Triarylamino polyamide containing condensed ring anthryl side group structure as well as preparation method and application thereof
A triarylamine-based polyamide and anthracene-based technology, which is applied in the preparation of carboxylic acid amides, amino compounds, and organic compounds, can solve the problems of poor heat resistance and easy peeling of polymer films, and achieve good chemical resistance. Stability, excellent electrochromic performance and memory performance, effect of improving adhesive strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
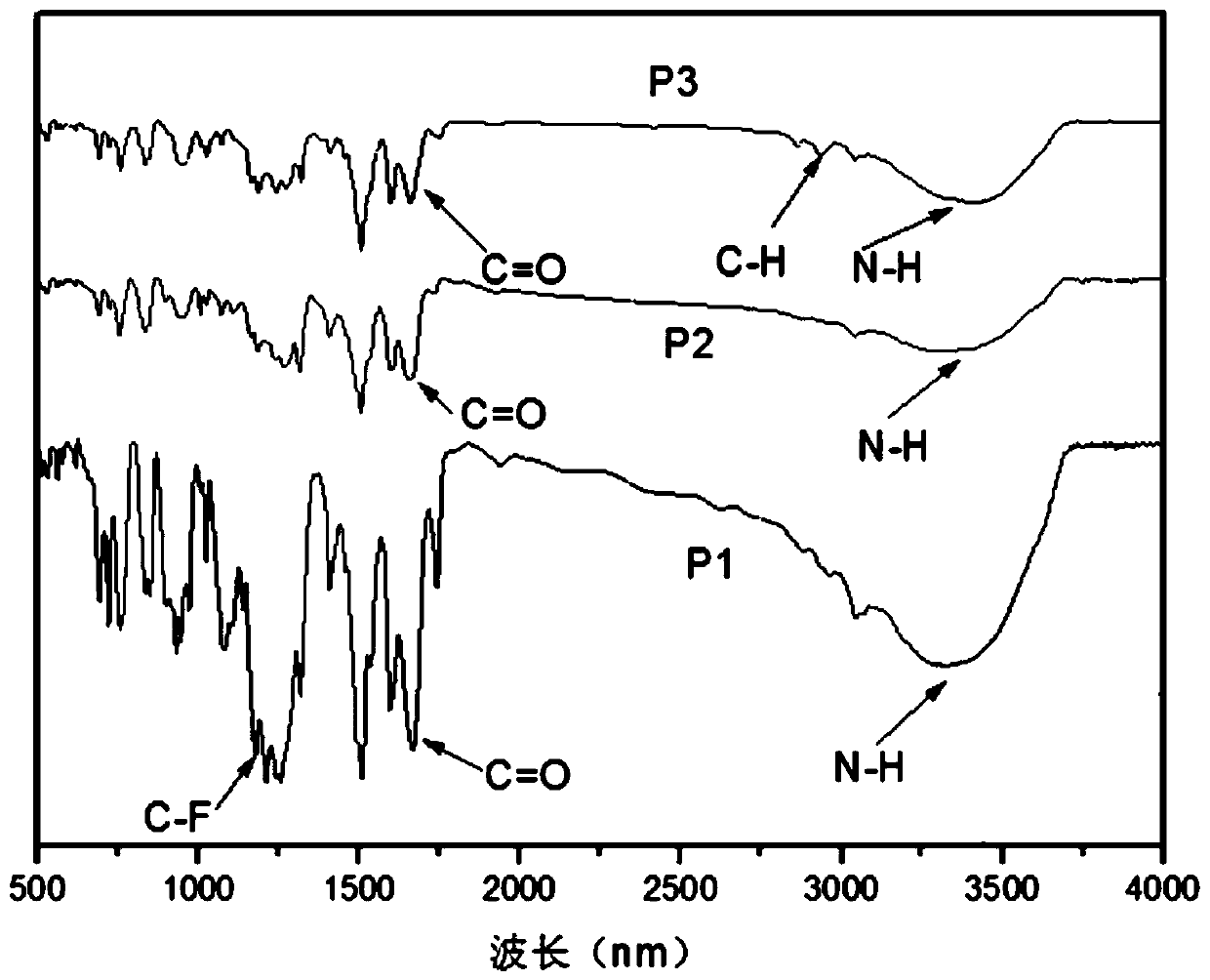
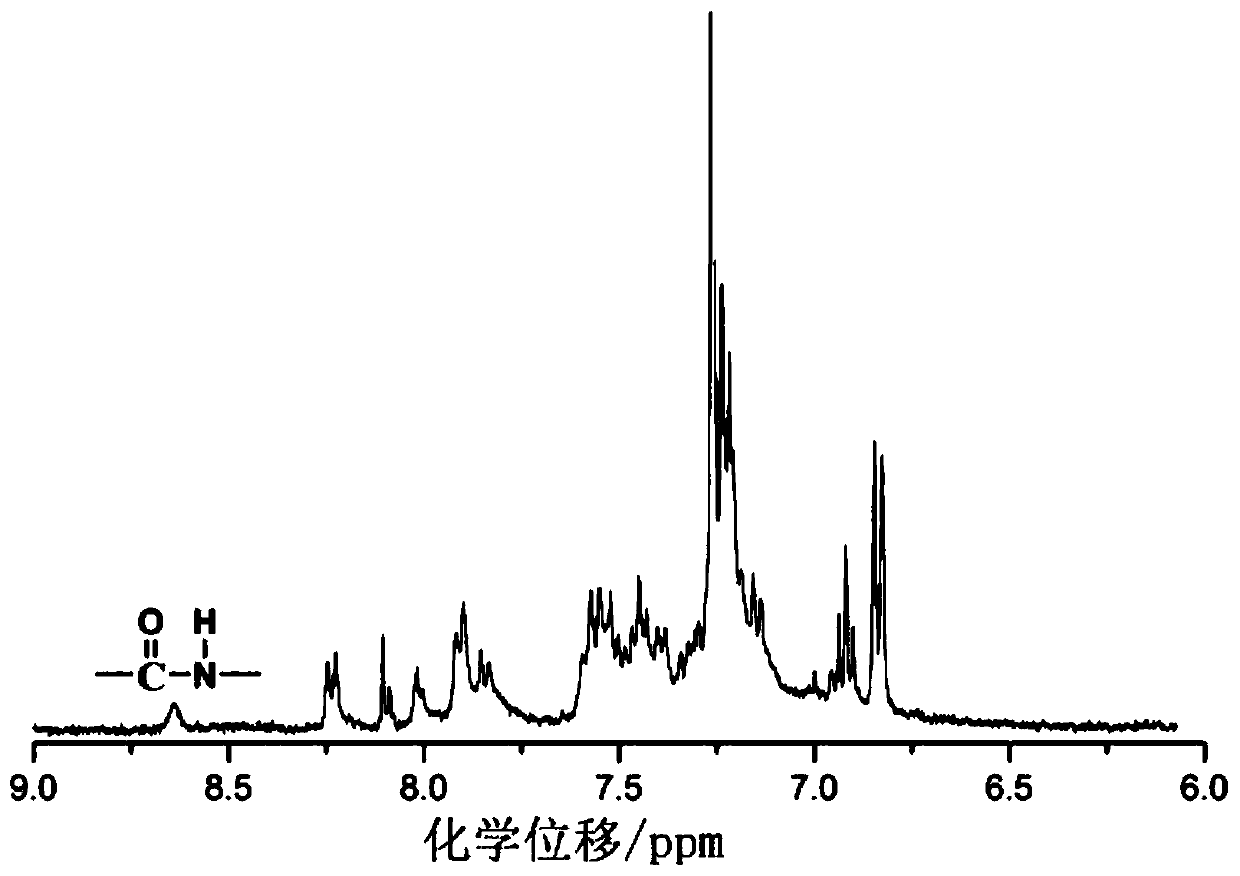
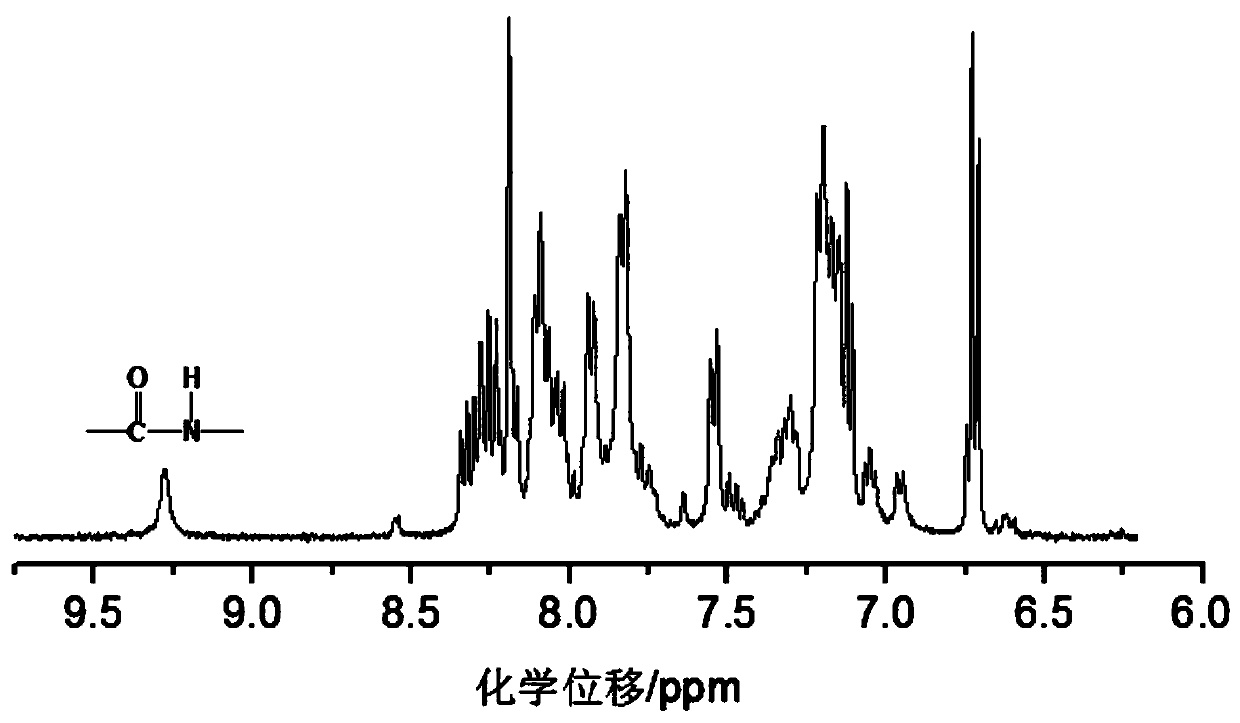
[0046] Specific embodiments 1. In this embodiment, a triarylamine-based polyamide containing a fused ring anthracenyl side group structure is a triarylamine-based polyamide P1 containing a fused ring anthracenyl side group structure. Triarylamine-based polyamide P2 or triarylamine-based polyamide P3 containing fused ring anthracene side group structure;
[0047] The structural formula of triarylamino polyamide P1 containing fused ring anthracene side group structure is:
[0048] In the formula, n is an integer of 6 to 13;
[0049] The structural formula of triarylamino polyamide P2 containing fused ring anthracene side group structure is:
[0050] In the formula, n is an integer of 6 to 20;
[0051] The structural formula of triarylamino polyamide P3 containing fused ring anthracene side group structure is:
[0052] In the formula, n is an integer of 10 to 25.
specific Embodiment approach 2
[0053] Specific embodiment 2. In this embodiment, a method for preparing a triarylamine-based polyamide containing a fused ring anthracenyl side group structure is carried out according to the following steps:
[0054] (1), under nitrogen atmosphere, add solvent DMSO to p-bromoaniline and cesium fluoride, under the condition of stirring and constant pressure, add p-fluoronitro at a rate of 1-2 drops per second, then heat up to Perform constant temperature reaction at 110°C, cool to room temperature after the reaction is complete, place the reaction product in distilled water at 24-25°C, stir until the crude product precipitates, then filter the crude product, wash the crude product with water at 99-100°C for 2- 3 times, then placed in a vacuum drying oven for drying, then recrystallized with acetic acid, then filtered out the crystallized product, and vacuum dried the crystallized product to obtain a yellow powder, which was 4-bromo-N, N-bis(4 - nitrophenyl) aniline;
[0055]...
specific Embodiment approach 3
[0065] Specific embodiment three: the difference between this embodiment and specific embodiment two is: if the diacid is 2,2-bis(4-carboxyphenyl)hexafluoropropane, then the triarylamine group containing the fused ring anthracenyl side group structure The polyamide is a triarylamine-based polyamide P1 containing a fused ring anthracene side group structure. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Coloring time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com