Nickel-based CO hydrogenation catalyst, preparation method therefor and application of nickel-based CO hydrogenation catalyst
A technology of hydrogenation reaction and catalyst, applied in the direction of catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalyst, etc., which can solve the problem of high requirements for reaction equipment, high reaction temperature and complex preparation methods and other problems, to achieve high catalytic activity and stability, high content of active components, and reduce the effect of reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Specifically, the preparation method of the nickel-based CO hydrogenation catalyst may comprise the following steps:
[0025] Step A, prepare a mixed aqueous solution of nickel salt and aluminum salt, and the concentration of nickel salt in the solution is preferably 0.5-1.5 mol / L, so as to obtain a mixed salt solution. Wherein, the nickel salt solution is at least one of nickel nitrate, nickel acetate and nickel sulfate; the aluminum salt solution is at least one of aluminum nitrate and aluminum sulfate. The amount of nickel salt in the mixed salt solution is based on the mass of the methanation catalyst carrier, and is calculated based on the loading of nickel oxide as 55 to 90 wt%; if there is no special description, the loading in this application is The mass percentage calculated based on the mass of the methanation catalyst carrier.
[0026] Step B. According to the ratio of using 2 to 6 times the volume of the first part of the alkali solution per volume of the ...
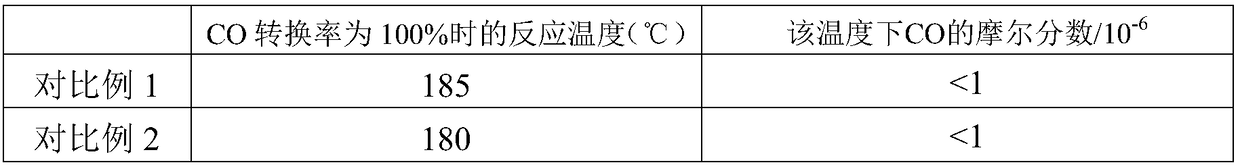
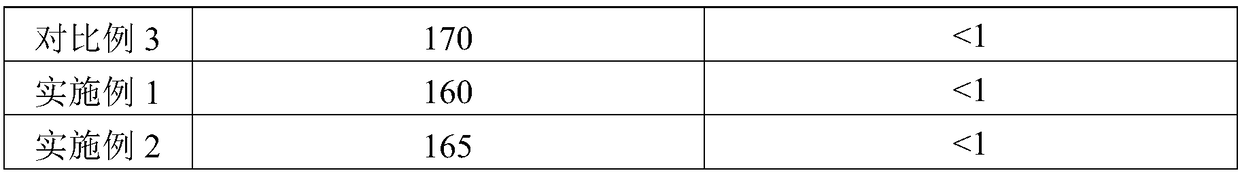
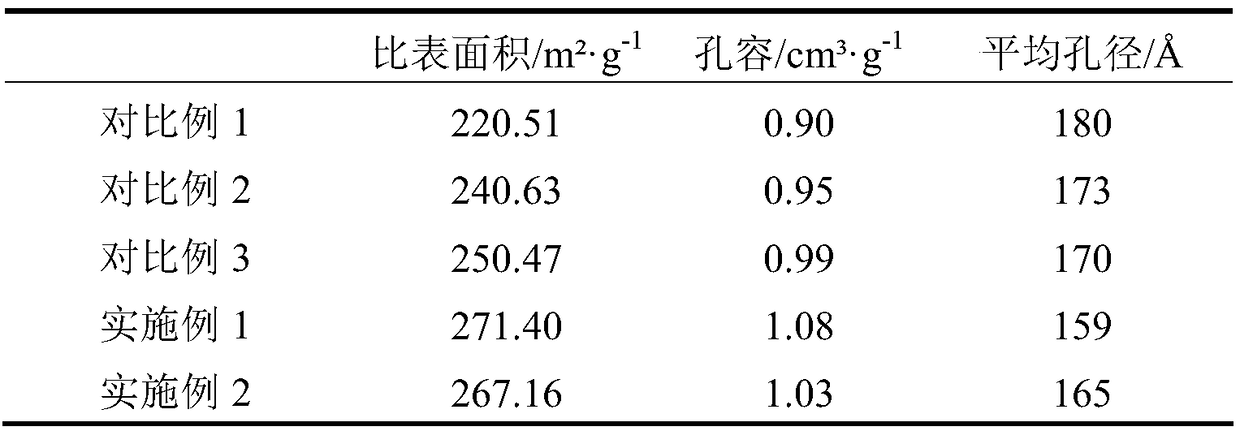
Embodiment 1
[0038] A nickel-based CO hydrogenation reaction catalyst with a nickel oxide loading of 66% is prepared by the co-precipitation method provided by the present invention, and the preparation method may include the following steps:
[0039] Step A1. Both 59.45 g of nickel nitrate hexahydrate and 58.84 g of aluminum nitrate hexahydrate were dissolved in 150 mL of deionized water to obtain a mixed salt solution.
[0040] Step B1, using a beaker as a reaction vessel, add 20mL of Na in the beaker with a concentration of 2mol / L 2 CO 3 solution and 20mL deionized water, then control the reaction temperature to 75-85°C, and under the stirring condition of 5-20r / s, the concentration of 2mol / L Na 2 CO 3 The mixed salt solution that solution and step A1 make adopts the mode of concurrent flow to join in the described beaker, and control Na 2 CO 3 The molar ratio of the solution to the mixed salt solution is 0.5-5:1, and at the same time, the pH value of the liquid in the reaction vess...
Embodiment 2
[0044] A nickel-based CO hydrogenation reaction catalyst with a nickel oxide loading of 75% is prepared by the co-precipitation method provided by the present invention, and the preparation method may include the following steps:
[0045] Step A2. Both 69.36 g of nickel nitrate hexahydrate and 44.13 g of aluminum nitrate hexahydrate were dissolved in 190 mL of deionized water to obtain a mixed salt solution.
[0046] Step B2, using a beaker as a reaction vessel, add 20mL of Na in the beaker with a concentration of 2mol / L 2 CO 3 solution and 20mL deionized water, then control the reaction temperature to 75-85°C, and under the stirring condition of 5-20r / s, the concentration of 2mol / L Na 2 CO 3 The mixed salt solution that solution and step A2 make adopts the mode of concurrent flow to join in the described beaker, and control Na 2 CO 3 The molar ratio of the solution to the mixed salt solution is 0.5-5:1, and at the same time, the pH value of the liquid in the reaction vess...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com