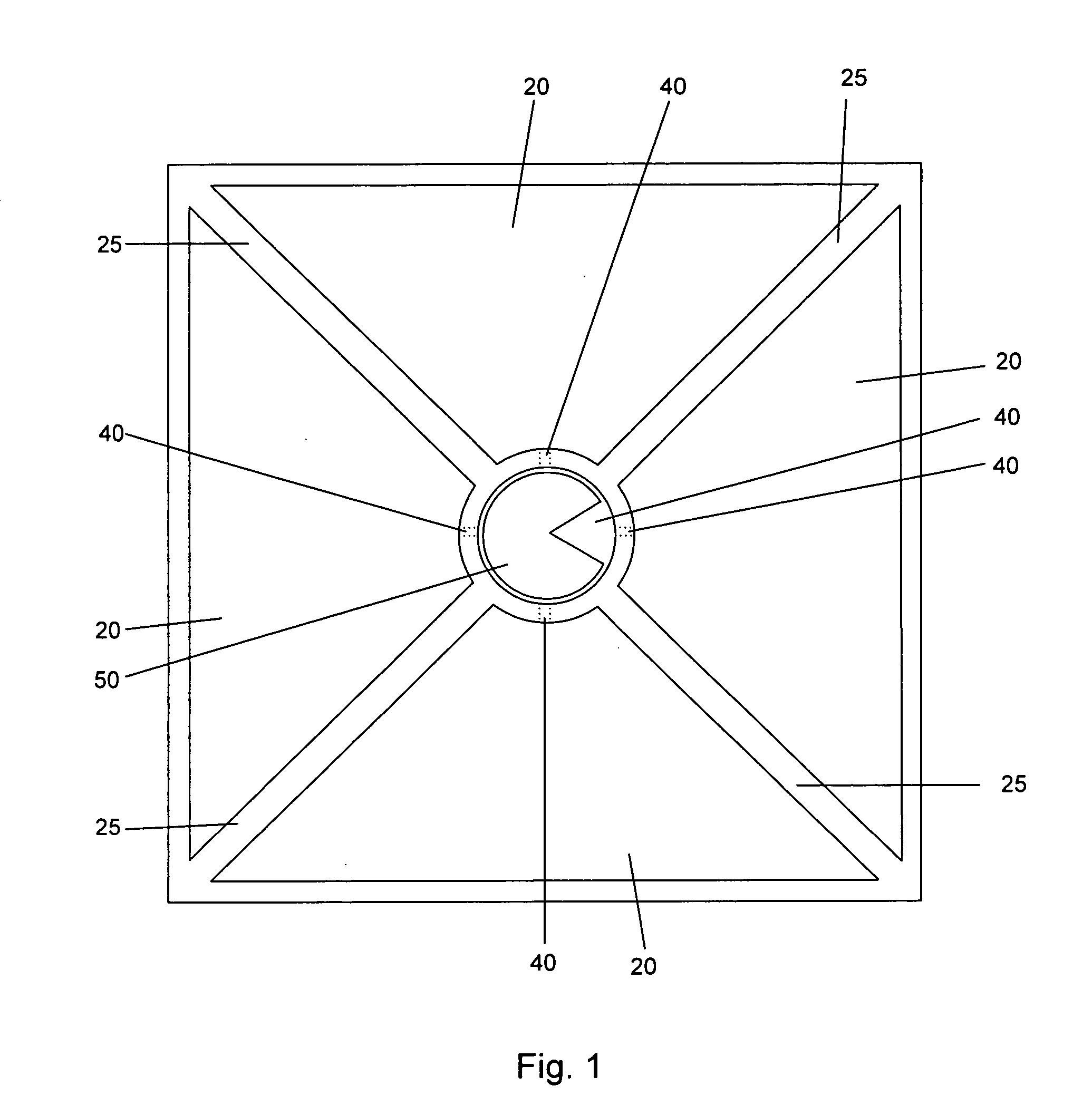
PCR method and related apparatus
a pcr and dna target technology, applied in the field of pcr method, can solve the problems of false positive and false positive, prone to contamination, and inability to analyze multiple dna targets within a sample in the same reaction tube through pcr (known as multiplexing), so as to reduce human error in methods and the potential for contamination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
One-Tube QRT-PCR
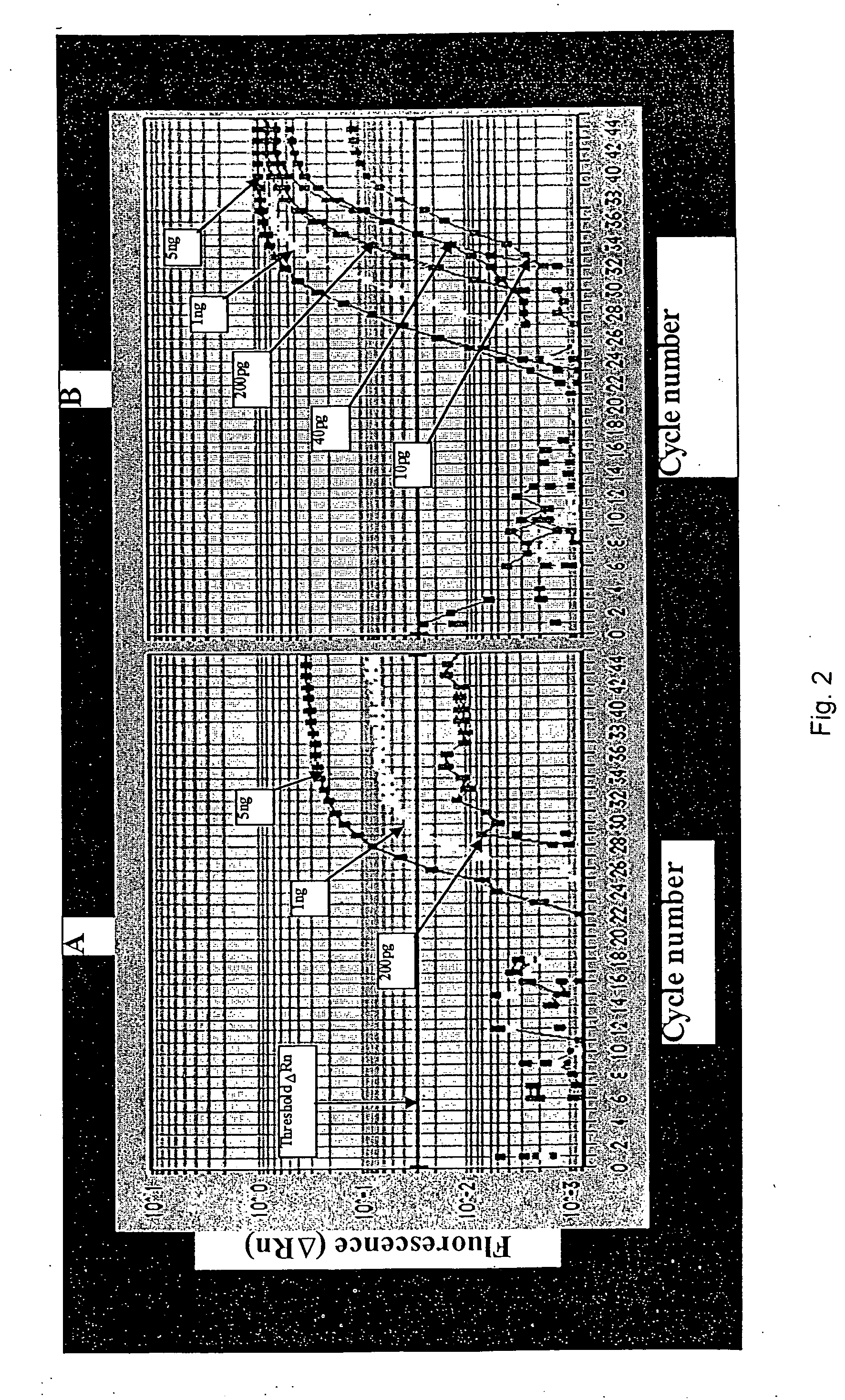
[0070] With the introduction of real-time, fluorescence-based 5′ nuclease PCR (Gibson, U. E., C. A. Heid and P. M. Williams. 1996. A novel method for real time quantitative RT PCR. Genome Res. 6:995-1001; Heid, C. A., J. Stevens, K. J. Livak and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994) and instruments such as the ABI PRISMT™ 7700 (TaqMan®) sequence detector (Applied Biosystems, Foster City, Calif., USA), quantitative RT-PCR is now a widely accepted method for measuring gene expression levels. Quantitative RT-PCR is a sensitive technique and is particularly useful for the analysis of samples containing limited amounts of nucleic acids, such as in clinical tissues (Collins, C., J. M. Rommens, D. Kowbel, T. Godrey, M. Tanner, S. I. Hwang, D. Polikoff, G. Nonet et al. 1998. Positional cloning of ZNF217 and NABC1: genes amplified at 20q13.2 and overexpressed in breast carcinoma. Proc. Natl. Acad. Sci. USA 95:8703-8708). When quantitating ...
example 2
Quantitative RT-PCR in Less than Twenty Minutes
[0078] The following is a one-tube two-step assay for quantitative reverse transcription followed by polymerase chain reaction (QRT-PCR), which can be completed in less than twenty minutes using Cepheid's Smart Cycler. Current methods of QRT-PCR for the 5′ fluorogenic assay in the Applied Biosystem's 7700 require more than two hours. By altering primer and probe concentrations and utilizing the fast ramping ability of the Smart Cycler, the reverse transcriptase reaction time was reduced to 2 minutes and the PCR time was reduced to 16 minutes using a 1 second denaturation and a 6 second extension for 40 cycles.
[0079] PCR reactions were designed for β-glucuronidase (β-gus) and carcinoembryonic antigen (CEA) cDNA respectively in 25 μl volumes with the following final concentrations: 400 nM each β-gus PCR primer (GUS-F, 5′-CTC ATT TGG AAT TTT GCC GAT T-3′ (SEQ ID NO: 3); (GUS-R, 5′-CCG AGT GAA GAT CCC CTT TTT A-3′)(SEQ ID NO: 4) or CEA p...
example 3
Rapid QRT-PCR: Multiplexed Assay
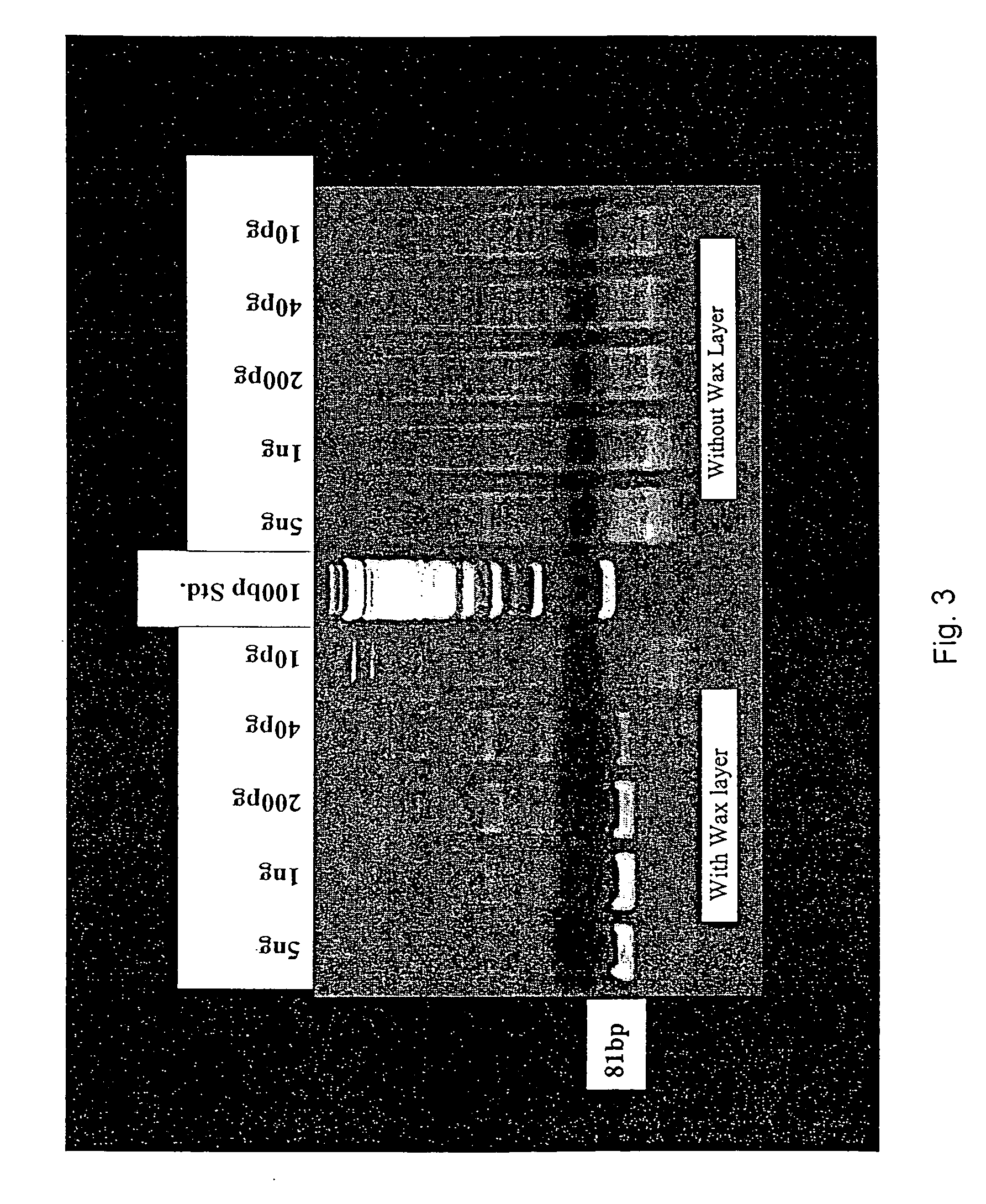
[0093] The Smart Cycler is currently capable of 4-color fluorescence detection and therefore allows multiplexing of QRT-PCR reactions. One goal is to multiplex internal controls for RT-PCR, an endogenous reference gene control to correct for RNA input, and the target gene (for example CEA) all in one tube. Initial tests multiplexing βGUS and CEA worked well at moderate CEA mRNA levels but failed when very low levels of CEA were present. Thus, the sensitivity of this reaction was not adequate for micrometastasis detection. One method to overcome this is to limit the amount of PCR primer used for the endogenous control gene. Theoretically this allows the rare CEA mRNA species to more effectively compete for PCR reagents, especially in later cycles. Attempts to do this with β-Gus, or a second endogenous control gene (18s ribosomal RNA), also failed to give adequate sensitivity.
[0094] It was hypothesized that the problem lay in the initial cycles, when...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com