Viologen derivative electrochromic material and preparation method thereof
A technology of electrochromic materials and derivatives, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as liquid electrolyte leakage, and achieve electrolyte leakage, good designability, and high contrast Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
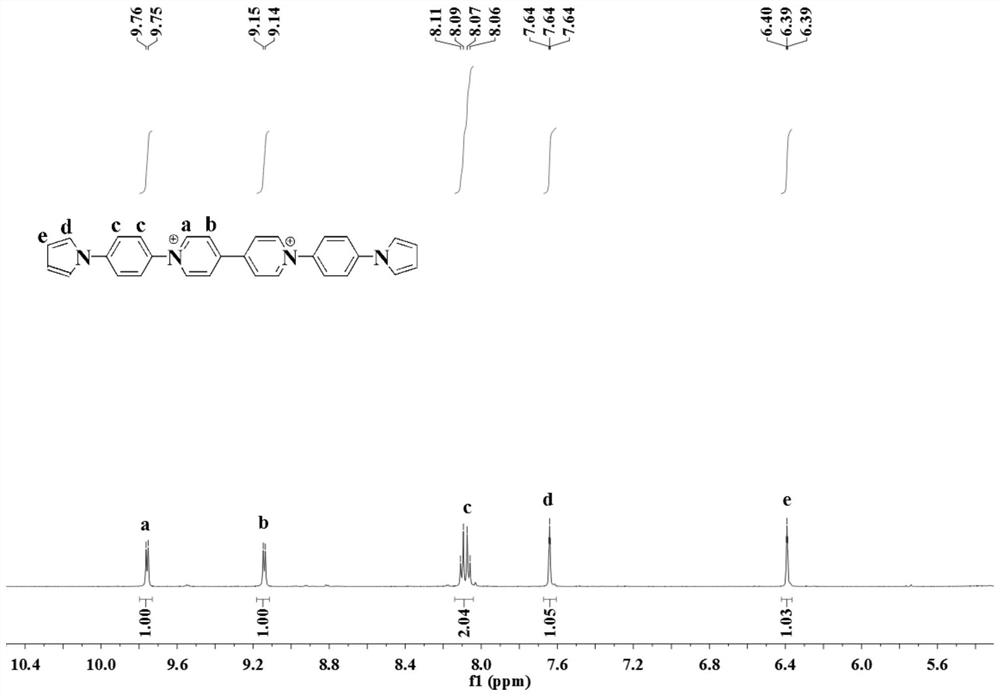
[0056] A preparation method of viologen derivative electrochromic material (A) 1,1'-(4-pyrrolylphenyl)-4,4'-bipyridine, comprising the following steps:
[0057] 1) Add 2.1g of pyrrole, 40mL of DMF, 8.9g of K to a 250mL three-neck flask 2 CO 3 , at 100°C N 2 Stir in the atmosphere for 30min, then add 5.4g of p-fluoronitrobenzene, and heat to reflux for 5h; after the reaction is completed, cool the solution to room temperature, add cold water, precipitate, and filter; wash the precipitate with water for 3 times, and dry; 100mL of diethyl ether was treated to obtain N-(4-nitrophenyl)pyrrole with a yield of 66%;
[0058] MS: m / z=188.1
[0059] 1 H NMR (600MHz, Chloroform-d) δ8.24(d,2H),7.88(d,2H),7.32(d,2H),6.34(t,2H).
[0060] Instruments used: mass spectrometry (MS) liquid chromatography-mass spectrometry, specification Agilent1100, produced by Bruker Company, Germany; hydrogen spectrum (1H NMR) nuclear magnetic resonance spectrometer, specification AVANCE III HD400, produc...
Embodiment 2
[0081] A method for preparing viologen derivative electrochromic material 1,1'-(4-pyrrolylphenyl)-4,4'-bipyridine, comprising the following steps:
[0082] 1) Add 4.2g of pyrrole, 80mL of DMF, 17.9g of K to a 250mL three-necked flask 2 CO 3 , at 100°C N 2 Stir in the atmosphere for 30min, then add 10.8g of p-fluoronitrobenzene, and heat to reflux for 5h; after the reaction is completed, cool the solution to room temperature, add cold water, precipitate, and filter; wash the precipitate with water for 3 times, and dry; 100mL of diethyl ether was treated to obtain N-p-nitrophenylpyrrole with a yield of 68.9%;
[0083] MS: m / z=188.1
[0084] 1 H NMR (600MHz, Chloroform-d) δ8.24(d,2H),7.88(d,2H),7.32(d,2H),6.34(t,2H).
[0085] Instruments used: mass spectrometry (MS) liquid chromatography-mass spectrometry, specification Agilent1100, produced by Bruker, Germany; hydrogen spectrum (1H NMR) nuclear magnetic resonance spectrometer, specification AVANCE III HD400, produced by Bru...
Embodiment 3
[0105] A preparation method of viologen derivative electrochromic material (B) 1,1'-(4-carbazolylphenyl)-4,4'-bipyridine, comprising the steps of:
[0106] 1) Add 4.18g carbazole, 40mL dry DMSO, 5.18g K 2 CO 3 , at N 2 Stir in the atmosphere for 10min, then add 3.6g of p-fluoronitrobenzene, heat up to 110°C, and heat to reflux for 15h; after the reaction is completed, cool the solution to room temperature, add 150mL of methanol to precipitate, and obtain yellow crystal N-p-nitrophenylcarba Azole, yield 75.4%;
[0107] MS: m / z=288.1
[0108] 1 H NMR (600MHz, Chloroform-d) δ8.36(d,2H),8.33(d,2H),8.04(d,2H),7.90(d,2H),7.39(t,2H),7.32(t, 2H).
[0109] Instruments used: mass spectrometry (MS) liquid chromatography-mass spectrometry, specification Agilent1100, produced by Bruker, Germany; hydrogen spectrum (1H NMR) nuclear magnetic resonance spectrometer, specification AVANCE III HD400, produced by Bruker, Germany.
[0110] 2) Add 5.2g of N-p-nitrophenylcarbazole, 0.2g of Pd / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com