2,4,4'-tribromodiphenyl ether hapten preparation method and application thereof
A technology of tribrominated diphenyl ether and dibrominated diphenyl ether, applied in the field of biochemistry, can solve problems such as interference, rareness, low concentration, etc., and achieve the effects of fast speed, high yield and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
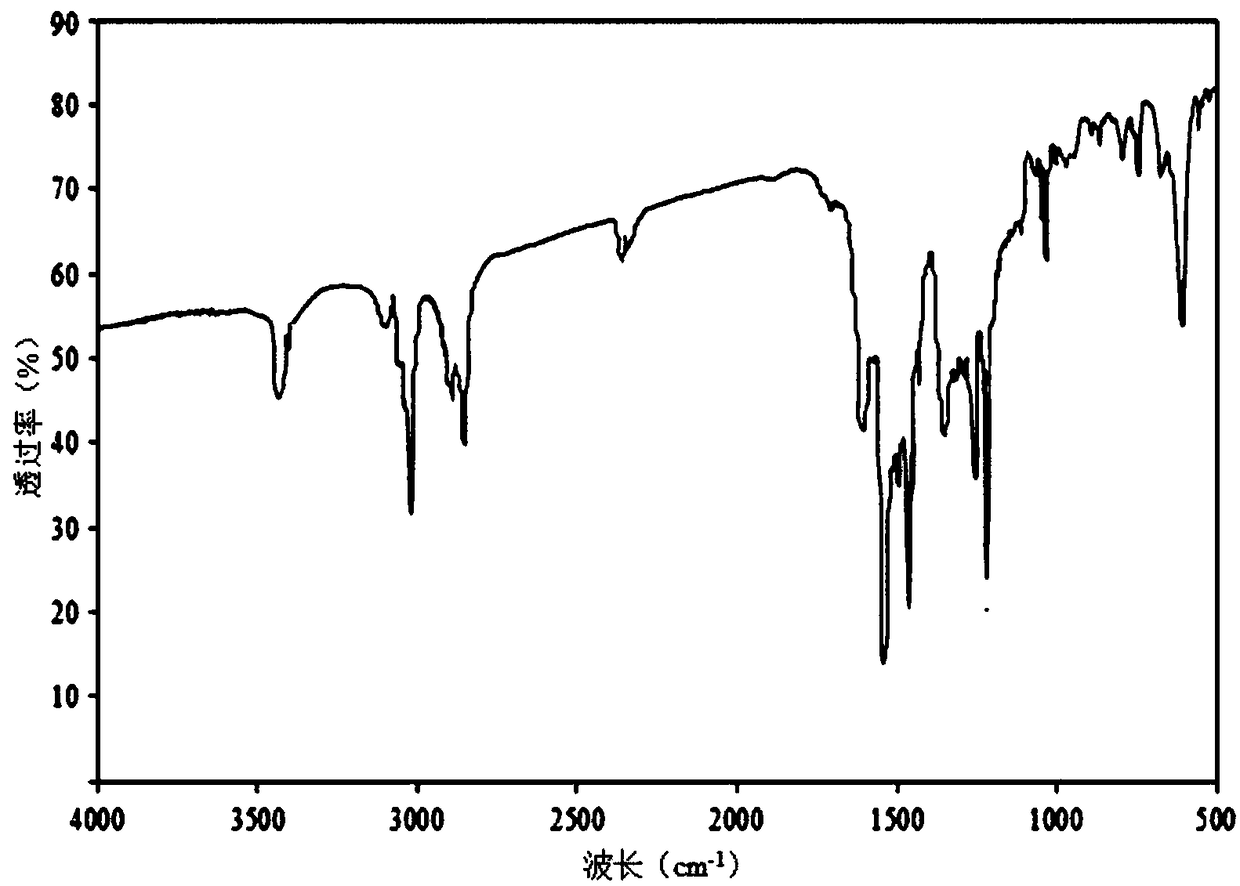
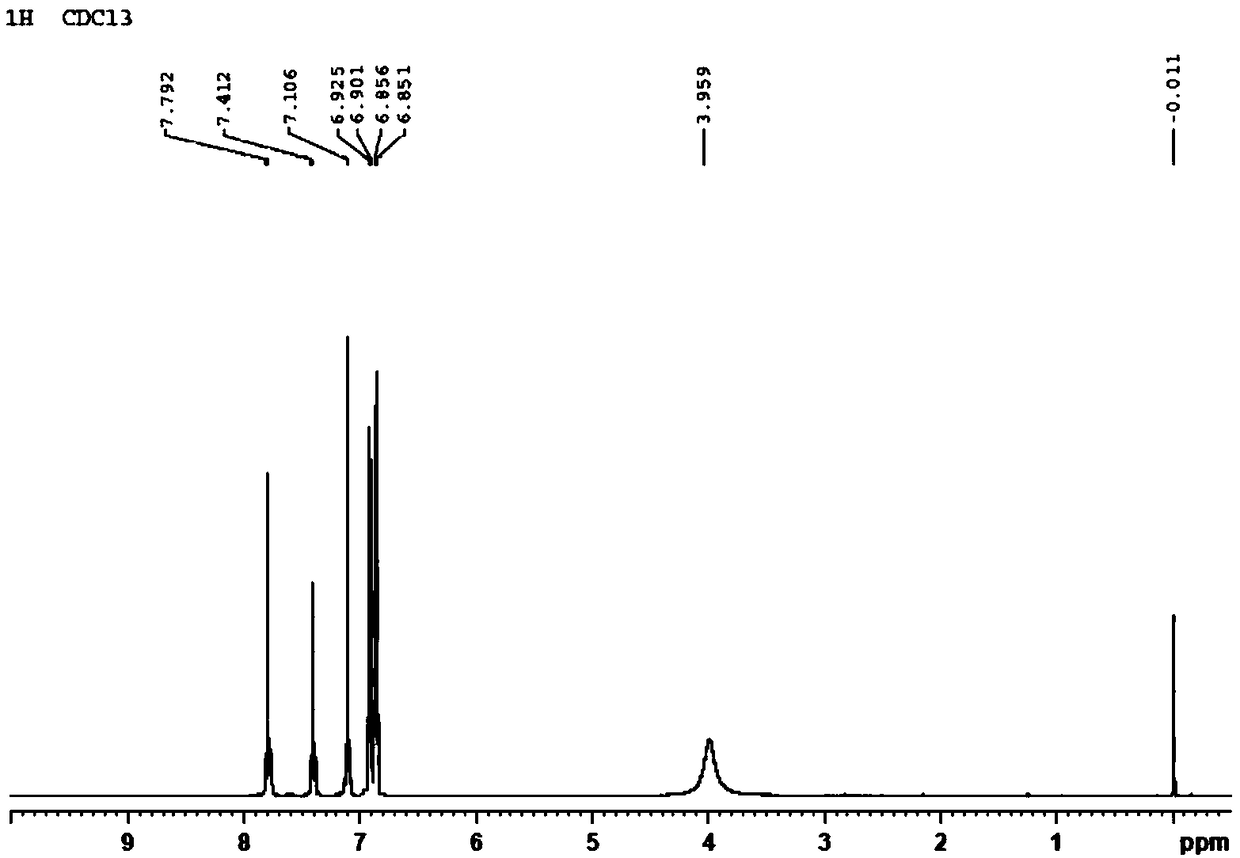
[0031] Example 1 Preparation of BDE-28 Hapten
[0032] Dissolve 1.04g of 2,4-dibromophenol (4.13mmol) in 8mL of dry DMF, place in an ice bath and cool down to 0°C, add 0.199g of sodium hydride (8.28mmol) in batches, and stir in the ice bath for 20min , placed at room temperature for 10min; then slowly added 0.583g p-fluoronitrobenzene (4.13mmol), and stirred at 90°C for 2h; 2 Cl 2 After extraction (25mL×3), the organic extracts were combined and washed with anhydrous MgSO 4 Drying, rotary evaporation and concentration, and after separation and purification by silica gel column chromatography (eluent: n-hexane: ether = 2:3 (v / v)), the orange-yellow oily product 4-nitro-2',4' was obtained -Dibromodiphenyl ether, the yield is 66.1%.
[0033]The above 4-nitro-2′,4′-dibromodiphenyl ether (1.30mmol, 0.485g) was dissolved in 8mL of n-butylamine, formic acid (10.4mmol, 0.4mL) and platinum carbon (0.770g, 5% carbon loading) as a catalyst, the mixture was heated and stirred at 90°C ...
Embodiment 2
[0035] The preparation of embodiment 2 BDE-28 coating former
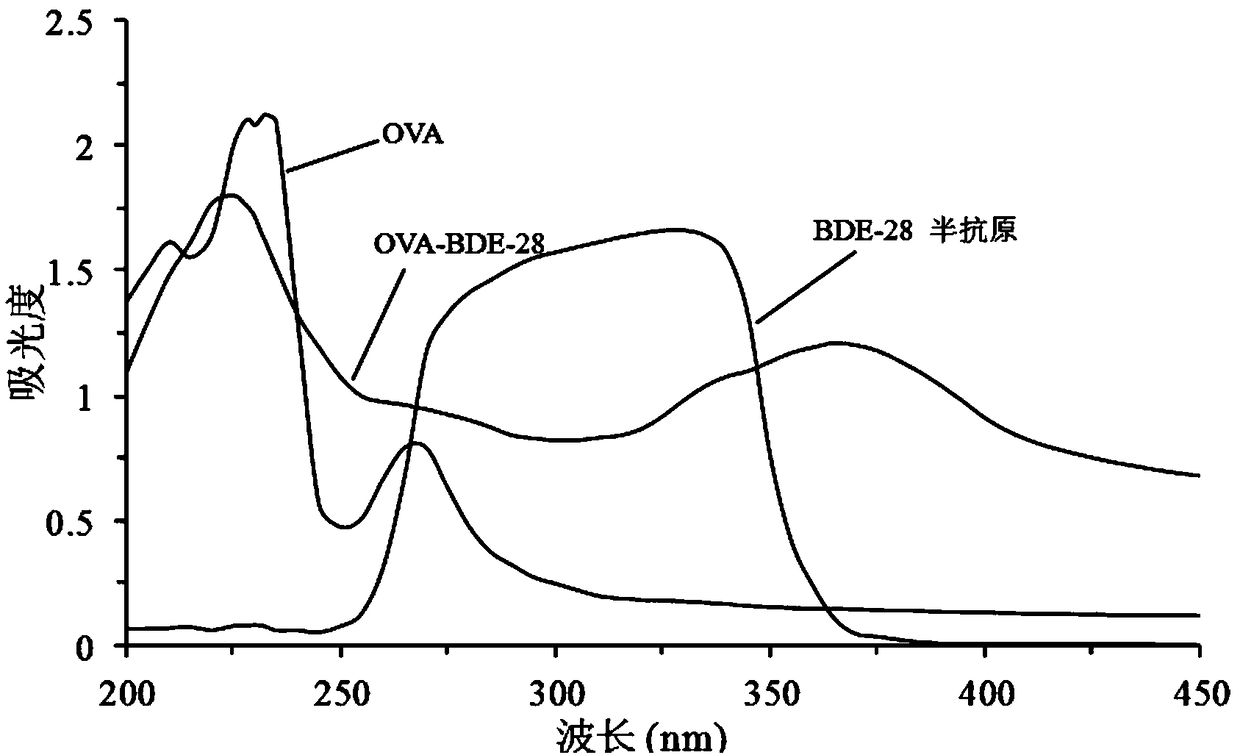
[0036] The BDE-28 hapten prepared in Example 1 is used to establish a variety of immunoassay methods and complete the detection of BDE-28 in environmental samples. One of the key uses is exactly to be used with carrier proteins such as bovine serum albumin (BSA), ovalbumin ( OVA), keyhole limpet hemocyanin (KLH) and other macromolecular protein carriers are coupled to prepare the BDE-28 artificial whole antigen. Dialdehyde method, diazotization method. Through the above-mentioned BDE-28 hapten (BDE-28-NH 2 ) is coupled with OVA to prepare the preparation route of BDE-28 coating agent as shown in formula (Ⅲ),
[0037]
[0038] The specific operation steps are as follows: take 0.0343gBDE-28-NH 2 (0.1mmol) into a 25mL Erlenmeyer flask, add 1mL N,N-dimethylformamide (DMF) dropwise, stir well and add dropwise to 10mL, 0.08mmol OVA solution (0.01M, pH7.40 phosphoric acid salt buffer solution), then slowly add 0.03...
Embodiment 3
[0040] Example 3 Immunoassay detection
[0041] Utilize the BDE-28 coated original prepared in embodiment 2 and polyclonal antibody (the polyclonal antibody used is obtained by BDE-28 artificial antigen successively immunizing New Zealand white rabbit several times) to establish the immune PCR method based on gold nanobiological probe (GNPs -iPCR), the specific operation steps of the GNPs-iPCR method are as follows:
[0042] a. Treat the PCR tube with 0.8% glutaraldehyde solution to enhance its surface adsorption, 20μL / well, incubate at 37°C for 6h, and use ddH 2 O Repeatedly wash the tube 3 times, 200μL / well, dry it and set aside; b. Dilute the original BDE-28 coating solution with 0.05M, pH9.60 carbonate buffer, add it to the PCR tube for coating, 20μL / well , overnight at 4°C; c. Pour off the original dilution of BDE-28 coating, wash the tube 3 times with PBST (0.01M, pH7.40 phosphate buffer + 0.05% Tween 20), 200μL / well, add 3% OVA (dissolved in 0.01M, pH 7.40 phosphate b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com