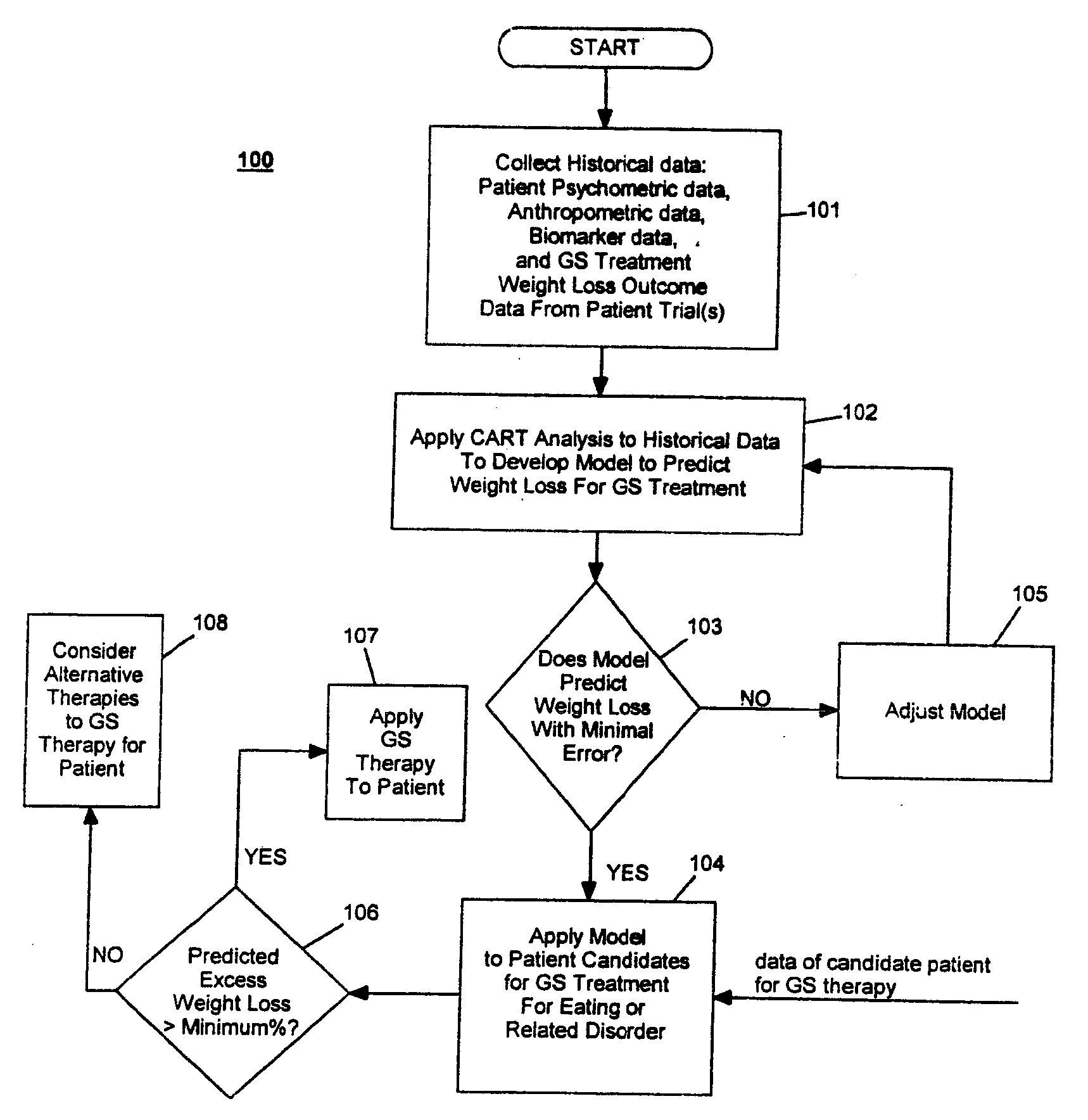
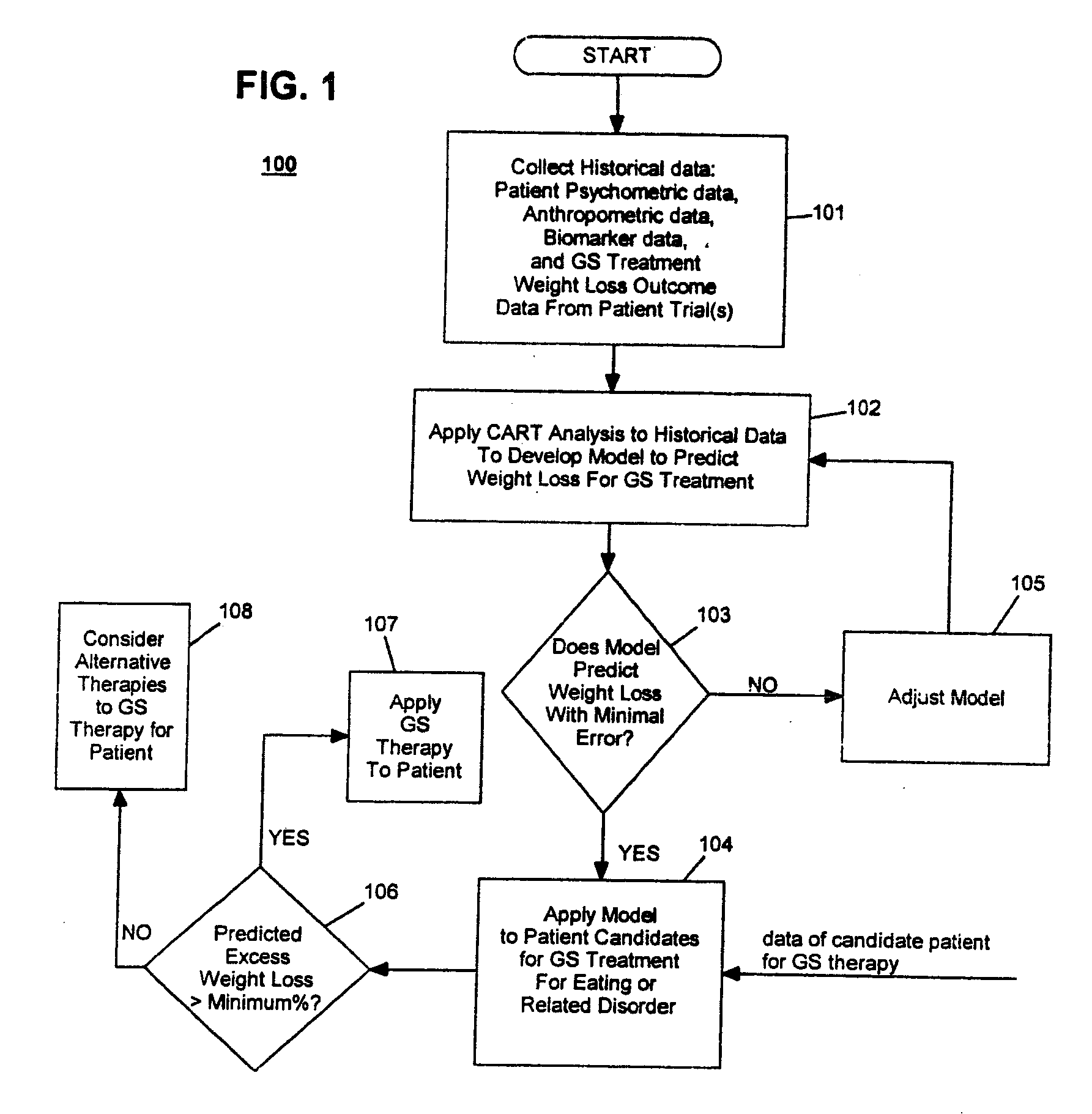
Method for screening and treating patients at risk of medical disorders
InactiveUS7194301B2Timely useAccurate predictionMedical simulationMedical data miningMedical disorderTreatment modality
Owner:TRANSNEURONIX INC
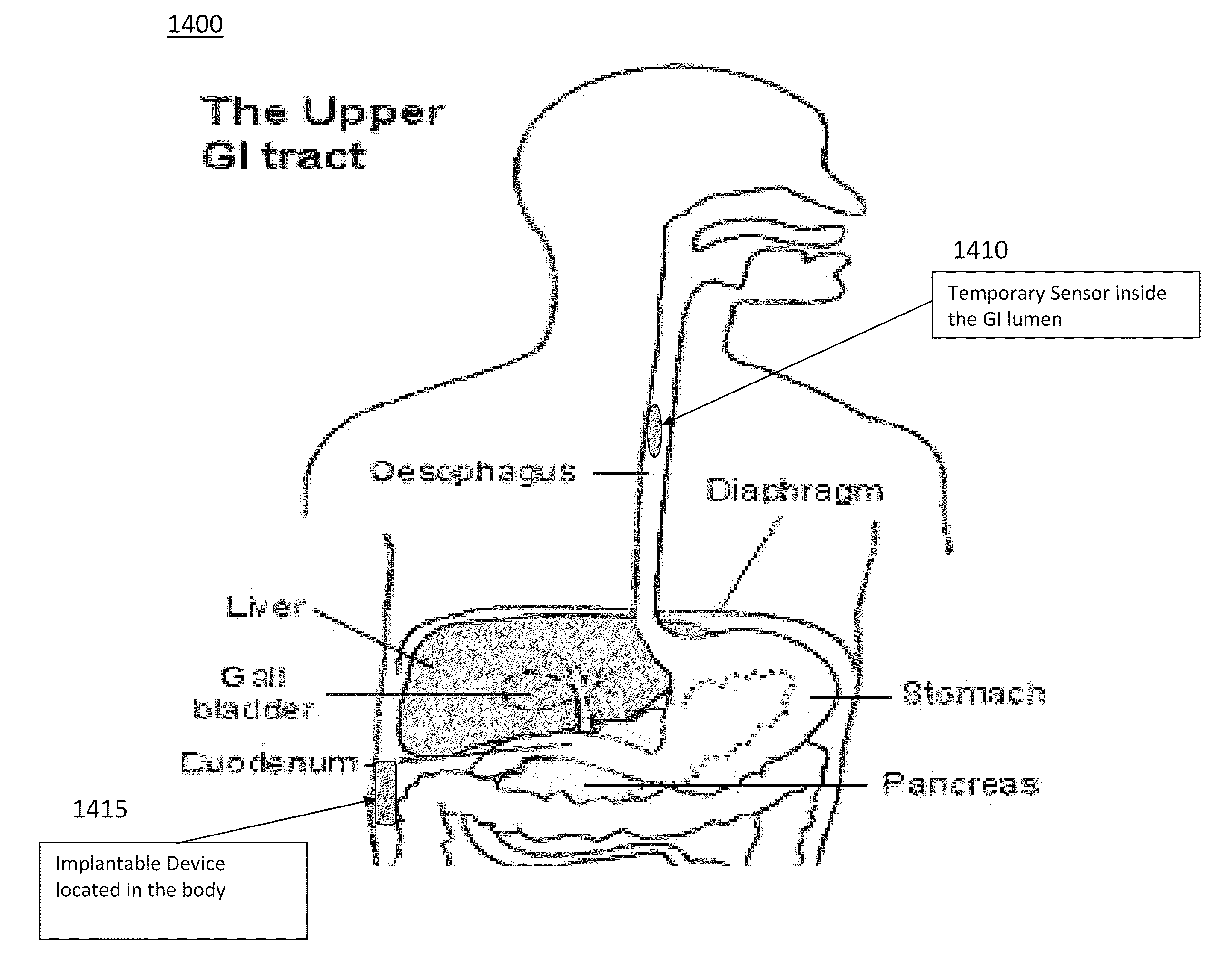
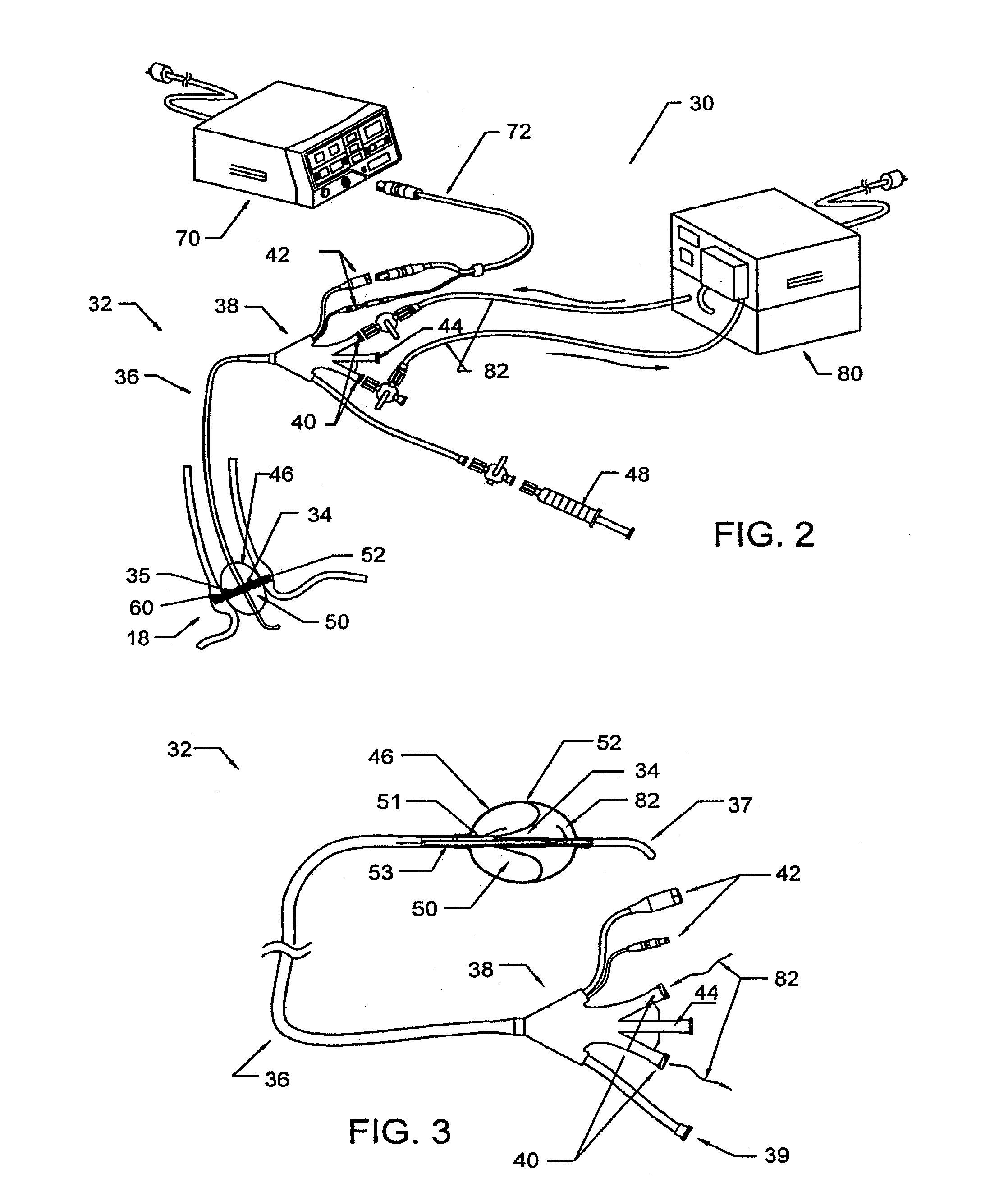
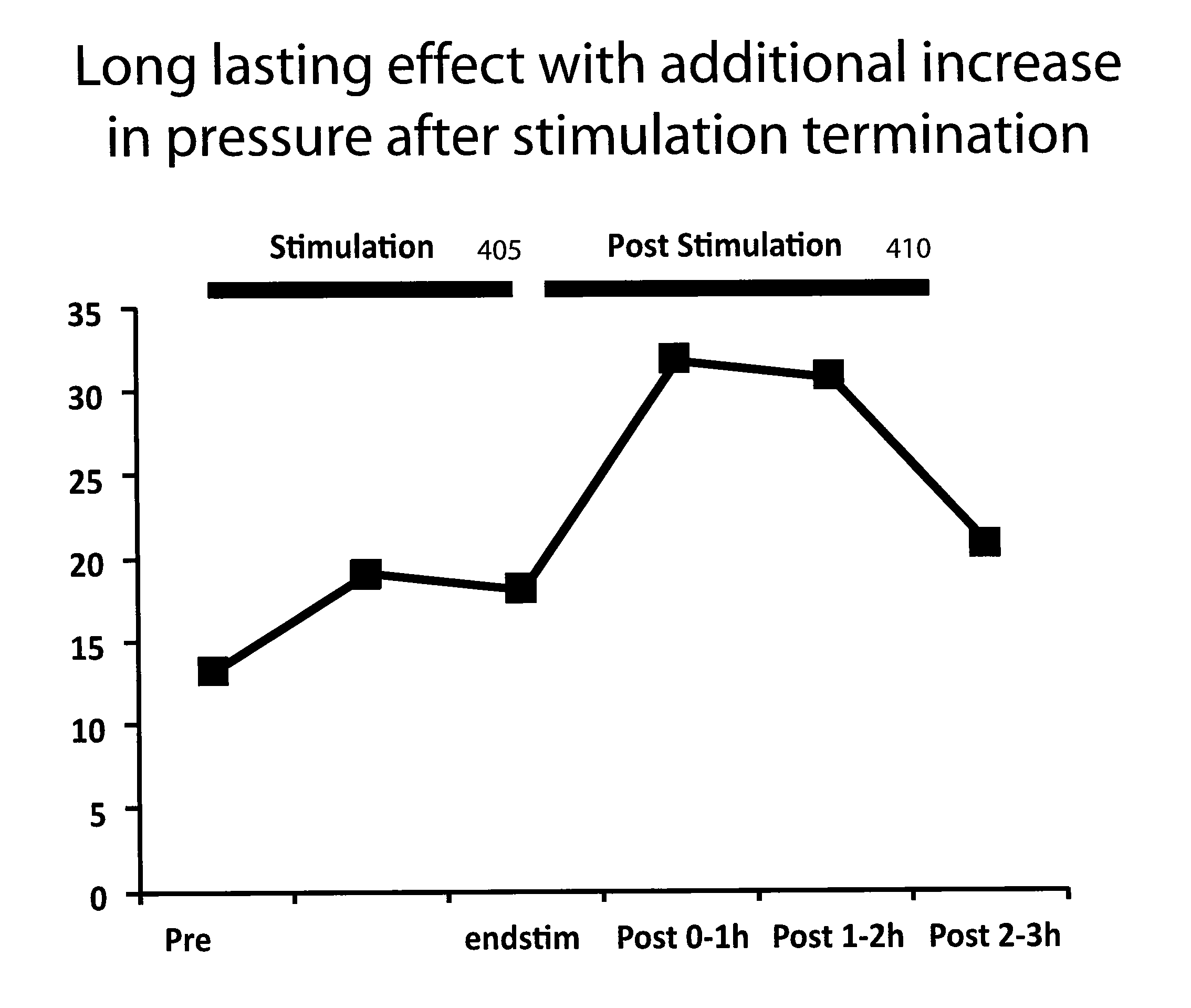
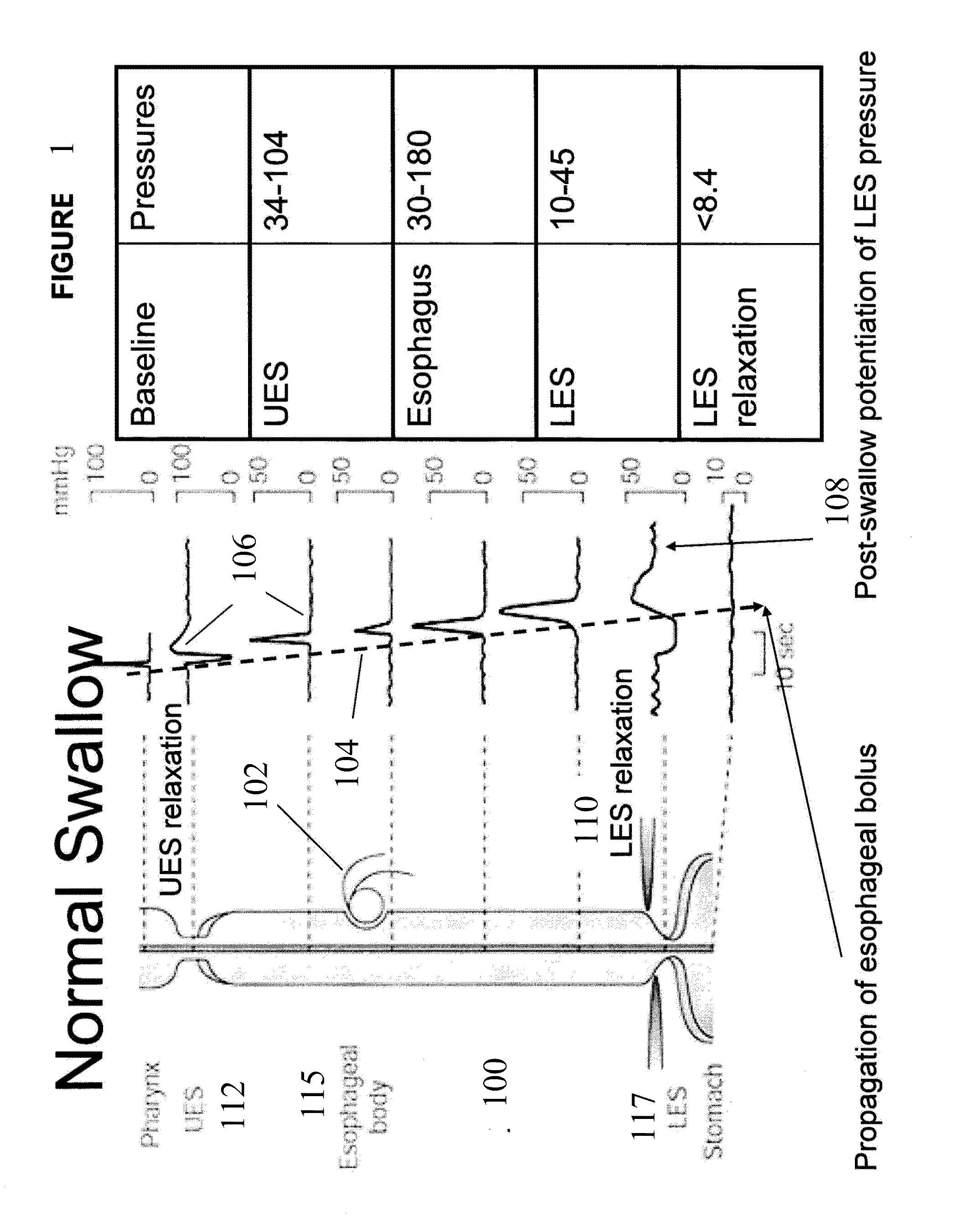
Device and implantation system for electrical stimulation of biological systems
ActiveUS8447403B2Less energyFunction increaseImplantable neurostimulatorsDiagnostic recording/measuringLower esophagusPhysical medicine and rehabilitation
The present specification discloses devices and methodologies for the treatment of nocturnal GERD. Individuals with nocturnal GERD may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
Porcine circovirus and Helicobacter combination vaccines and methods of use
InactiveUS20060029617A1Viral antigen ingredientsMicrobiological testing/measurementDiseasePorcine Circoviruses
The present invention is based on the discovery of novel species of the genus Helicobacter that are associated with gastro-esophageal ulceration in pigs. In particular, a novel species, H. cerdo, has been used as a source of antigenic material for the development of vaccine for the treatment of the gastro-esophageal disorders. Most advantageously, the novel Helicobacter and the porcine circoviruses associated with PMWS in pigs are useful for providing combination vaccines whereby immunogens derived from both types of pathogens may be codelivered to the target animal to stimulate the generation of protective antibodies and immunity. The invention, therefore, provides vaccines that are useful for the tratment of gastro-esophageal ulceration and PMWS in porcines. The present invention includes, therefore, multivalent immunogenic compositions and vaccines, multivaccine kits, and combined immunization or vaccination methods which make it possible to use such combined immunization or vaccination programmes.
Owner:MERIAL LTD
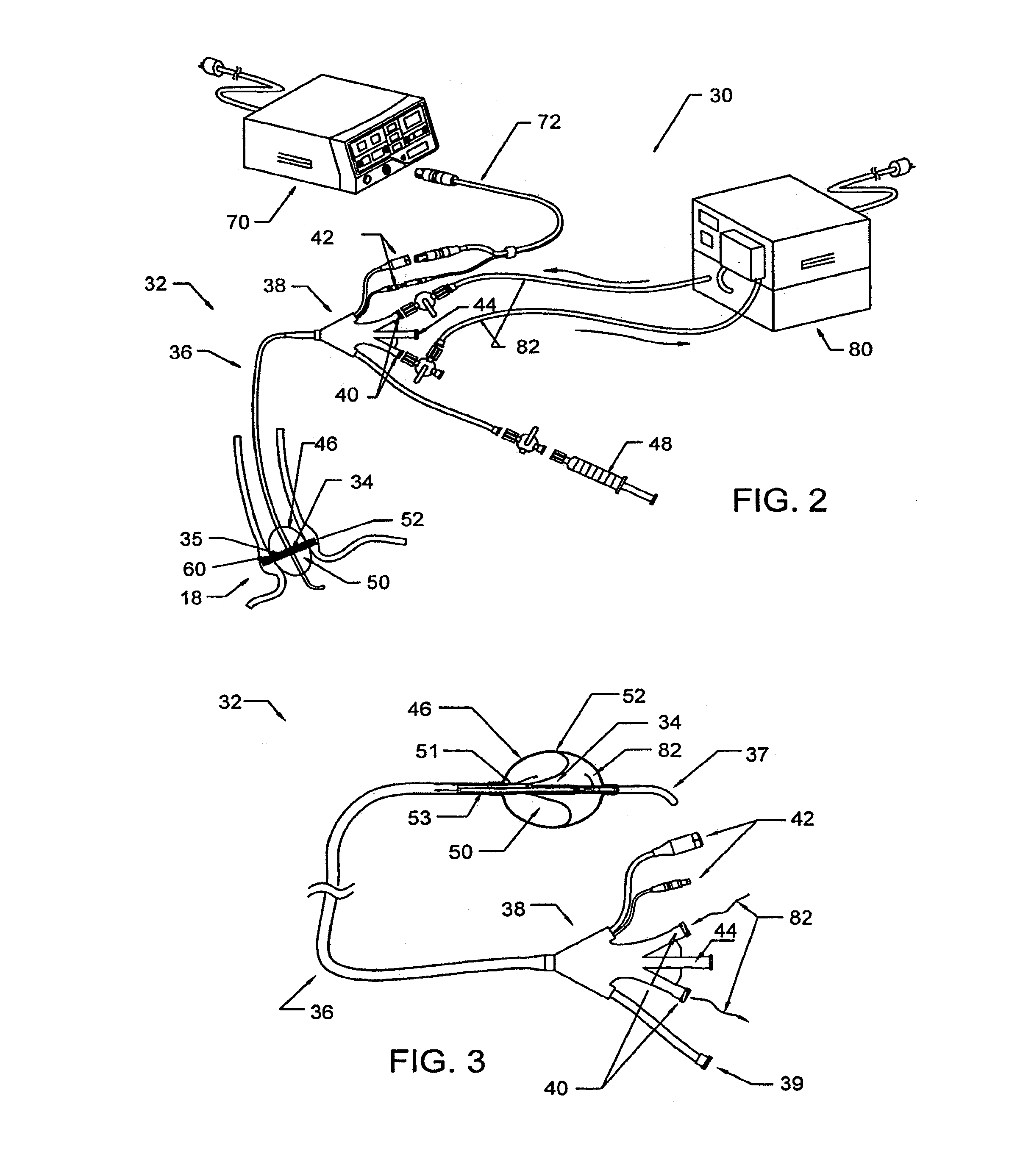
Method and apparatus employing ultrasound energy to treat body sphincters
InactiveUS20130072928A1Prevent and delay openingDecreased tissue complianceUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyAcoustic energySphincter
Methods and apparatus for treating gastroesophageal reflex and other luminal conditions provide for delivering acoustic energy to a body lumen to remodel tissue surrounding the body lumen. In the case of treating GERD, a catheter carrying an ultrasonic or other vibrational transducer is introduced to the lower esophageal sphincter, and acoustic energy is delivered to the sphincter in order to tighten or bulk the sphincter such that reflex is reduced.
Owner:RECOR MEDICAL INC
Method for Screening and Treating Patients at Risk of Medical Disorders
InactiveUS20070244375A1Reduced health costAccurate predictionElectrotherapySurgeryMorbidly obeseProper treatment
Method for screening patients to predict which patients at risk of a medical disorder, such as morbid obesity, gastrointestinal problems, or gastroesophageal problems, will be responders, and conversely, which patients will not, to achieve a favorable outcome from therapy for that disorder. This method supports an intervention strategy for patients having weight or gastrointestinal problems that will cut health costs. It enables patients and care-givers alike to more efficiently use their time, efforts and resources by enabling an early selection of an appropriate treatment modality for a given patient. Its application also extends to other implantable medical devices and therapies using them.
Owner:MEDTRONIC TRANSNEURONIX
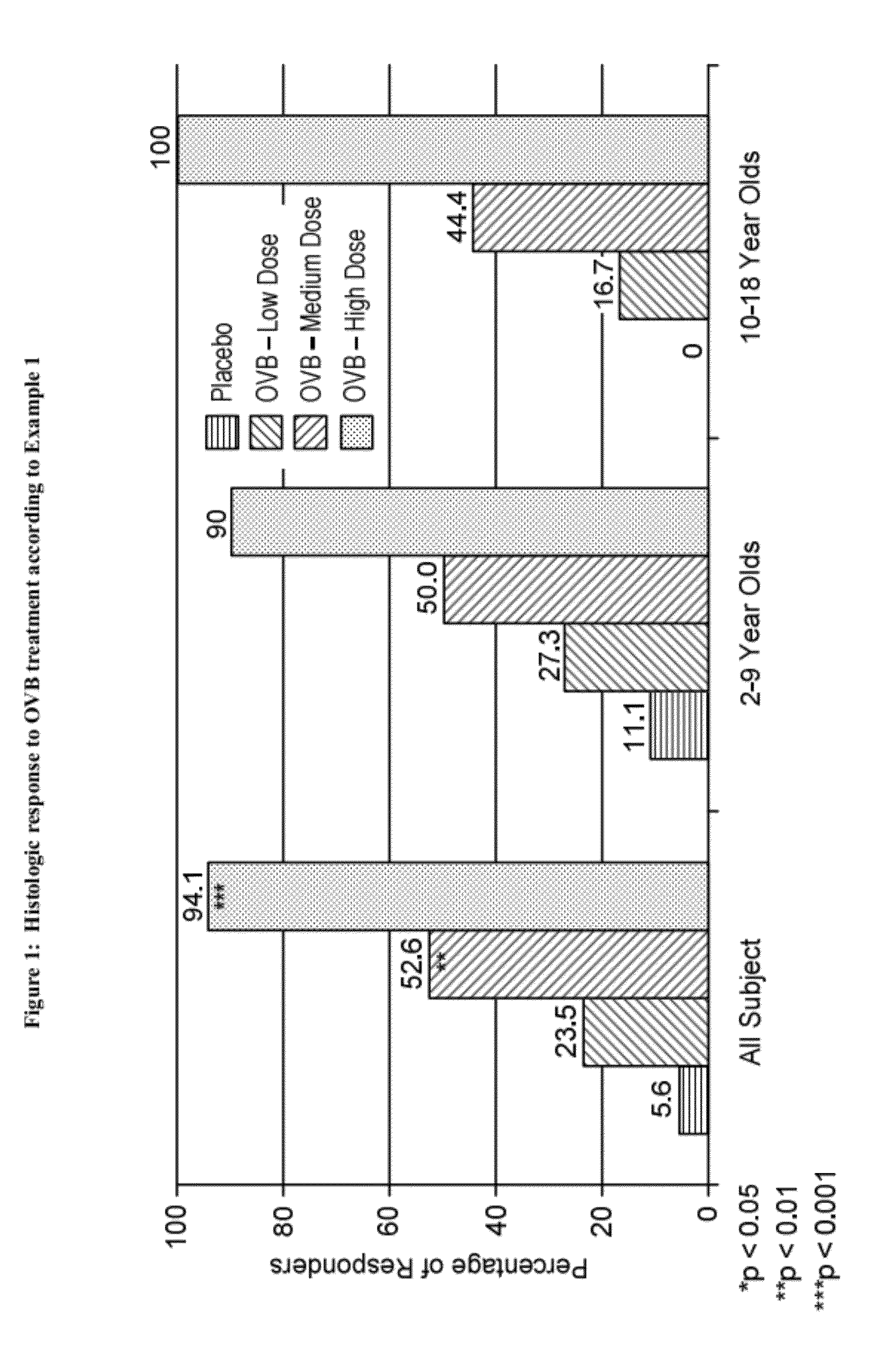
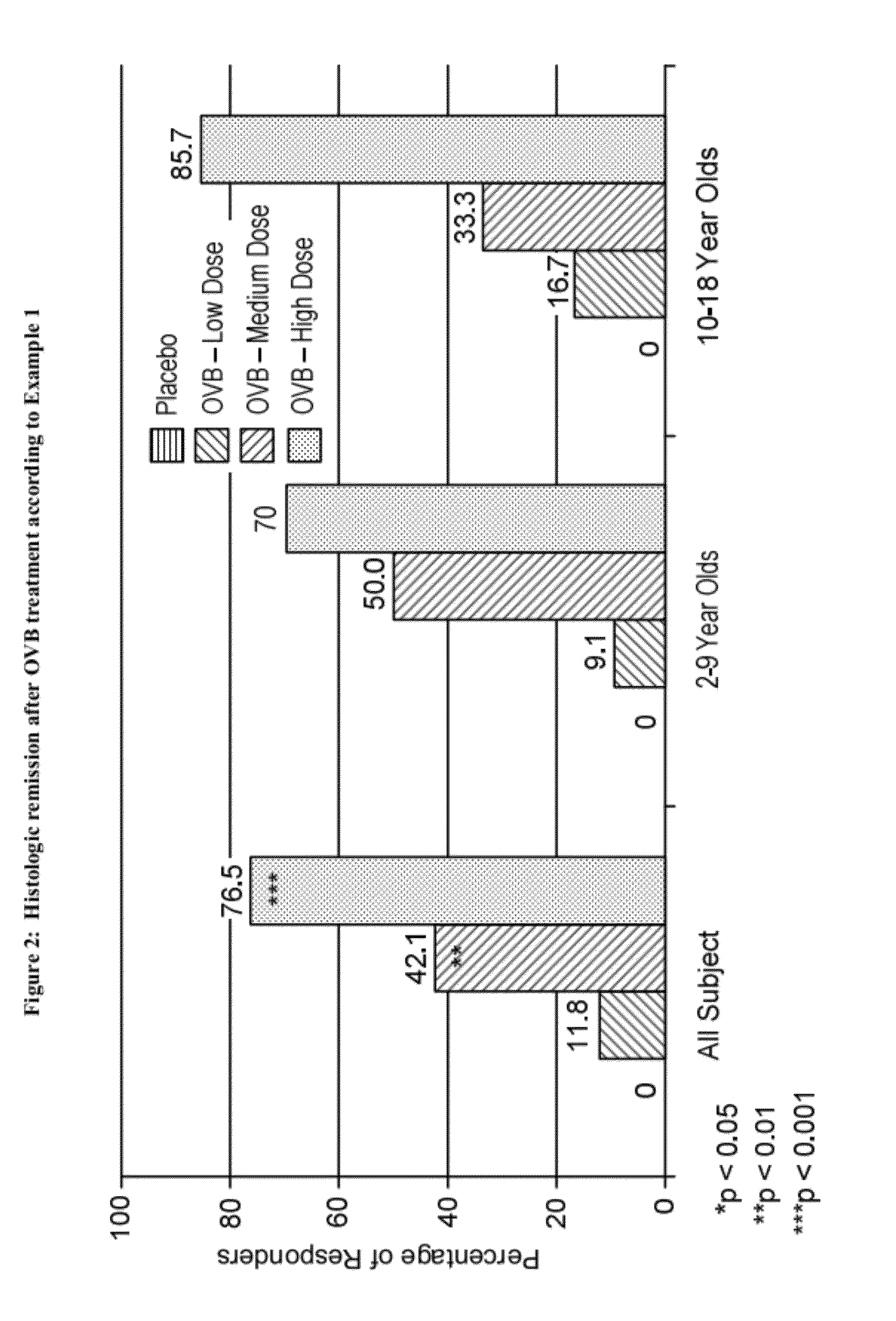
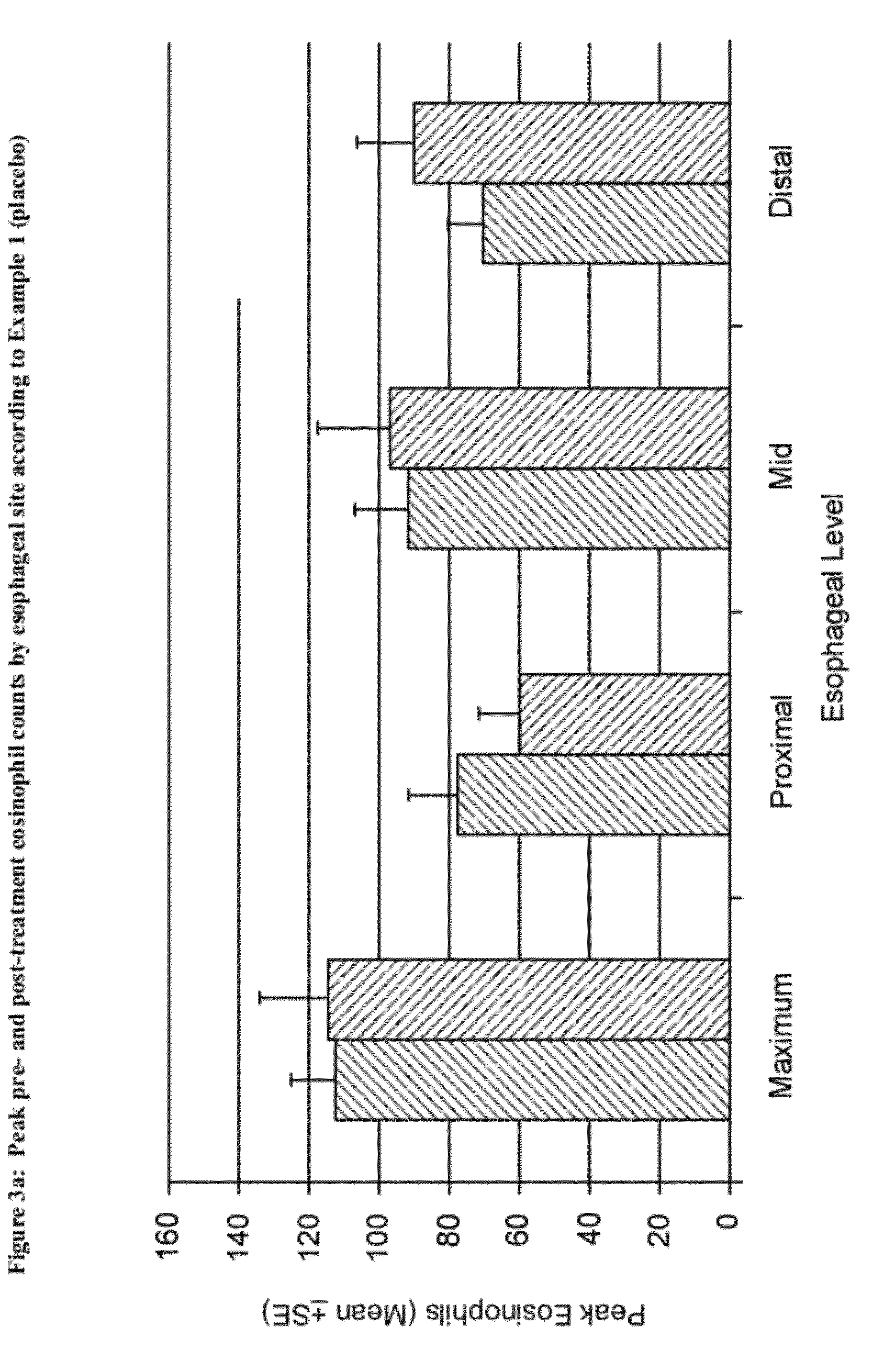
Methods of treatment for esophageal inflammation
Provided herein are methods for treating gastrointestinal inflammation, for example, esophageal inflammation, or reduction of eosinophilic infiltration of the esophagus and / or reducing local and systemic exposure and / or side effects resulting therefrom. Provided herein are methods for diagnosing gastrointestinal inflammation, for example, esophageal inflammation, or reduction of eosinophilic infiltration of the esophagus. Also provided herein are methods for inducing remission of eosinophilic infiltration of the esophagus
Owner:MERITAGE PHARMA INC
Intraluminal methods of ablating nerve tissue
InactiveUS20130131668A1Prevent and delay openingReduce complianceUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyAcoustic energySphincter
Methods and apparatus for treating gastroesophageal reflex and other luminal conditions provide for delivering acoustic energy to a body lumen to remodel tissue surrounding the body lumen. In the case of treating GERD, a catheter carrying an ultrasonic or other vibrational transducer is introduced to the lower esophageal sphincter, and acoustic energy is delivered to the sphincter in order to tighten or bulk the sphincter such that reflex is reduced.
Owner:RECOR MEDICAL INC
Device and implantation system for electrical stimulation of biological systems
ActiveUS20110307028A1Not induce dysphagiaIncrease pressureImplantable neurostimulatorsDiagnostic recording/measuringLower esophagusPhysical medicine and rehabilitation
The present specification discloses devices and methodologies for the treatment of transient lower esophageal sphincter relaxations (tLESRs). Individuals with tLESRs may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
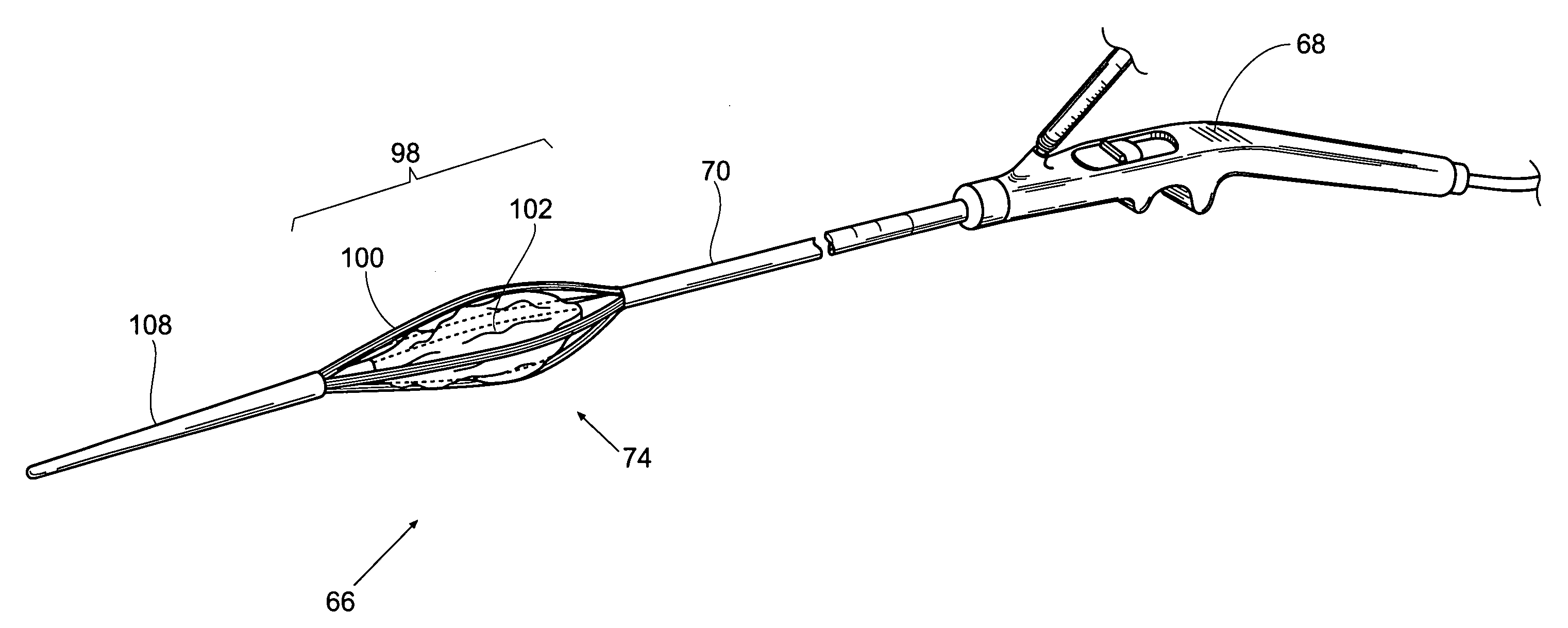
Esophageal balloon catheter with visual marker
ActiveUS20060224114A1Easily select and steerPrecise positioningStentsBalloon catheterEndoscopeVisual perception
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
High specific gravity intragastric device
The teachings are directed to an intragastric device comprising a flexible and expandable bladder having a predetermined shape upon expansion for contacting the antrum of the stomach of a subject. The device is designed to avoid passage of any part of the device beyond the pylorus and lower esophageal sphincter while the bladder is expanded during use. In these embodiments, the bladder can contain a high specific gravity material when expanded; wherein, the high specific gravity material contributes to an in vivo specific gravity of the device that ranges from about 1.2 g / ml to about 2.1 g / ml and functions to direct the device to the pyloric antrum of the subject during use of the device. Moreover, these embodiments can include a filling material comprising a biocompatible fluid component and a hydrogel component to make the device substantially leakproof and contribute to the in vivo specific gravity of the device.
Owner:HANCOCK JOHN
Systems and methods for treating obesity and other gastrointestinal conditions
InactiveUS20090118699A1Reduce morbidityIncrease satietyElectrotherapyPeptide/protein ingredientsReflexREFLEX DECREASE
Owner:RESPIRATORY DIAGNOSTICS
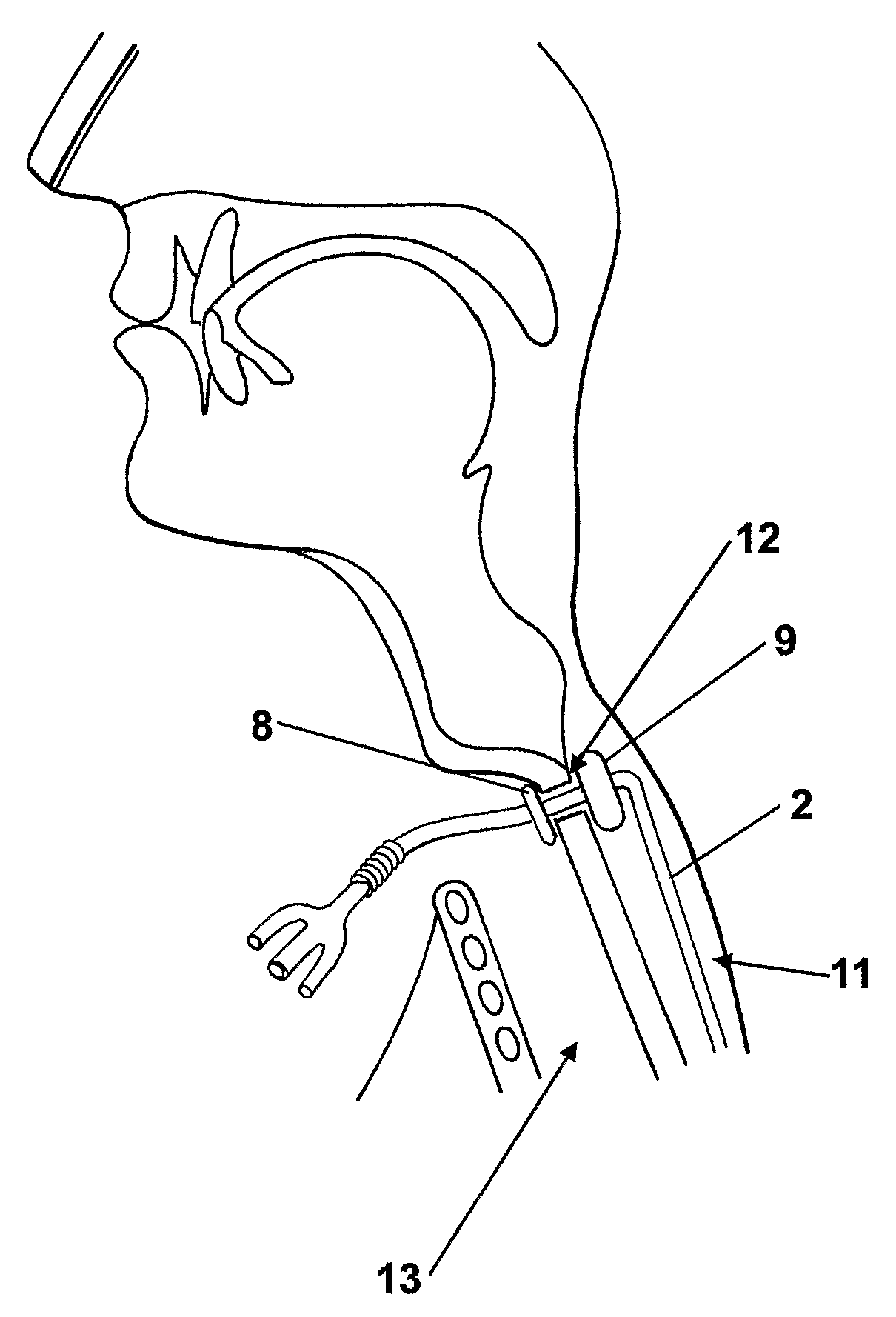
Catheter
InactiveUS20090171280A1Comfortable to wearFew infectionRespiratorsStentsTracheo-oesophageal fistulaCatheter device
A tracheo-oesophageal fistula catheter (1) comprises an elongate conduit (2) having a proximal and a distal end, the conduit (2) comprising a plurality of lumens commencing adjacent to the proximal end; means (8, 9) for substantially sealing a tracheo-oesophageal fistula, the means being attached to the conduit (2) adjacent to the proximal end and comprising two elements (8, 9) to be placed on either side of the fistula; and at least one expandable balloon (7) attached to the conduit (2) adjacent to the distal end, and in fluid connection with at least one of said lumens; wherein at least one lumen is a gastric lumen, the gastric lumen extending along the full length of the conduit and having an outlet (6) at the distal end of the conduit (2), and wherein at least one element of the means for sealing the fistula comprises an expandable balloon in fluid connection with at least one of said lumens.
Owner:CITY HOSPITALS SUNDERLAND NHS TRUST
Device and Implantation System for Electrical Stimulation of Biological Systems
ActiveUS20140228911A1Not induce dysphagiaIncrease pressureImplantable neurostimulatorsCatheterLower esophagusSphincter
The present specification discloses devices and methodologies for the treatment of transient lower esophageal sphincter relaxations (tLESRs). Individuals with tLESRs may be treated by implanting a stimulation device within the patient's lower esophageal sphincter and applying electrical stimulation to the patient's lower esophageal sphincter, in accordance with certain predefined protocols. The presently disclosed devices have a simplified design because they do not require sensing systems capable of sensing when a person is engaged in a wet swallow and have improved energy storage requirements.
Owner:PARAS HLDG LLC
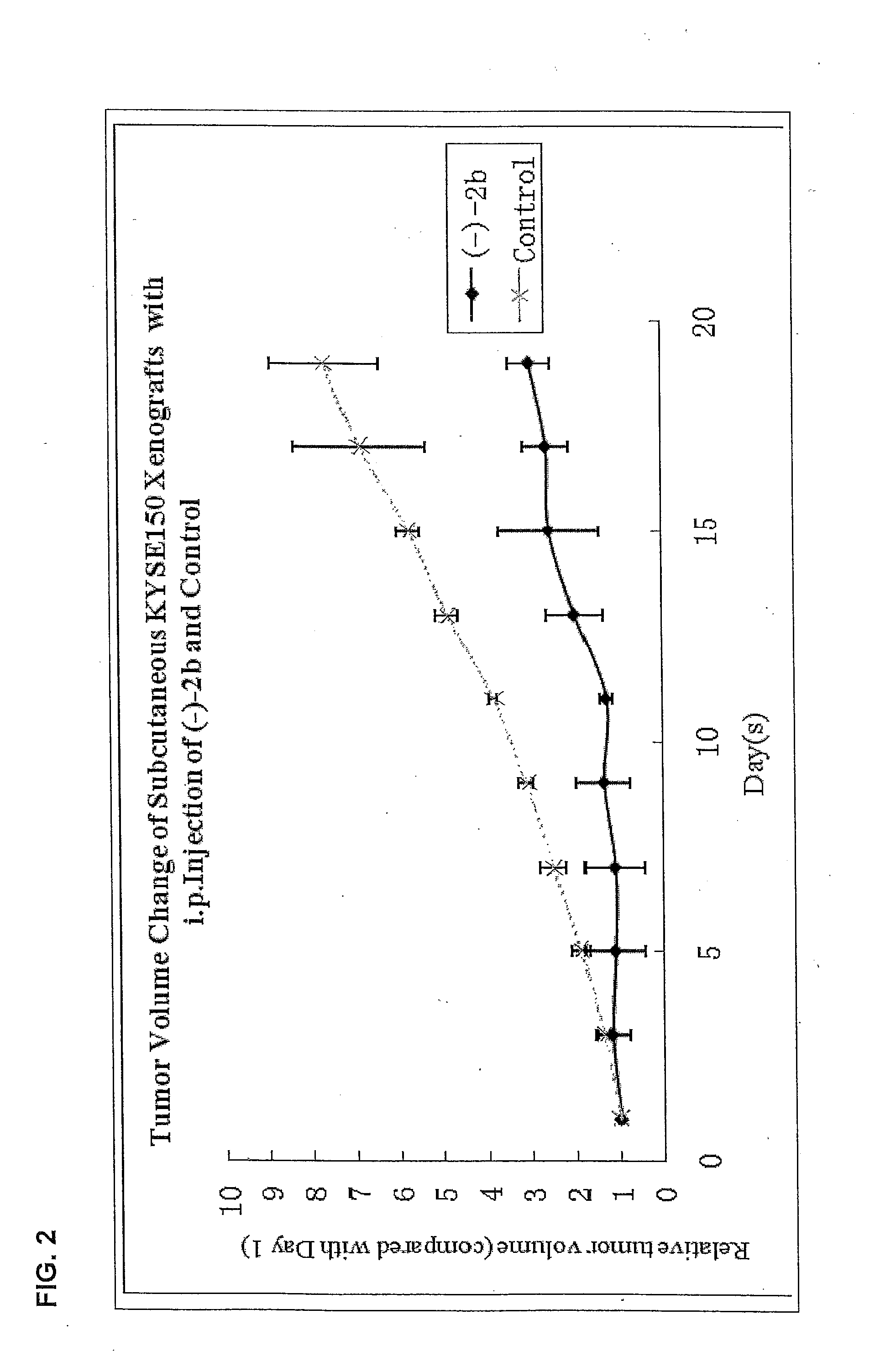
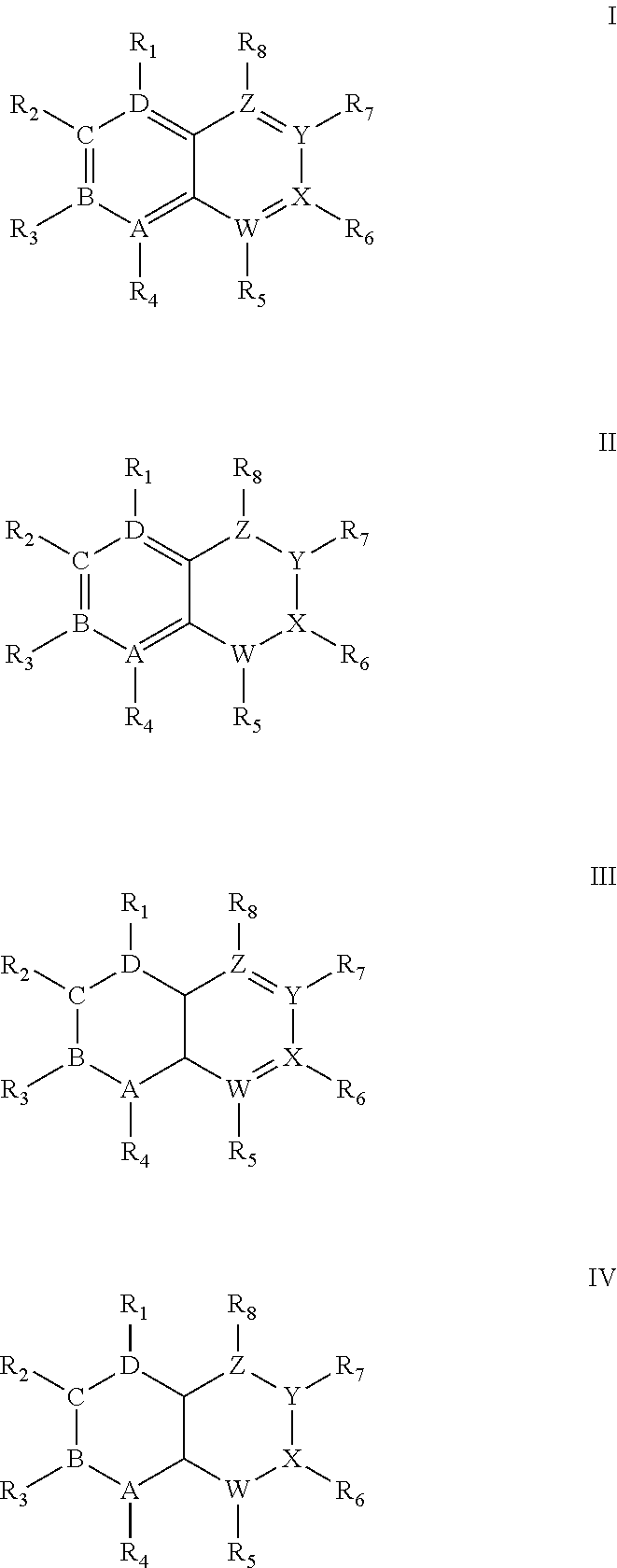
Quinoline derivatives as Anti-cancer agents
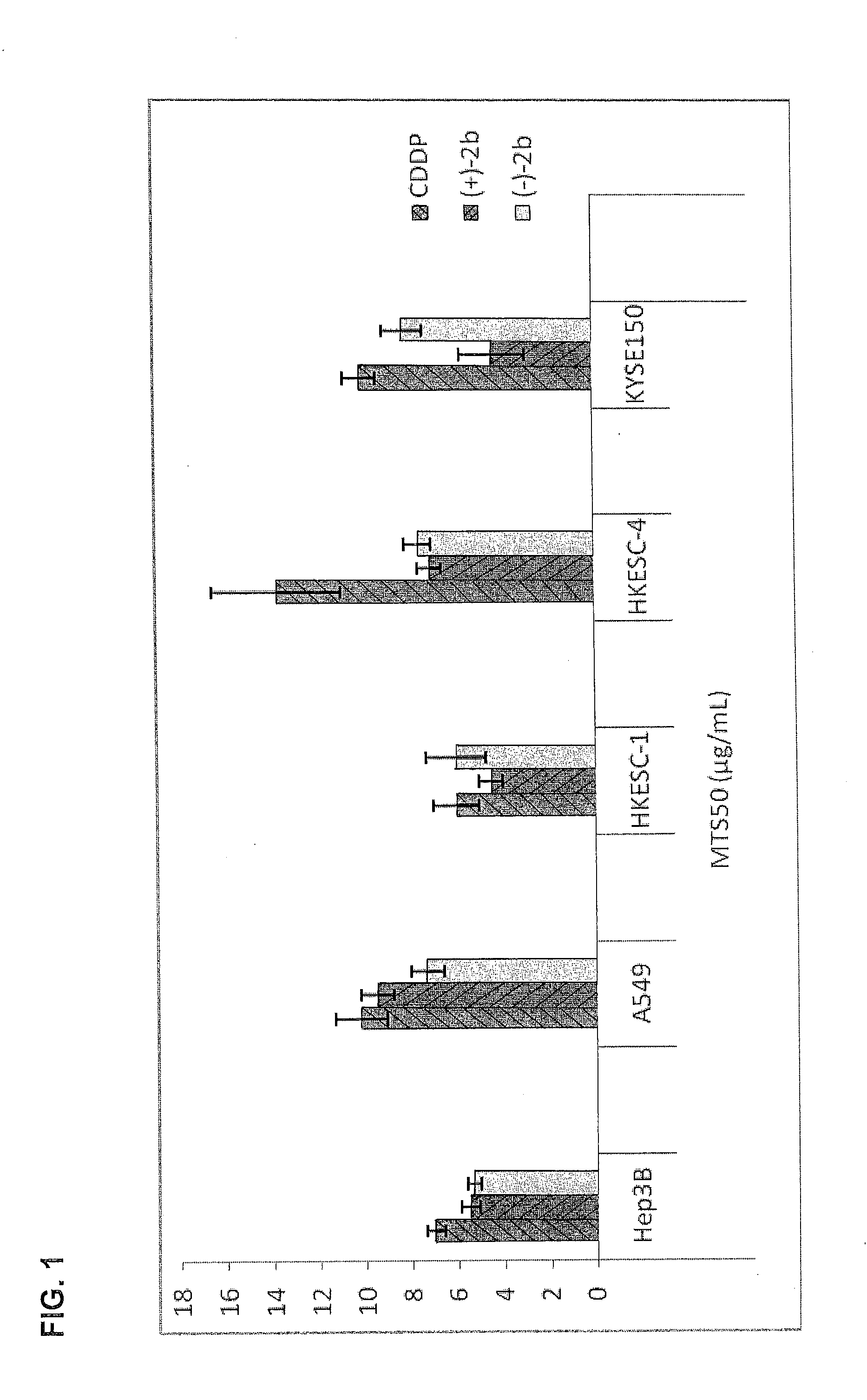
Quinoline derivatives showing anticancer activities against cancer cell lines of hepatocellular carcinoma (Hep3B), lung carcinoma (A549), esophageal squamous cell carcinoma (HKESC-1, HKESC-4 and KYSE150). The quinoline derivatives have a backbone structure of the following formulas:
Owner:THE HONG KONG POLYTECHNIC UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com