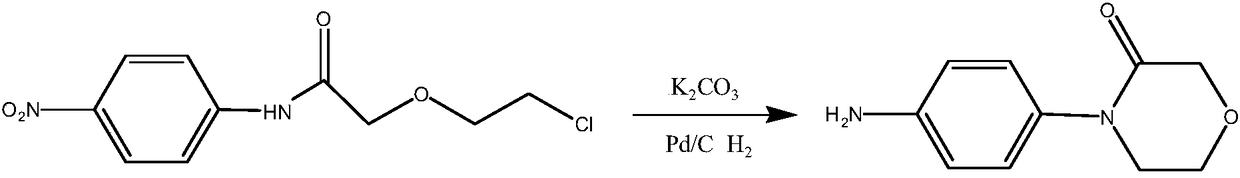
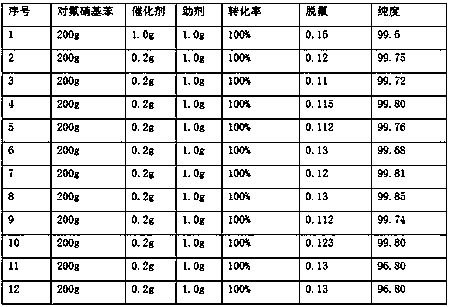
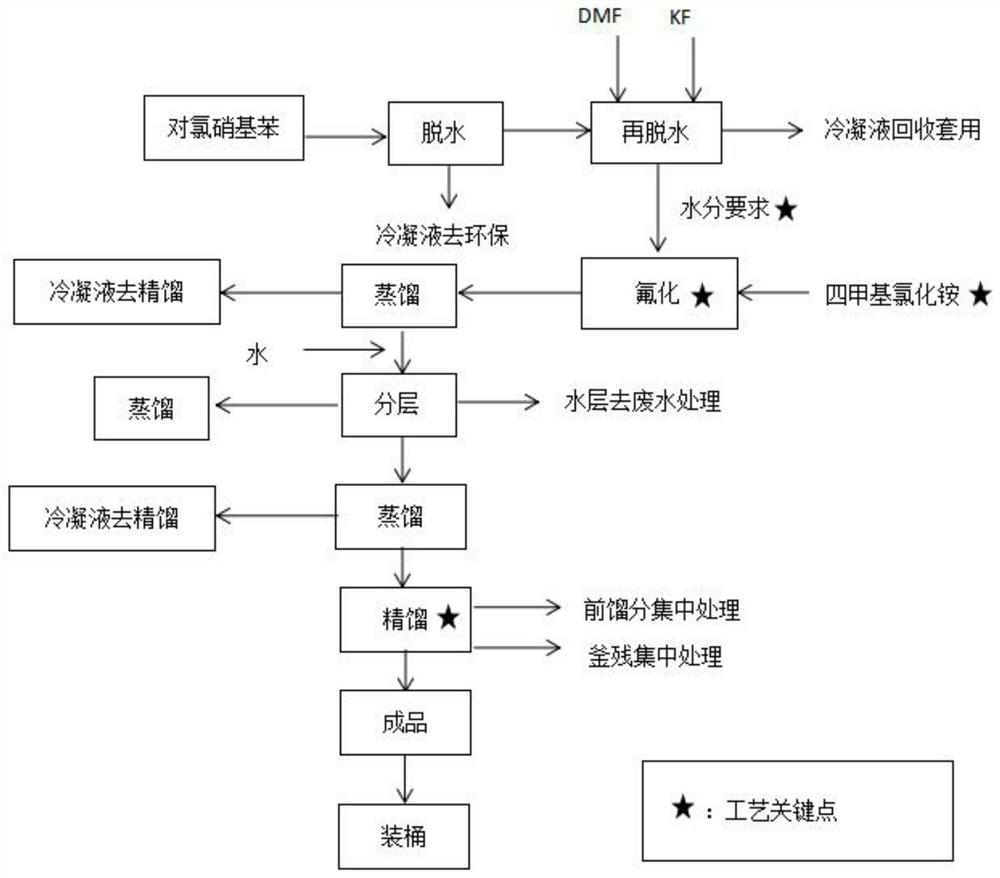
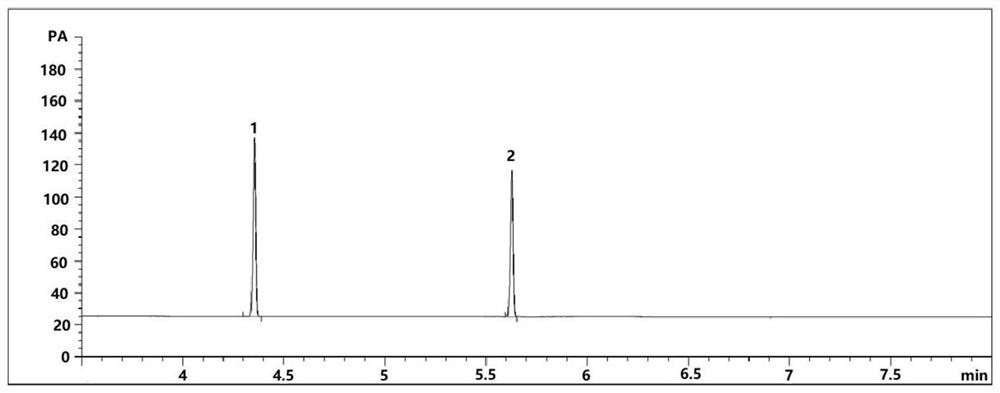
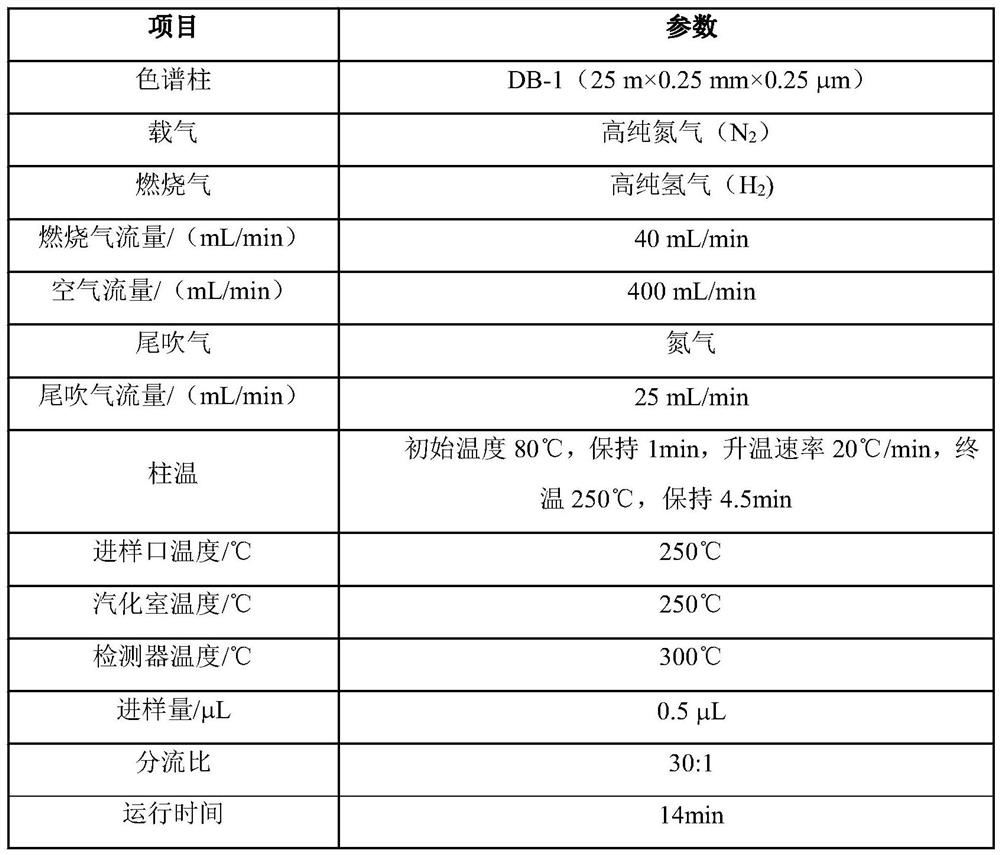
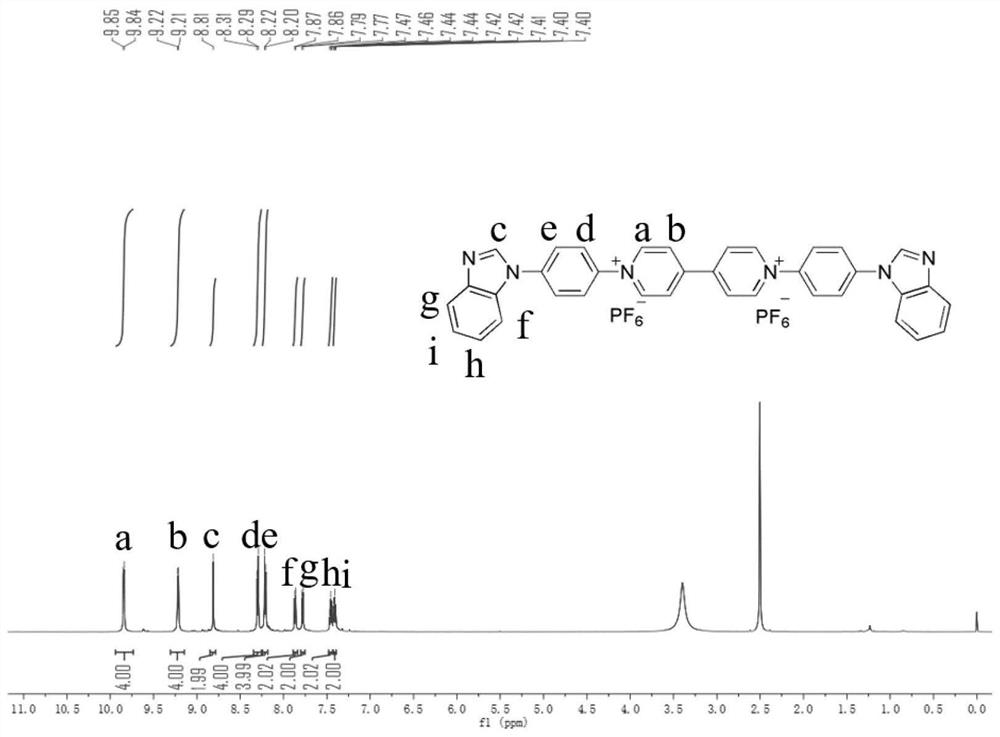
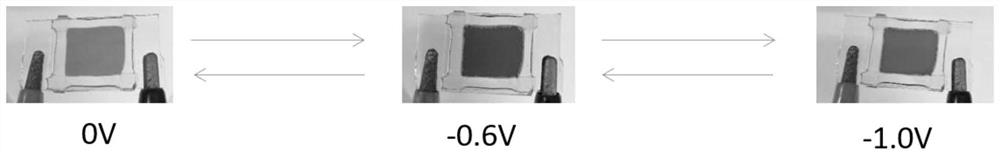
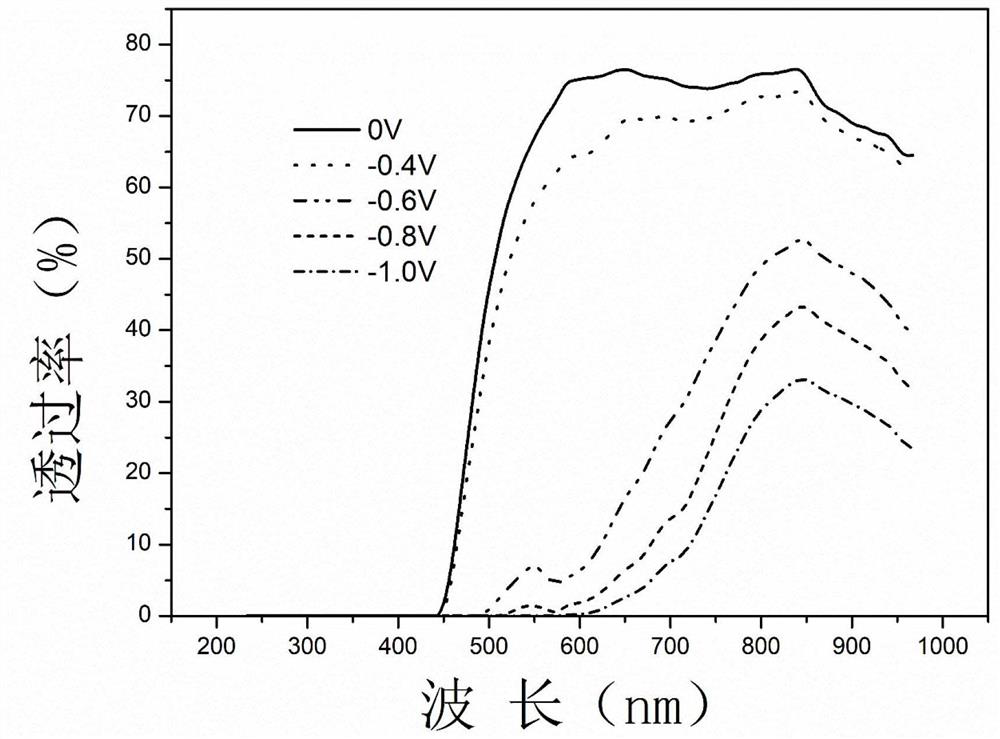
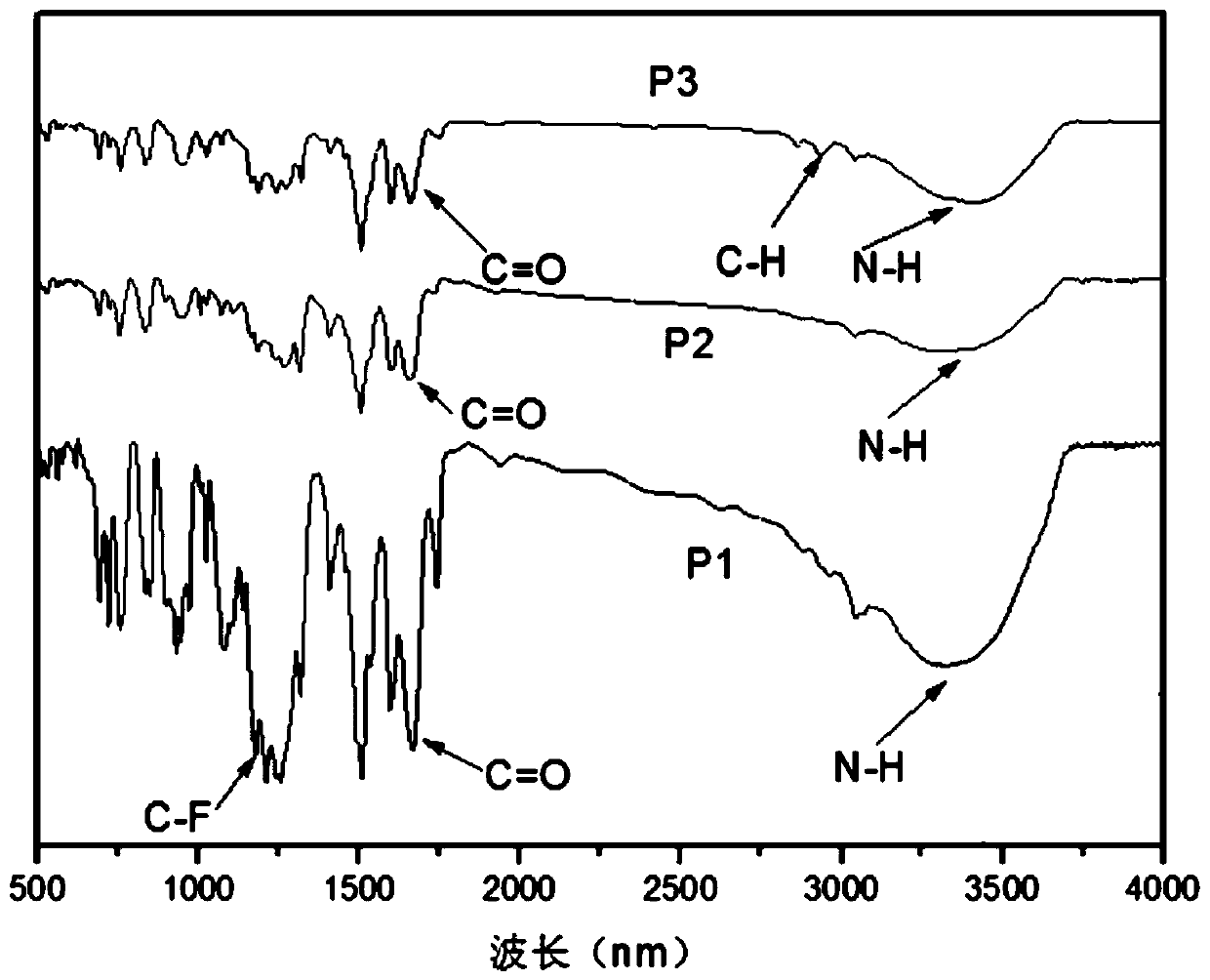
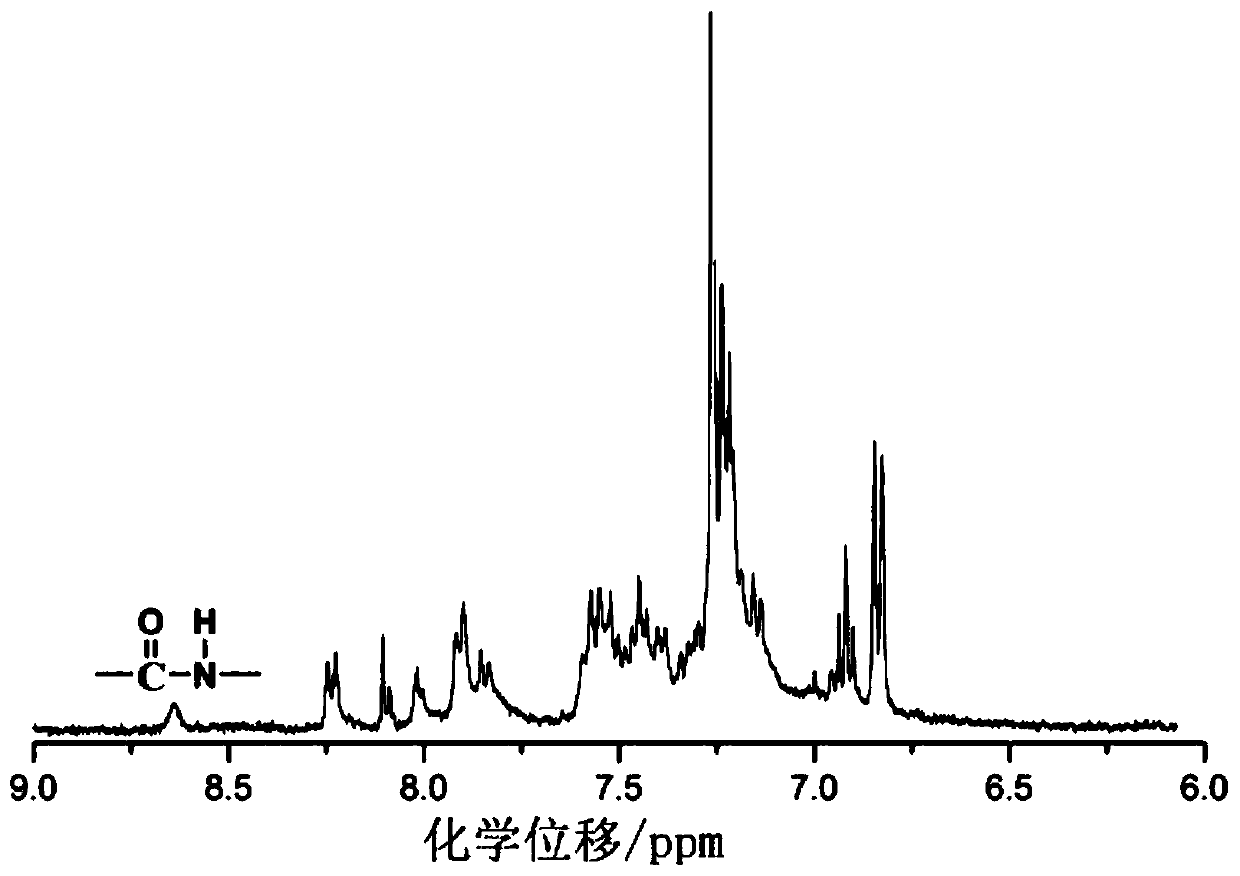
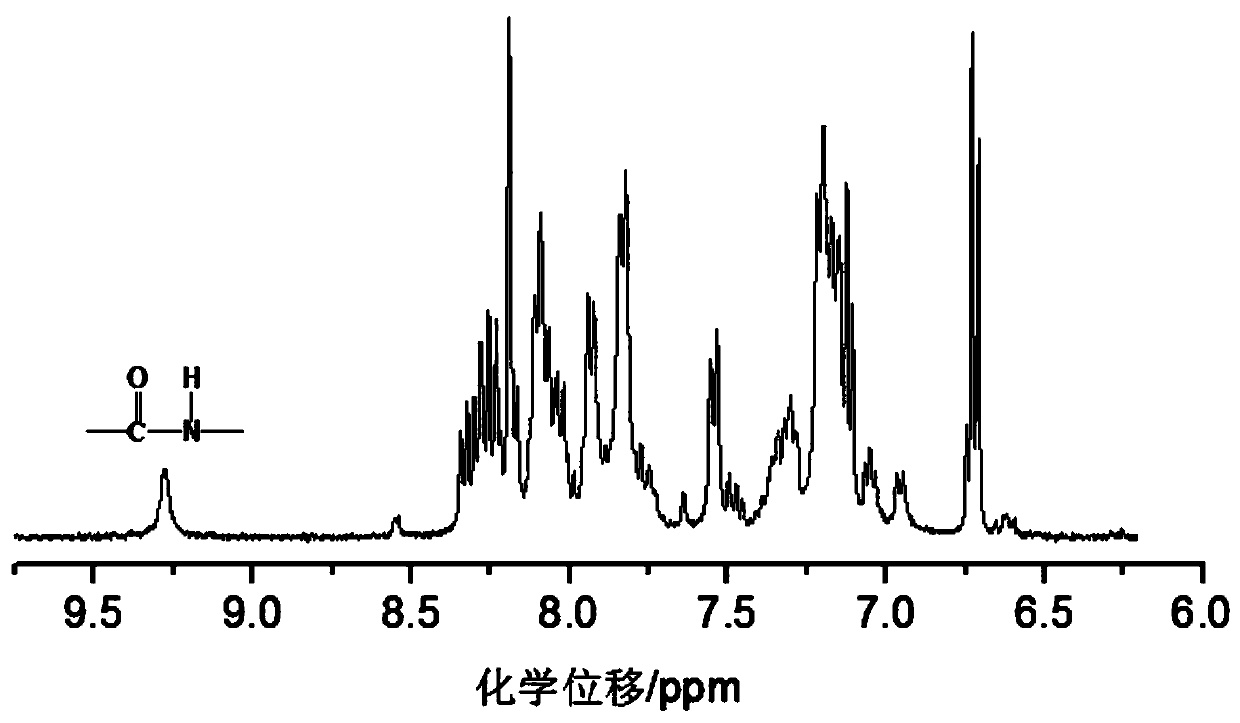
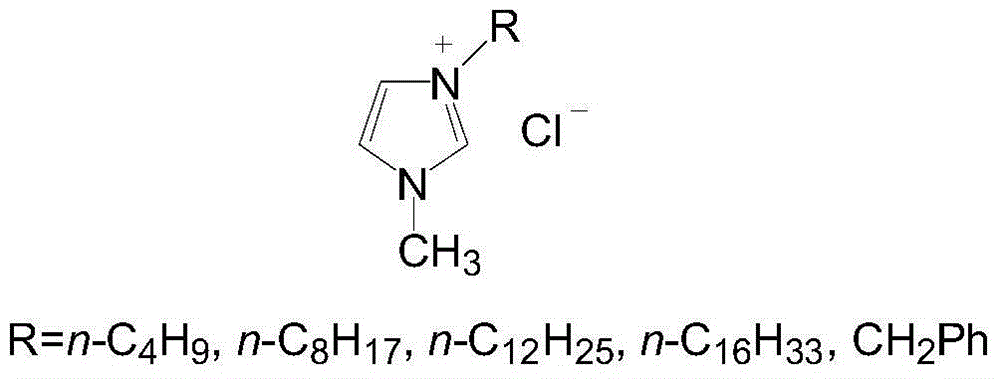
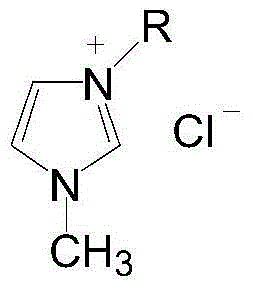
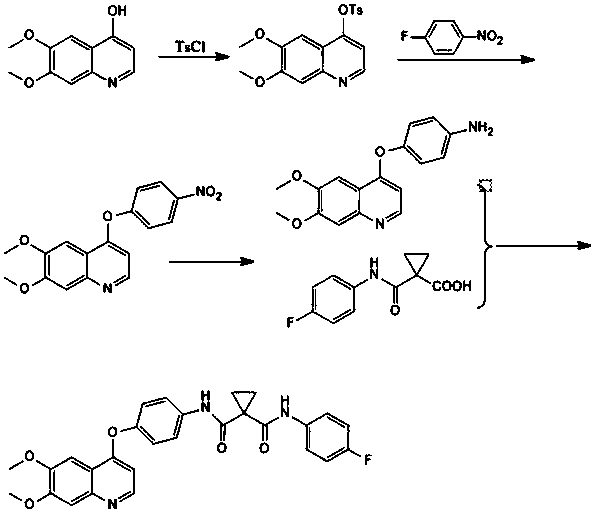
Patents
Literature
47 results about "P-fluoronitrobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
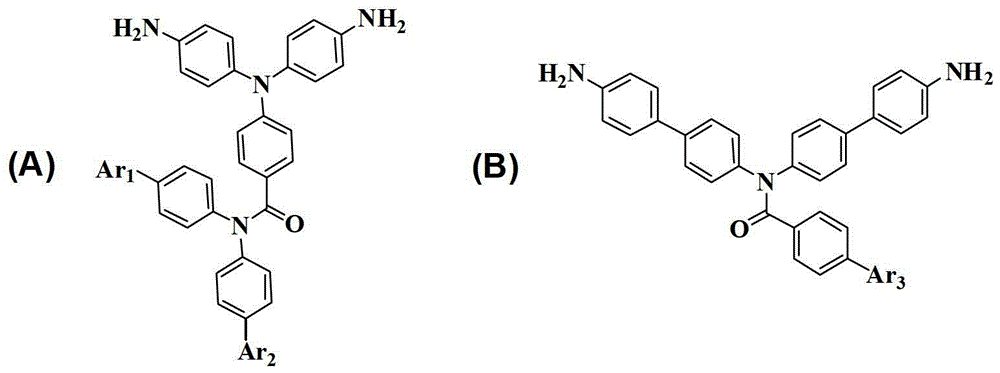
Diamine monomer containing diphenylamine-fluorene, preparation method and application of same in polyimide preparation
ActiveCN105085281AAvoid accumulationReduce the interaction forceOrganic compound preparationAmino compound preparationPolymer scienceTwo step
The invention discloses diamine monomer containing diphenylamine-fluorene, a preparation method and the application of same in polyimide preparation and belongs to the technical field of organic compound preparation. The monomer is N, N-dual (4-aminophenyl)-9, 9-dimethyl fluorene. A synthetic method comprise the following two steps that 1, under the action of cesium fluoride, 2 amino-9, 9-dimethyl fluorene and p-fluoronitrobenzene are subjected to nucleophilic substitution, and the N, N-dual (4-aminophenyl)-9, 9-dimethyl fluorene is obtained; then Pd / C serves as a catalyst and hydrazine hydrate serves as a reducing agent, and the N, N-dual (4-aminophenyl)-9, 9-dimethyl fluorene is obtained. According to the application, the N, N-dual (4-aminophenyl)-9, 9-dimethyl fluorene diamine monomer is involved into reaction with various dianhydride, and polyimide is obtained through the preparation. According to the diamine monomer containing the diphenylamine-fluorene, the preparation method and the application of same in polyimide preparation, by means of the structure peculiar to the diamine monomer, obtained polymer has good solubleness, and the unique photoelectric property is achieved; the diamine monomer and the polyimide obtained with the diamine monomer have vast application prospect in such fields as electroluminescence, cavity transmission and electrochromism.
Owner:JILIN UNIV
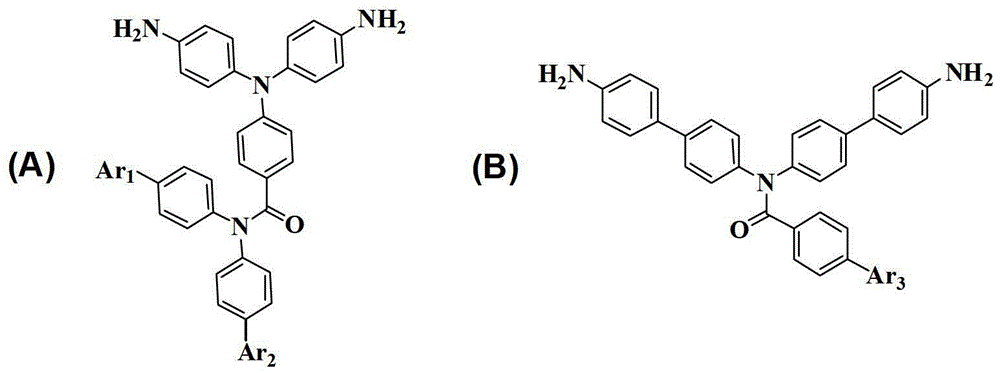
Aromatic diamine compound containing imide structure as well as preparation method and application thereof
ActiveCN103145581ASimple processImprove thermal performanceOrganic compound preparationCarboxylic acid amides preparationSolubilityPolyester
The invention discloses an aromatic diamine compound containing an imide structure as well as a preparation method and application thereof. The method for preparing the aromatic diamine compound containing the imide structure comprises the following steps of: preparing a rigid non-planar distorted structural intermediate containing the imide structure by high reaction activity of acyl chloride and active hydrogen; introducing different chemical groups through the Suzuki reaction of halogen elements and boric acid ester; utilizing fluoronitrobenzene to react with amino so as to preparing a binitro product by reaction of fluoronitrobenzene and amino; and reducing to obtain a novel aromatic diamine compound containing the imide structure. The synthesizing method is simple, easy to purify and high in yield; the synthesized compound has unique fluorescent characteristic, excellent thermal property and solubility property, can be used as a photoelectric material and a monomer for synthesizing novel high-performance polymers such as polyamide, polyimide, polyamide imide and polyester imide.
Owner:SUN YAT SEN UNIV
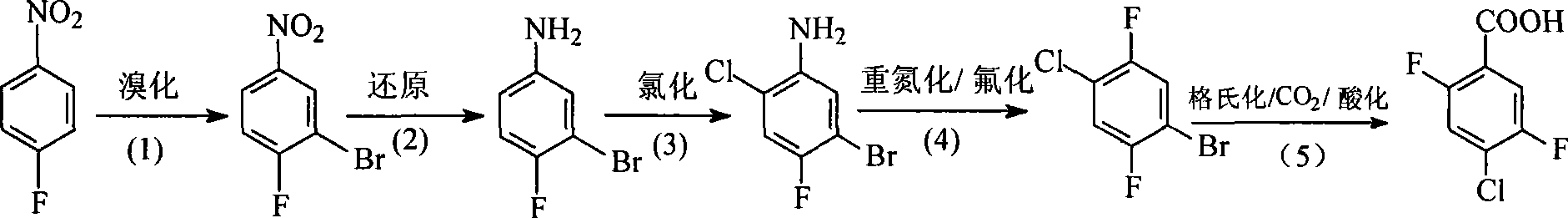
Preparation method of 4-chloro-2,5-difluorobenzoic acid
InactiveCN101381301AOrganic compound preparationCarboxylic compound preparationGrignard reagentP-fluoronitrobenzene
The invention relates to a preparation method for 4-chloro-2,5-difluorobenzoic acid. The preparation method comprises the following steps that: p-fluoronitroben-zene is used as a starting material and is subjected to bromization to obtain 3-bromo,4-fluoro-nitrobenzene; the 3-bromo,4-fluoro-nitrobenzene is reduced to obtain 3-bromo,4-fluoro-aniline; the 3-bromo,4-fluoro-aniline is chlorized to obtain 3-bromo,4-fluoro,6-chloro-aniline; the 3-bromo,4-fluoro,6-chloro-aniline is subjected to diazotization and then fluorized to obtain 4-chloro-2,5-difluorobromobenzene; and the 4-chloro-2,5-difluorobromobenzene is made into a bromobenzene grignard reagent and then is added into carbon dioxide to obtain a target substance after acidification. The preparation method prepares the 4-chloro-2,5-difluorobenzoic acid with high purity (more than 98 percent) through a easy large scale of commercial preparation method and lays a foundation for commercial application of the 4-chloro-2,5-difluorobenzoic acid.
Owner:EAST CHINA UNIV OF SCI & TECH
Method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction
ActiveCN101585771ATake advantage ofAvoid direct separationOrganic chemistryOrganic compound preparationChlorobenzenePotassium fluoride
The invention discloses a method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction, comprising steps of: generating a mixture mainly composed of parachloronitrobenzene, o-chloronitrobenzene and m-chloronitrobenzene in process of parachloronitrobenzene, o-chloronitrobenzene by chlorobenzene nitration method; removing low-boiling-point substances by evaporation; then adding anhydrous potassium fluoride and catalyst to the mixture for fluoro-reaction at 150 DEG. C to 250 DEG. C; after reaction, filtering to remove potassium chloride, after rectifying to acquire parachloronitrobenzene, o-chloronitrobenzene, the residual portion via recrystallization acquires m-chloronitrobenzene; the catalyst is quaternary ammonium salt or calixarene; charge rate of chloronitrobenzene mixture and mixture is 1:0.01 to 1. The method of the invention is simple to operate, in scale and changes waste material into things of value, which realizes zero discharge. The method is characterizedin sustainable development, energy saving, consumption reduction and environmentally friendly property.
Owner:浙江省常山长盛化工有限公司
Preparation method of 5-chloro-2-methoxy cyanophenyl
InactiveCN102786439ALow priceEasy to getPreparation by cyanide reactionP-fluoronitrobenzeneNitrobenzene
The invention discloses a preparation method of 5-chloro-2-methoxy cyanophenyl, relating to the technical field of medicine intermediates. The method is characterized by using fluoronitrobenzene as an initial raw material, and conducting bromination, nitro reduction, diazotization-Sandmeyer, Williamson substitution and carbonitriding to synthesize 5-chloro-2-methoxy cyanophenyl. The 5-chloro-2-methoxy cyanophenyl obtained by the method is a white powdery solid with the purity of 99.5%, the feed stock conversion each step respectively reaches 100%, and the total yield of the whole process reaches 48%.
Owner:CHANGZHOU UNIV
4,4'-diamido-4''-ferrocenyl triphenylamine and preparation method thereof
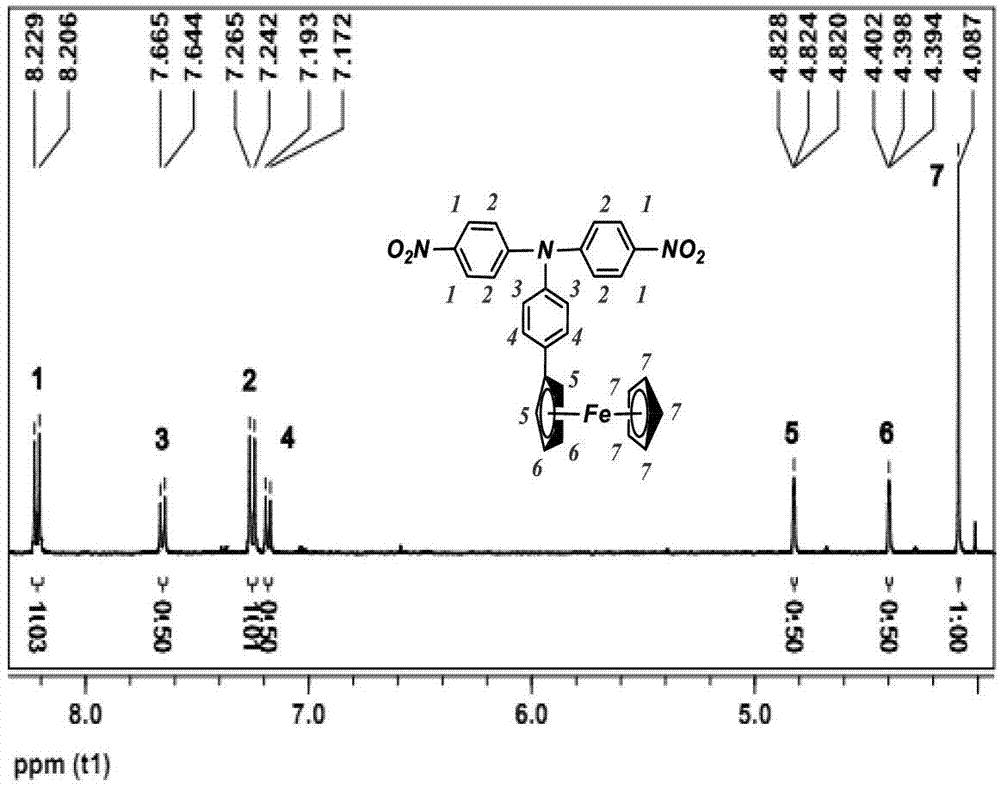
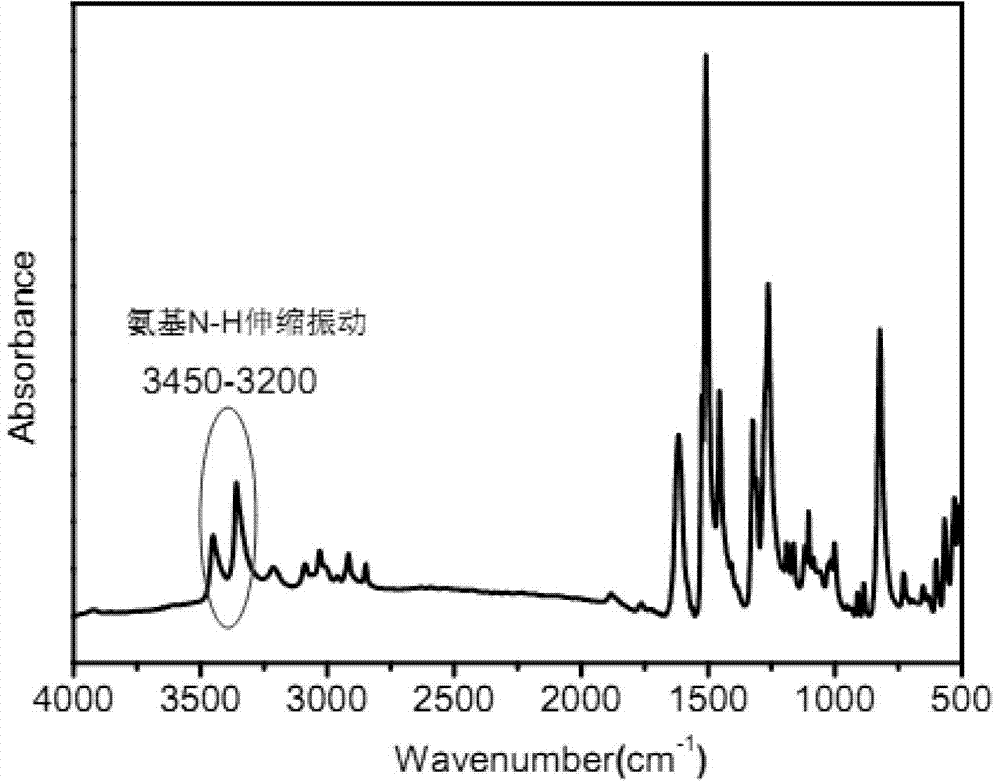
The invention provides 4,4'-diamido-4''-ferrocenyl triphenylamine and a preparation method of 4,4'-diamido-4''-ferrocenyl triphenylamine, belonging to the technical field of triphenylamine. The preparation method comprises the steps of taking 4-fluoronitrobenzene and 4-aminophenyl ferrocene as raw materials, adding a certain dose of catalyst, reacting for 12-72h at 110-180 DEG C to obtain 4,4'-binitro-4''-ferrocenyl triphenylamine, and then reducing dinitro-compound to 4,4'-diamido-4''-ferrocenyl triphenylamine by using target carbon as the catalyst and hydrazine hydrate as a reducing agent. 4,4'-diamido-4''-ferrocenyl triphenylamine provided by the invention is simple in synthetic process, low in requirements for the equipment, and high in yield, and the compound is not easy to be oxidized during the preparation process, thereby being suitable for mass production.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of apixaban and intermediates thereof
ActiveCN107955002AHigh purityAtom utilization is highOrganic chemistryOrganic solventP-fluoronitrobenzene
The invention discloses a preparation method of apixaban and intermediates thereof. The invention provides a preparation method of an apixaban intermediate I. The preparation method of the apixaban intermediate I comprises the step of performing nucleophilic substitution reaction on an apixaban intermediate II and p-fluoronitrobenzene in an organic solvent in the presence of an alkali to obtain the apixaban intermediate I. The preparation method has short steps, simple and safe operation, simple post-treatment steps, environmental friendliness and high total yield, and the obtained product hashigh purity, low production cost and high atomic utilization, and is suitable for industrial production. The formula is shown in the description.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Preparation method of chemical intermediate N-isopropyl p-fluoroaniline
ActiveCN111072498ANo pollution in the processHigh yieldAmino compound purification/separationOrganic compound preparationPtru catalystIsopropyl
The invention discloses a preparation method of a chemical intermediate that is N-isopropyl p-fluoroaniline, which is characterized in that the method comprises adding p-fluoronitrobenzene, a hydrogenation catalyst and acetone into a reaction kettle, heating to 55-75 DEG C, and introducing hydrogen into the reaction kettle to perform hydrogenation reduction reaction; after the reaction is finished, filtering the reaction system to recover the waste catalyst, and layering the filtrate obtained by filtering to obtain wastewater; and then performing rectification to obtain the target product N-isopropyl p-fluoroaniline; the hydrogenation catalyst is a composite catalyst, and the composite catalyst takes a nickel-doped carbon aerogel / TiO2 composite material as a carrier and Pt as a catalytic active component. The method disclosed by the invention is simple in process, economical, environment-friendly and high in product yield.
Owner:SHANGYU XIES CHEM IND
Triphenylamine-based polymer containing benzoxazine structure, as well as preparation method and application thereof
ActiveCN109593172AImprove solubilityImprove film formationOrganic chemistryMaterial analysis by observing effect on chemical indicatorPolymer scienceNitration
The invention provides a triphenylamine-based polymer containing a benzoxazine structure, as well as a preparation method and application thereof, and aims at solving the problems that an existing triphenylamine-based polymer has poor heat resistance and an electrochromic polymer film has poor film attachment performance. The preparation method comprises the following steps: reacting raw materialsincluding N,N-diphenyl-1,4-phenylenediamine and N,N'-diphenyl-benzidine with p-fluoronitrobenzene to realize nitration; reducing nitryl into amino, and synthesizing a polymer by using bisphenol A, paraformaldehyde and the prepared triphenylamine monomer as raw materials through a condensation reaction under a certain condition. The polymer has excellent electrochromic performance and memory performance, can be applied to the field of electrochromism, has good presence in explosive detection, and can be applied to the field of electrochromic polymers.
Owner:HEILONGJIANG UNIV
Prepn process of nitro aromatic amine compound
ActiveCN1740141ALow costEasy to useOrganic compound preparationAmino compound preparationDistillationNitrobenzene
The present invention belongs to the field of preparation technology of aromatic amine as hole transmission material intermediate. The preparation process of nitro aromatic amine compound includes the following steps: reacting one of phenylamine, diphenylamine and triphenylamine, strong alkali and p-fluoro nitrobenzene or p-chloro nitrobenzene in certain molar proportion in N, N-dimethyl formamide as solvent inside some reactor at 90-130 deg.c for 5-16 hr; filtering in the hot state to eliminate reaction produced impurity; decompression distillation to eliminate DMF and to obtain initial product; and re-crystallization with methanol, ethanol or acetonitrile to obtain the nitro aromatic amine compound. The present invention has the advantages of cheap material, simple post-treatment, high product yield, high product purity and being suitable for industrial production.
Owner:TIANJIN UNIV
Triarylamine-containing oxazine polymer and preparation method and application thereof
ActiveCN109651284AImprove solubilityImprove film formationOrganic chemistryMaterial analysis by observing effect on chemical indicatorPolymer scienceNitration
A triarylamine-containing oxazine polymer and a preparation method and application thereof are disclosed. The invention relates to a triarylamine-containing oxazine polymer and a preparation method and application thereof. The invention is to solve the problem that the conventional triarylamine-based polymer has poor heat resistance and an electrochromic polymer film has poor film attaching ability. According to the invention, N,N'-di-naphthyl-1,4-phenylenediamine, which is used as a raw material, reacts with p-fluoronitrobenzene to realize nitration, and then nitro group is reduced to an amino group; and bisphenol A, paraformaldehyde and the prepared triphenylamine-based monomer are used as raw materials to undergo a condensation reaction under a certain conditions to synthesize a polymer. The polymer has excellent electrochromic property and memory performance, can be applied in the field of electrochromism, and also performs well in explosives detection. The invention is applied inthe field of electrochromic polymers.
Owner:HEILONGJIANG UNIV
Multifunctional triarylamine-containing oxazine polymer and preparation method and application thereof
ActiveCN109593171AImprove solubilityImprove film formationOrganic chemistryMaterial analysis by observing effect on chemical indicatorPolymer scienceNitration
The invention relates to a multifunctional triarylamine-containing oxazine polymer, a preparation method and application thereof, and aims at solving the problems that conventional triarylamine-containing polymers have poor heat resistance and electrochromic polymer thin films have poor film attaching ability. The preparation method comprises the following steps of: reacting N4,N4-di-naphthalene-2-base-biphenyl-4,4-diamine used as a raw material with p-fluoronitrobenzene to realize nitration, then reducing a nitro group into an amino group, carrying out condensation reaction under certain conditions to synthesize the polymer by using a triphenylamine-based monomer prepared from bisphenol A and paraformaldehyde as a raw material. The polymer has excellent electrochromism property and memoryproperty, can be applied to the field of electrochromism, has good performance in explosive detection and is applied to the field of electrochromic polymers.
Owner:HEILONGJIANG UNIV
Preparation method of 4-(4-aminophenyl)-3-molindone
ActiveCN108658888AAvoid passivation effectsReduce generationOrganic chemistryEnvironmental resistanceHydrobromide
The invention provides a preparation method of 4-(4-aminophenyl)-3-molindone. The preparation method has the advantages that reaction steps with high risk and high environmental pressure are omitted,especially the use of mixed acid and chloroacetyl chloride is avoid, so that the pressure of environmental protection is reduced; in addition, the use of strong oxidants such as potassium permanganateis avoided, so that the production of by-products is reduced, the synthesis yield is high, the product quality is good and the purification of reaction post treatment is facilitated; besides, all thesteps related in the reaction process are easy to carry out, so that the reaction in which passivation effect of functional groups and the like are difficult to generate in an existing method is avoided, for example, the route which takes p-nitroaniline as a raw material to carry out substitution reaction is almost impossible to perform; besides, the selected raw materials such as p-fluoronitrobenzene and bromoethylamine hydrobromide, disclosed by the invention are easy to obtain; compared with the molindone and other raw materials involved in the existing method, the preparation method is low in price and is suitable for industrialized production.
Owner:上海科利生物医药有限公司
Method for producing p-fluoroaniline by solvent-free catalytic hydrogenation method
InactiveCN108997138AImprove reaction efficiencyIncrease profitOrganic compound preparationChemical recyclingPhosphateSlag
The invention provides a method for producing p-fluoroaniline by a solvent-free catalytic hydrogenation method. The method comprises that p-fluoronitrobenzene as a raw material undergoes a catalytic hydrogenation reaction in an autoclave under the condition of no solvent and use of a catalyst and a phosphate to produce p-fluoroaniline. The method utilizes a solvent-free hydrogenation method. Compared with the existing solvent hydrogenation method, the method greatly increases reaction efficiency, obviously improves the equipment utilization rate, effectively prevents the energy consumption andequipment cost caused by the solvent recovery, belongs to the pollution-free environment-friendly processes and effectively prevents the production of a large amount of wastewater and waste slag. Thenoble metal catalyst Pt / C is prepared through simple processes and can be recycled in the catalyst reaction and the metal in the catalyst can be recovered and recycled so that the production cost isfurther reduced. The phosphate can be used as a dehydrogenation inhibitor and can improve a reaction conversion rate.
Owner:济南和润化工科技有限公司
Synthetic analysis and detection method for industrially producing p-fluoronitrobenzene
PendingCN114487194AImprove accuracyHigh sensitivityOrganic chemistryComponent separationPtru catalystMeth-
The invention discloses a synthesis analysis detection method for industrial production of p-fluoronitrobenzene, and relates to the technical field of p-fluoronitrobenzene production. Parachloronitrobenzene is used as a raw material, potassium fluoride and a solvent N, N-dimethylformamide are adopted, under the action of a phase transfer catalyst tetramethylammonium chloride, fluorination is adopted to replace one-step synthesis, reactants are collected in real time in the synthesis process, gas chromatography is used for quantitative analysis, and the content of impurities in the composition is controlled. The method comprises the following steps: dehydrating parachloronitrobenzene, adding N, N-dimethylformamide, and carrying out vacuum dehydration; adding potassium fluoride in batches according to time intervals for dehydration and reaction, adding a phase transfer catalyst tetramethylammonium chloride, and carrying out heating reflux reaction to obtain a crude product p-fluoronitrobenzene; and dissolving the reactant with methanol, filtering, carrying out gas chromatographic analysis, and calculating the conversion rate by adopting a peak area normalization method. The raw materials are subjected to dehydration treatment, the feeding mode, the feeding amount and the reaction time are optimized, and the conversion rate of the reaction is controlled in combination with a gas chromatographic analysis method.
Owner:YUNNAN YUNTIANHUA
Purpurine derivative electrochromic and electrofluorescent color-changing material as well as preparation method and application thereof
ActiveCN114133380AGood discoloration effectHigh optical contrastOrganic chemistryTenebresent compositionsNitro compoundNitrobenzene
The invention discloses a viologen derivative electrochromic and electrofluorescent discoloration bifunctional material as well as a preparation method and application thereof. The preparation method comprises the following steps: by taking 4, 4 '-dipyridyl as a raw material, carrying out nucleophilic substitution reaction on the 4, 4'-dipyridyl and 1-chloro-2, 4-dinitrobenzene to generate Zincke salt; carrying out nucleophilic substitution reaction on p-fluoronitrobenzene and a nitrogen-containing aromatic heterocyclic ring to generate a nitro compound, and reducing the nitro compound into an amino compound by using hydrazine hydrate as a reducing agent; and performing Zincke reaction on products of the two-step reaction to generate a target product. According to the invention, a benzimidazole group with an electron donating effect is introduced into a viologen derivative, and forms an electron donating-electron withdrawing effect with an electron-deficient bipyridine group, so that the color change voltage is reduced, and the optical contrast is improved; meanwhile, an electrofluorescence discoloration function is introduced into the viologen derivative.
Owner:SOUTH CHINA UNIV OF TECH
Novel preparation method of p-fluoronitrobenzene
ActiveCN108586257AReasonably adjust the dosage ratioImprove carrier strengthOrganic chemistryOrganic compound preparationBenzeneOrganic synthesis
The invention discloses a novel preparation method of p-fluoronitrobenzene, and relates to the field of organic synthesis. The method concretely comprises the following steps of firstly crushing durian shells; adding ethanol; performing ball milling to prepare durian shell powder; then, mixing the durian shell powder with urea; performing sintering treatment at a certain temperature to obtain mixed powder; adding the mixed powder into a tetrabutylammonium chloride solution for stirring and still standing treatment; then, performing drying to obtain a catalyst; adding reaction raw materials ofpara-nitrochloro-benzene, potassium fluoride and the catalyst into a reaction kettle; performing sealing; performing reaction at a certain temperature; after the reaction is completed, performing water washing and layering treatment; performing organic layer rectification to obtain a target product. The method disclosed by the invention has the advantages that the operation is simple; a solvent isnot used in the reaction process; the cost is effectively reduced; the product yield is high.
Owner:SHANGYU XIES CHEM IND
Triarylamino polyamide containing condensed ring anthryl side group structure as well as preparation method and application thereof
ActiveCN110982064ARetain performanceLarge conjugate areaAmino preparation from aminesOrganic compound preparationPolymer scienceP-fluoronitrobenzene
The invention discloses triarylamino polyamide containing a condensed ring anthryl side group structure as well as a preparation method and application of thereof, and relates to triarylamino polyamide containing a condensed ring anthryl side group structure as well as a preparation method and application thereof. The invention aims to solve the problems of poor heat resistance and poor electrochromic polymer film attaching capacity of existing triarylamine polyamide. The method comprises the following steps: taking p-bromoaniline as a raw material, carrying out nitration reaction on p-bromoaniline and p-fluoronitrobenzene, carrying out condensation reaction on a product of the nitration reaction and 9-anthraylboronic acid, and then carrying out binitro reduction to obtain diamino; and carrying out condensation reaction on the diamino and different diacids to generate polyamide. The triarylamino polyamide provided by the invention has excellent electrochromic performance and good stability, and is applied to the field of electrochromic polymers.
Owner:HEILONGJIANG UNIV
Method for preparing 4-nitrotriphenylamine
InactiveCN101538210ALow toxicitySimple stepsAmino compound preparation by condensation/addition reactionsOrganic solventP-fluoronitrobenzene
The invention discloses a method for preparing 4-nitrotriphenylamine, which comprises the following steps that: diphenylamine and alkali are reacted in an organic solvent, then fluoronitrobenzene is added into the organic solvent; and the product is obtained by reaction at 10 to 30 DEG C; and the organic solvent is one or more of dimethyl sulfoxide, N, N-dimethyl acetamide, dimethylimidazolidinone and methylpyrrolidone. The method preferably selects the solvent to ensure that the reaction can be performed at room temperature and has no byproducts, is simple and effective, has low cost, convenient use of raw materials, short reaction time and high yield, and is easy to apply to industrialized production.
Owner:EAST CHINA UNIV OF SCI & TECH +1
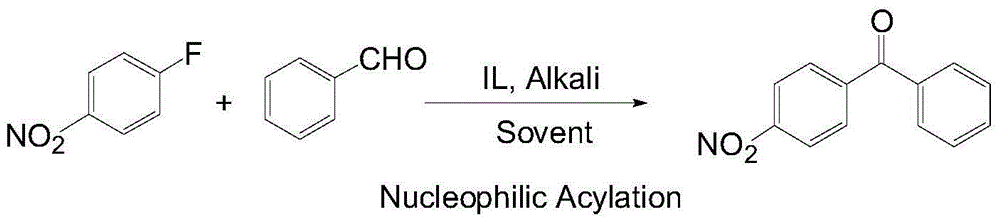
Novel method for synthesizing p-nitrobenzophenone
InactiveCN104926662AAchieve recyclingReduce energy consumptionOrganic chemistryOrganic compound preparationP-fluoronitrobenzeneBenzaldehyde
The invention relates to a novel method for synthesizing p-nitrobenzophenone. The method is characterized in that p-fluoronitrobenzene and benzaldehyde are adopted as raw materials; an imidazole ionic liquid is adopted as a catalyst precursor; and N-heterocyclic carbine is produced under the existence of alkali and is used for catalyzing a nucleophilic acylation reaction between the two substrates, such that p-nitrobenzophenone is produced. According to the invention, for a first time, ionic liquid is used as the nucleophilic acylation reaction catalytic precursor, such that catalyst recycling can be realized. Also, the synthesis method is implemented under a near room temperature, such that energy-consumption is low. The method also has the advantages of simple operation and high yield. When the ionic liquid is recycled 4 times, a p-nitrobenzophenone yield is still higher than 75%.
Owner:QINGDAO UNIV OF SCI & TECH
Preparation method of p-fluoroaniline
ActiveCN109053462AReduce generationHigh reaction yieldOrganic compound preparationAmino compound preparationP-fluoronitrobenzeneHydrogen atmosphere
The invention discloses a preparation method of p-fluoroaniline. The preparation method comprises the steps: under the catalysis of modified Raney nickel, a hydrogenation reduction reaction occurs ina hydrogen atmosphere in the p-fluoronitrobenzene, and after the reaction is finished, the p-fluoroaniline is obtained through post-treatment; the modified Raney nickel is Raney nickel modified by Moand Cr. The preparation method effectively reduces the occurrence of defluorination side reaction and improves the reaction yield by using the modified Raney nickel as a catalyst, and has industrial application prospects.
Owner:SHANGYU SUNFIT CHEM
Preparation method of cabozantinib
InactiveCN108264482AHigh reaction yieldImprove response qualityOrganic chemistryP-fluoronitrobenzeneCarboxylic acid
The invention relates to a preparation method of cabozantinib. The method comprises the following steps: taking 5,6-dimethoxy-4-hydroxyquinoline, TsCl, fluoronitrobenzene, and 1-(4-fluorophenyl)amino-carbonyl cyclopropane carboxylic acid as raw materials, and performing steps of a hydroxy protection reaction, a coupling reaction, a reduction reaction, and a condensation reaction to obtain the cabozantinib. The preparation method of cabozantinib has the advantages of high yield, low cost, less three wastes, easy operation, and safety, and is suitable for industrialization.
Owner:南京法恩化学有限公司
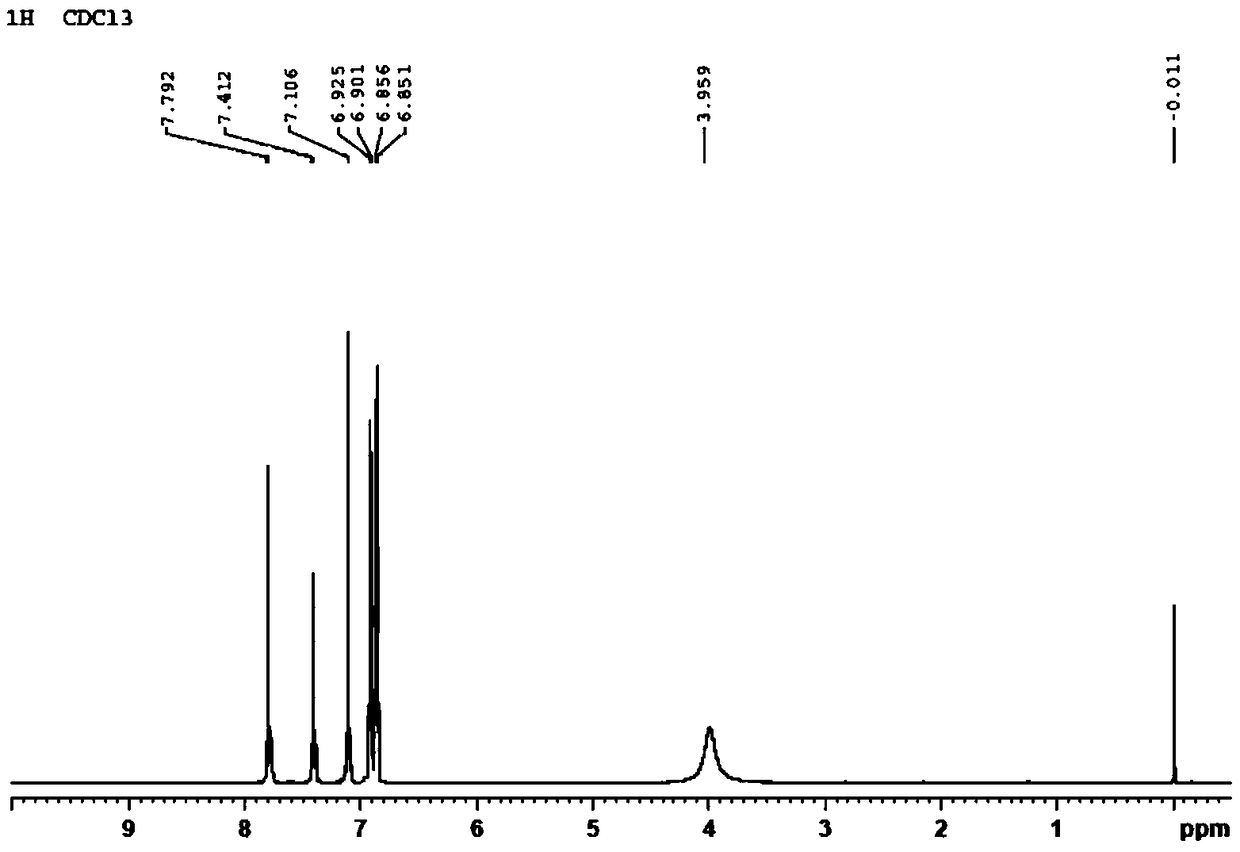
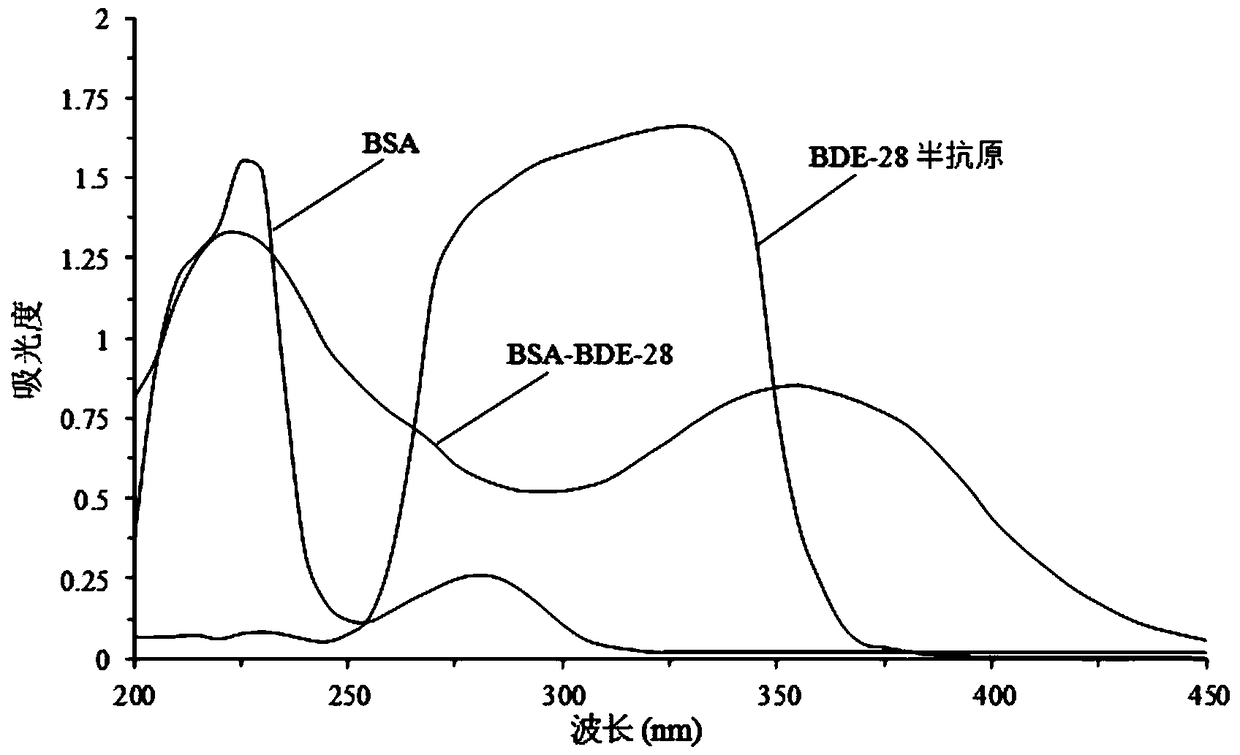
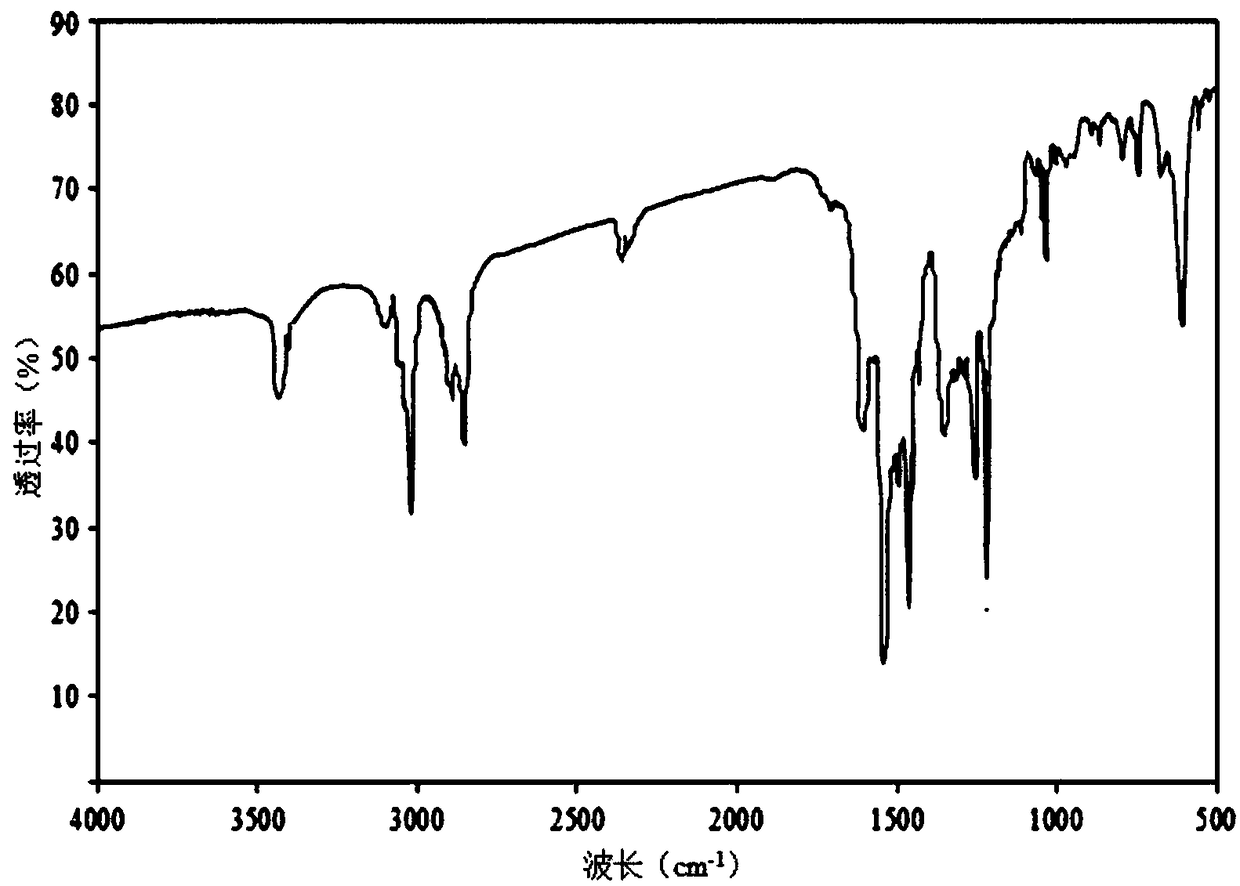
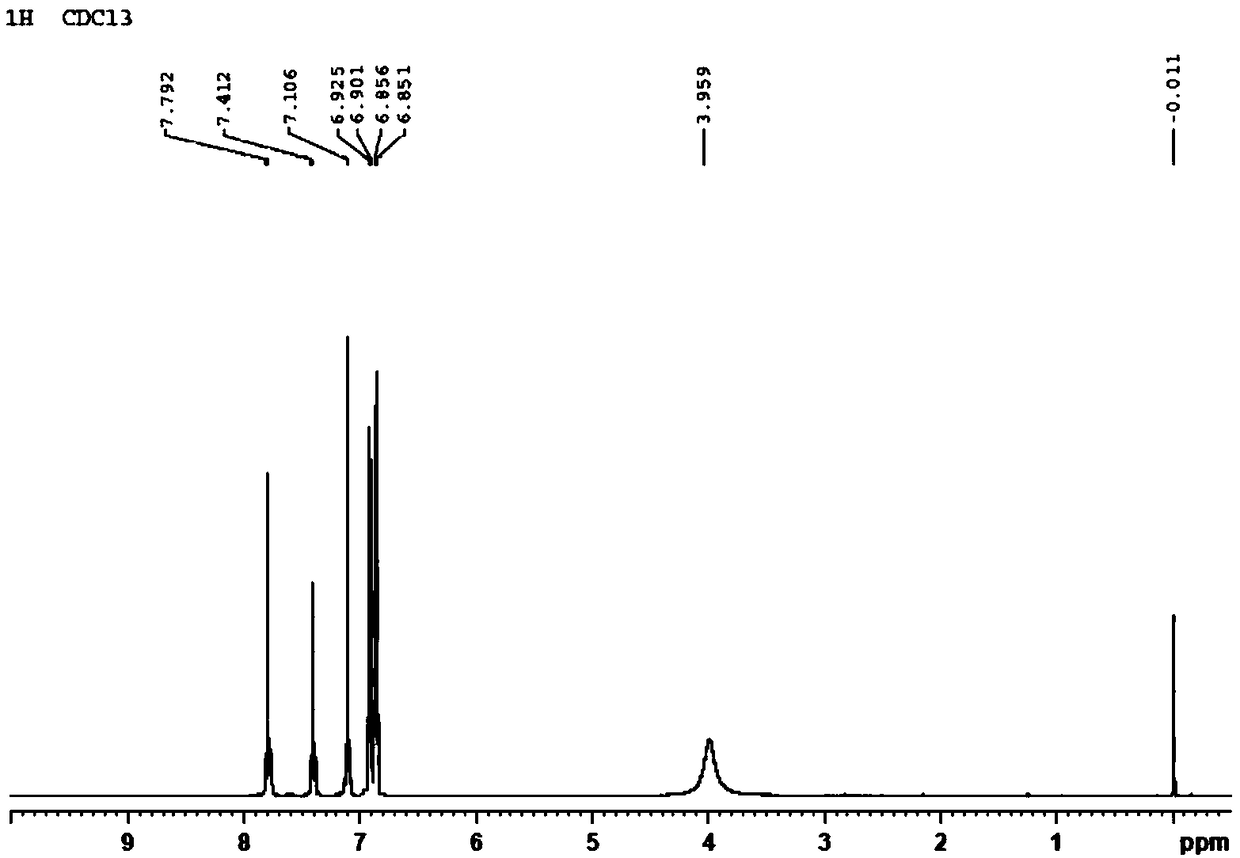
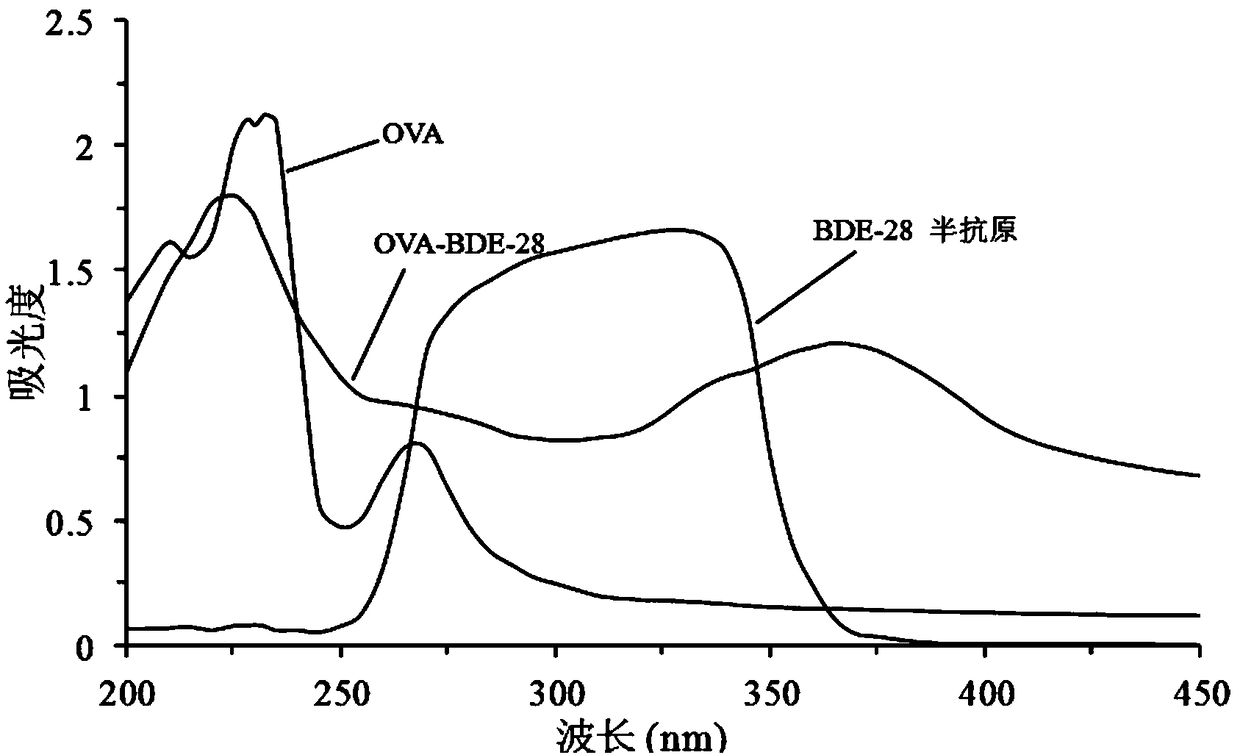
Preparation method and application of 2,4,4'-tribromobiphenyl ether immunogen
InactiveCN108752463AThe synthesis method is simpleSynthetic method is fastSerum albuminPeptide preparation methodsP-fluoronitrobenzeneBovine serum albumin
The invention discloses a preparation method and an application of a 2,4,4'-tribromobiphenyl ether immunogen. 4-nitro-2',4'-dibromodiphenyl ether is synthesized from 2,4-dibromophenol and 4-fluoronitrobenzene as raw materials through electrophilic substitution reaction; then, a 2,4,4'-tribromodiphenyl ether hapten containing an amino active group is prepared through hydrogenation reduction reaction, and the 2,4,4'-tribromobiphenyl ether immunogen is prepared from the 2,4,4'-tribromodiphenyl ether hapten and bovine serum albumin by coupling with a diazotization method. The preparation method ofthe 2,4,4'-tribromobiphenyl ether immunogen is rapid and simple, can be used for preparing 2,4,4'-tribromobiphenyl ether artificial polyclonal antibodies and has a significant effect on establishmentof immunoassay methods associated with 2,4,4'-tribromobiphenyl ether.
Owner:SHANGHAI JIAO TONG UNIV
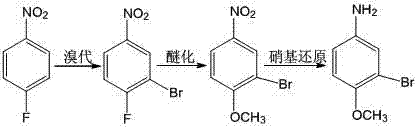
Method for preparing 3-bromo-4-methoxyaniline
ActiveCN102199099ALow priceEasy to getOrganic compound preparationAmino-hyroxy compound preparationBromineP-fluoronitrobenzene
The invention relates to the field of organic synthesis, in particular to a method for preparing 3-bromo-4-methoxyaniline. The method for industrially preparing 3-bromo-4-methoxyaniline comprises the step of: by taking p-nitrochlorobenzene as an initial raw material, synthesizing 3-bromo-4-methoxyaniline through three-step reactions of bromization, etherification and nitro-reduction. The 3-bromo-4-methoxyaniline obtained in the process is yellow powdery solid with the purity of 99.9 percent, the raw material conversion rate at each step respectively reaches 100 percent, and the overall yield rate throughout the whole process reaches 62 percent.
Owner:溧阳常大技术转移中心有限公司
2,4,4'-tribromodiphenyl ether hapten preparation method and application thereof
InactiveCN108727204AThe synthesis method is simpleHigh speedOrganic compound preparationOvalbuminP-fluoronitrobenzeneCarrier protein
The invention discloses a 2,4,4'-tribromodiphenyl ether hapten preparation method and application thereof. 2,4-dibromophenol and 4-fluoronitrobenzene are used as raw materials for electrophilic substitution reaction to synthesize 4-nitro-2',4'-dibromodiphenyl ether, and then through hydrogenation reduction reaction, a 2,4,4'-tribromodiphenyl ether hapten is prepared. By means of the method, the problem is solved that the 2,4,4'-tribromodiphenyl ether does not have an active group coupled to carrier protein, the method is simple, the speed is high, the yield is higher, the synthesized 2,4,4'-tribromodiphenyl ether hapten can be used for preparing a 2,4,4'-tribromodiphenyl ether artificial coating antigen and building a 2,4,4'-tribromodiphenyl ether Immunoassay method.
Owner:SHANGHAI JIAO TONG UNIV
Method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction
ActiveCN101585771BTake advantage ofAvoid direct separationOrganic chemistryOrganic compound preparationChlorobenzeneQuaternary ammonium cation
The invention discloses a method of comprehensively use of chloronitrobenzene mixture by fluoro-reaction, comprising steps of: generating a mixture mainly composed of parachloronitrobenzene, o-chloronitrobenzene and m-chloronitrobenzene in process of parachloronitrobenzene, o-chloronitrobenzene by chlorobenzene nitration method; removing low-boiling-point substances by evaporation; then adding anhydrous potassium fluoride and catalyst to the mixture for fluoro-reaction at 150 DEG. C to 250 DEG. C; after reaction, filtering to remove potassium chloride, after rectifying to acquire parachloronitrobenzene, o-chloronitrobenzene, the residual portion via recrystallization acquires m-chloronitrobenzene; the catalyst is quaternary ammonium salt or calixarene; charge rate of chloronitrobenzene mixture and mixture is 1:0.01 to 1. The method of the invention is simple to operate, in scale and changes waste material into things of value, which realizes zero discharge. The method is characterizedin sustainable development, energy saving, consumption reduction and environmentally friendly property.
Owner:浙江省常山长盛化工有限公司
Comprehensive development separation method of m-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture
ActiveCN110437073AReduce coke contentReduce pollutionOrganic chemistryOrganic compound preparationPotassium fluorideP-fluoronitrobenzene
The invention provides a comprehensive development separation method of m-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture. The comprehensive development separation method ofm-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture comprises following steps: the m-chloronitrobenzene, p-chloronitrobenzene, and o-chloronitrobenzene mixture, potassium fluoride, and tetrabutylammonium chloride are introduced into a distiller, under vacuum conditions, water vapour in the system is discharged, the temperature is increased to 140 to 145 DEG C, ultrasonicreaction is carried out, the temperature is reduced to 73 to 78 DEG C, water is added, and an oil layer and a water layer are obtained through separation; the oil layer is introduced into an ice waterbath for stirring crystallization, filtering is carried out to obtain filtrate o-fluoronitrobenzene; a crystal product obtained through separation is introduced into a rectifying tower, crystal melting is carried out, tower top temperature is controlled to be 205 to 210 DEG C, tower bottom temperature is controlled to be 235 to 240 DEG C, a non-condensing collector is adopted for rectification, and a p-fluoronitrobenzene crude product and a m-fluoronitrobenzene crude product are obtained; the p-fluoronitrobenzene crude product is heated to 150 to 180 DEG C, is cooled to 50 to 55 DEG C, and iscooled to 25 to 30 DEG C slowly for separation, a filtrate is collected, and purified p-fluoronitrobenzene is obtained; the m-fluoronitrobenzene crude product is cooled to 10 to 15 DEG C, is heated to 30 to 40 DEG C slowly, separation is carried out, and a crystal product is collected to obtain purified m-fluoronitrobenzene.
Owner:SHANGYU XIES CHEM IND
Prepn process of nitro aromatic amine compound
ActiveCN100417638CLow costEasy to useOrganic compound preparationAmino compound preparationDistillationNitrobenzene
The present invention belongs to the field of preparation technology of aromatic amine as hole transmission material intermediate. The preparation process of nitro aromatic amine compound includes the following steps: reacting one of phenylamine, diphenylamine and triphenylamine, strong alkali and p-fluoro nitrobenzene or p-chloro nitrobenzene in certain molar proportion in N, N-dimethyl formamide as solvent inside some reactor at 90-130 deg.c for 5-16 hr; filtering in the hot state to eliminate reaction produced impurity; decompression distillation to eliminate DMF and to obtain initial product; and re-crystallization with methanol, ethanol or acetonitrile to obtain the nitro aromatic amine compound. The present invention has the advantages of cheap material, simple post-treatment, high product yield, high product purity and being suitable for industrial production.
Owner:TIANJIN UNIV
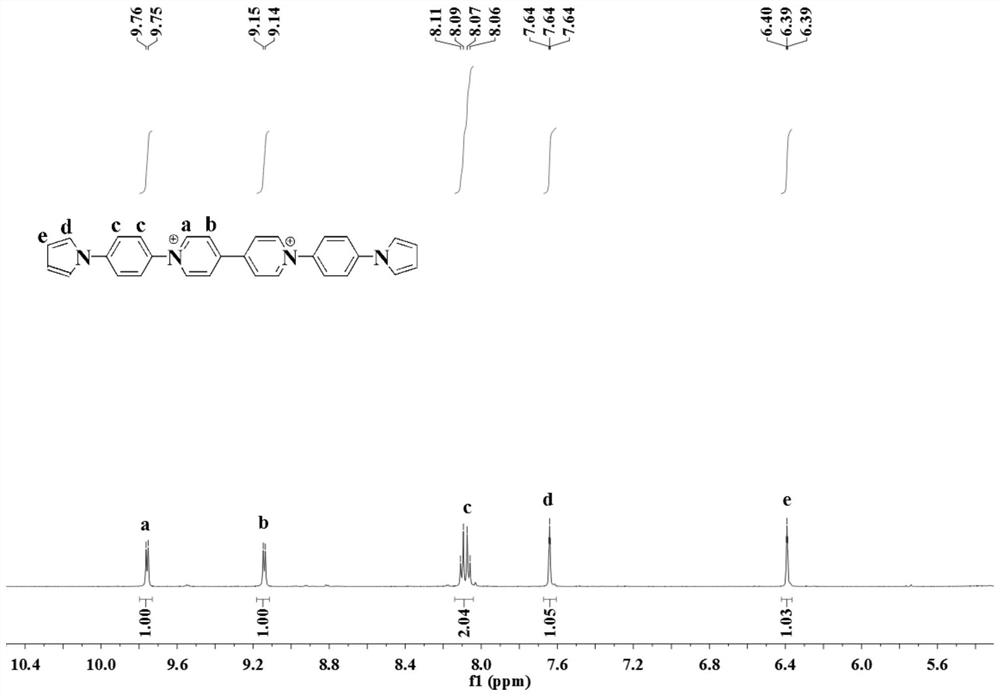
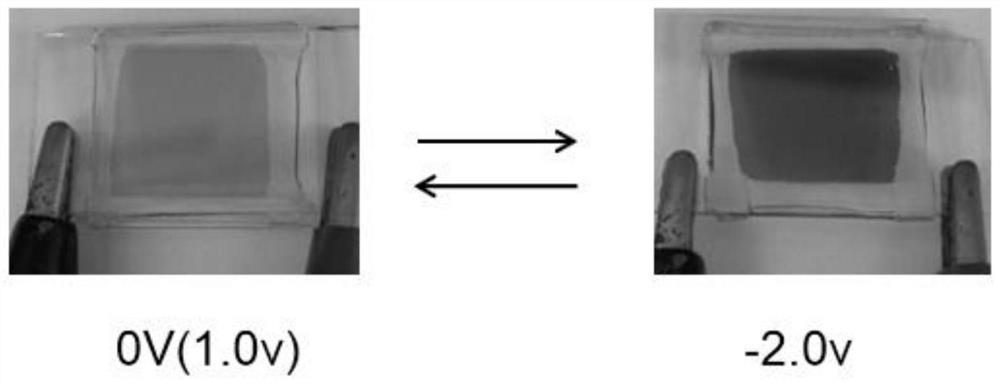
Viologen derivative electrochromic material and preparation method thereof
ActiveCN110105336BImprove the state of electron deficiencyReduce electrochromic voltageOrganic chemistryTenebresent compositionsElectrolytic agentNitro compound
The invention discloses a purpurine derivative electrochromic material and a preparation method thereof. The preparation method comprises the steps that 4,4'-dipyridine is adopted as a raw material and undergoes a Menschutkin reaction with 1-chloro-2,4-dinitrobenzene to synthesize a 1,1'-bis(2,4-dinitro)-4,4'-dipyridyl salt; then an N-heterocyclic compound and p-fluoronitrobenzene are subjected toaromatic nucleophilic amination to prepare a 4-nitrocompound, and then the 4-nitrocompound is reduced into a 4-amino compound; finally, the products obtained after the reactions in the two steps undergo a Zincke reaction to obtain a target product. A pyrrole group and carbazole are introduced into a derivative, the electron deficient state of the core group 4,4'-dipyridine of a purpurine compoundis improved, and the electrochromic voltage of the purpurine compound is reduced; moreover,a bipolar electrochromic device can be obtained, a higher contrast ratio is realized, multiple color changesare obtained, and the problem of electrolyte leakage of solution-type electrochromic devices can also be solved.
Owner:SOUTH CHINA UNIV OF TECH
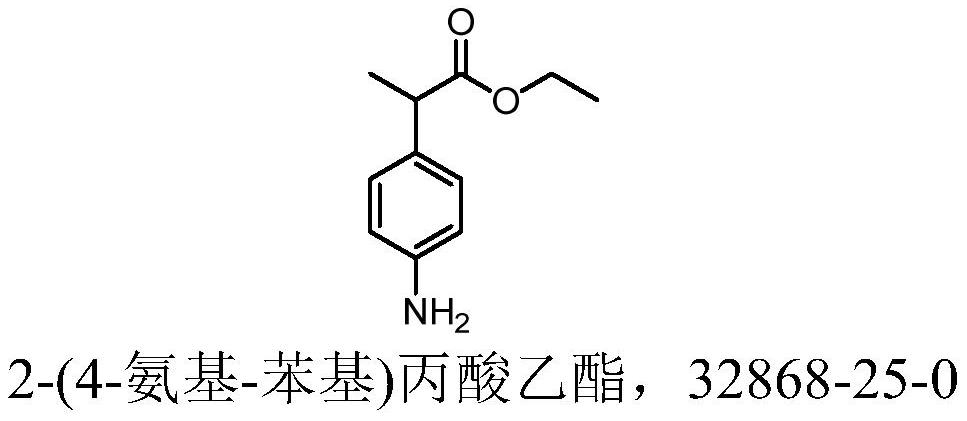
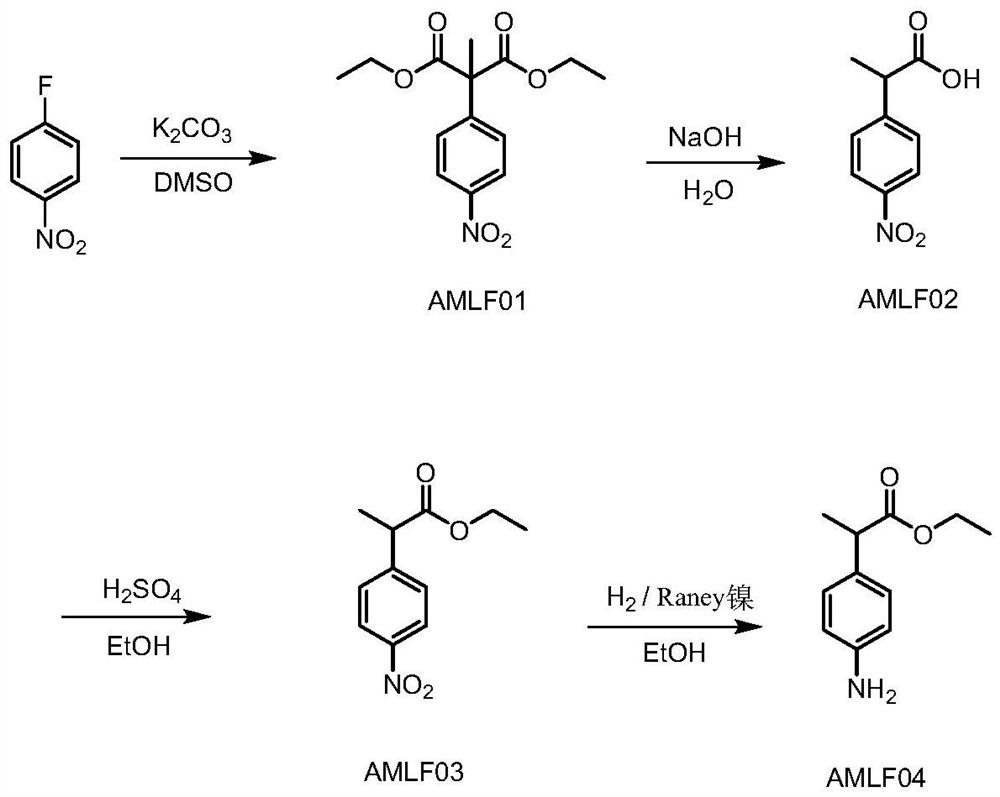
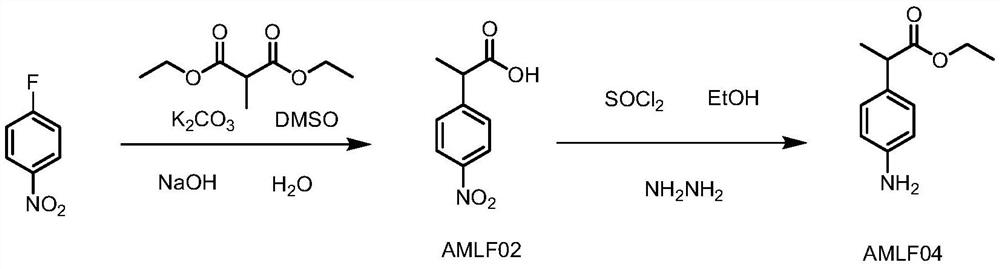
Preparation method of alminoprofen intermediate
InactiveCN113135831AImprove securityLow costOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisMethylmalonic acid
The invention discloses a preparation method of an alminoprofen intermediate, belongs to the field of organic chemical synthesis, and provides a new technical route for improving the defects of synthetic reaction of ethyl 2-(4-amino-phenyl)propionate: 1) taking p-fluoronitrobenzene and diethyl methylmalonate as raw materials, adding alkali, conducting heating, finishing the reaction, conducting cooling, conducting hydrolyzing with alkali, after the reaction is finished, conducting layering, conducting washing with water, extracting impurities, adjusting the pH value, extracting a product, and conducting concentrating to obtain AMLF02; 2) heating AMLF02 in a solvent to 55-75 DEG C, and dropwise adding thionyl chloride for esterification reaction, conducting cooling, adjusting the pH, directly adding a catalyst for reduction at the temperature of 70-80 DEG C, after the reaction is finished, conducting filtering with the aid of diatomite, neutralizing the filtrate to 7-8, conducting concentrating to remove ethanol, extracting a water phase with methylbenzene, and conducting concentrating to obtain AMLF04. The method has the beneficial effects that the problem of slow filtration is solved, extraction is omitted, time is saved, and cost is saved; direct layering is performed after reaction, the amount of hydrochloric acid is reduced, and the treatment difficulty of three wastes is reduced; and safe hydrazine hydrate replaces active nickel.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com