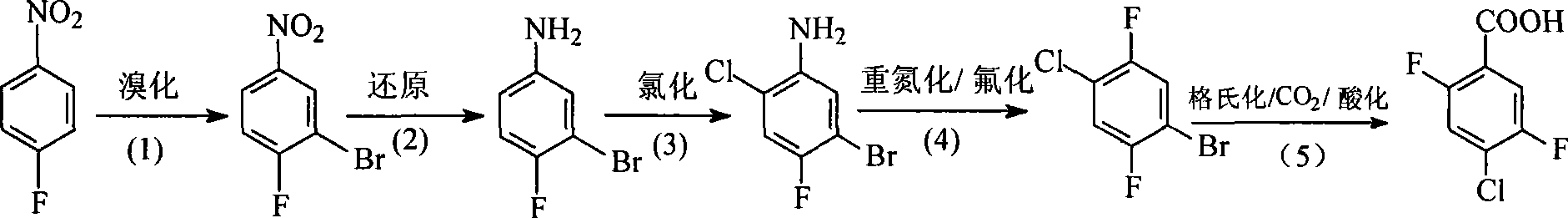
Preparation method of 4-chloro-2,5-difluorobenzoic acid
A technology of difluorobenzoic acid and difluorobromobenzene, which is applied in the field of preparation of fluorine-containing organic acids, can solve problems such as difficulty in preparation and purification, low purity of difluorobenzoic acid, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0021] A. Bromination
[0022] Add 399g of water and 510g of concentrated sulfuric acid (T≤60°C) into a 5L three-neck flask equipped with a spherical condenser, thermometer, and electric stirrer, and then add 90g (0.638mol) of p-fluoronitrobenzene, and control the temperature at 38-43 °C, 112 g (0.67 mol) of potassium bromate was added in batches, and the reaction time was about 12 hours. During the feeding process, follow up and measure the sample. When p-nitrofluorobenzene ≤ 2%, and the product content is above 98%, stop the reaction, cool, add 500g of water, cool to below 40°C, let stand for stratification, and wash the lower layer with water. Wash with weak alkali until neutral. Distilled under reduced pressure to obtain 110 g of 3-bromo-4-fluoronitrobenzene with a yield of 78.7% and a content of 98%. Melting point: 57~59℃, 1 H NMR (CDCl 3 ) δ (ppm): 7.29 to 8.46 (m, 3H).
[0023] B. Hydrogenation reduction
[0024] Add 600 mL of methanol, 200 g (0.913 mol) of 3-brom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com