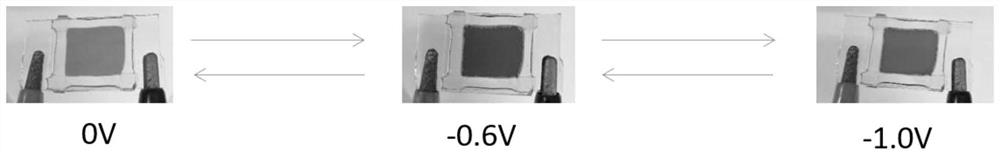
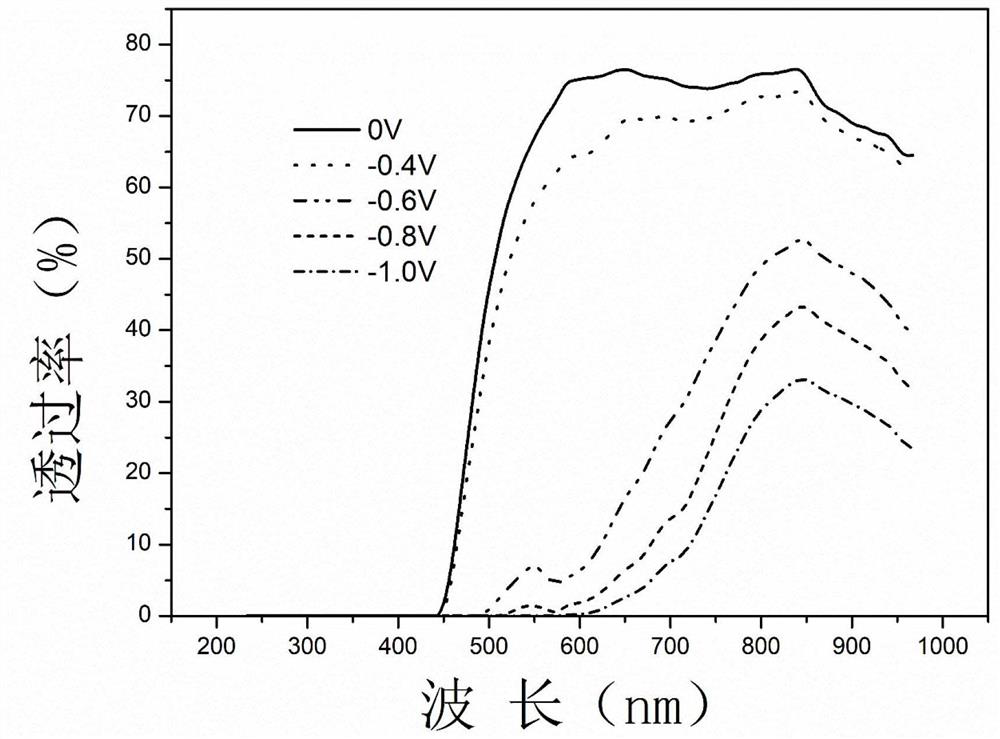
Purpurine derivative electrochromic and electrofluorescent color-changing material as well as preparation method and application thereof
An electrochromic and derivative technology, used in color-changing fluorescent materials, chemical instruments and methods, optics, etc., can solve the problems of reducing the electrochromic voltage, unable to give viologen derivatives electrofluorescence color performance, etc. Optical contrast, excellent discoloration performance, effect of reducing discoloration voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
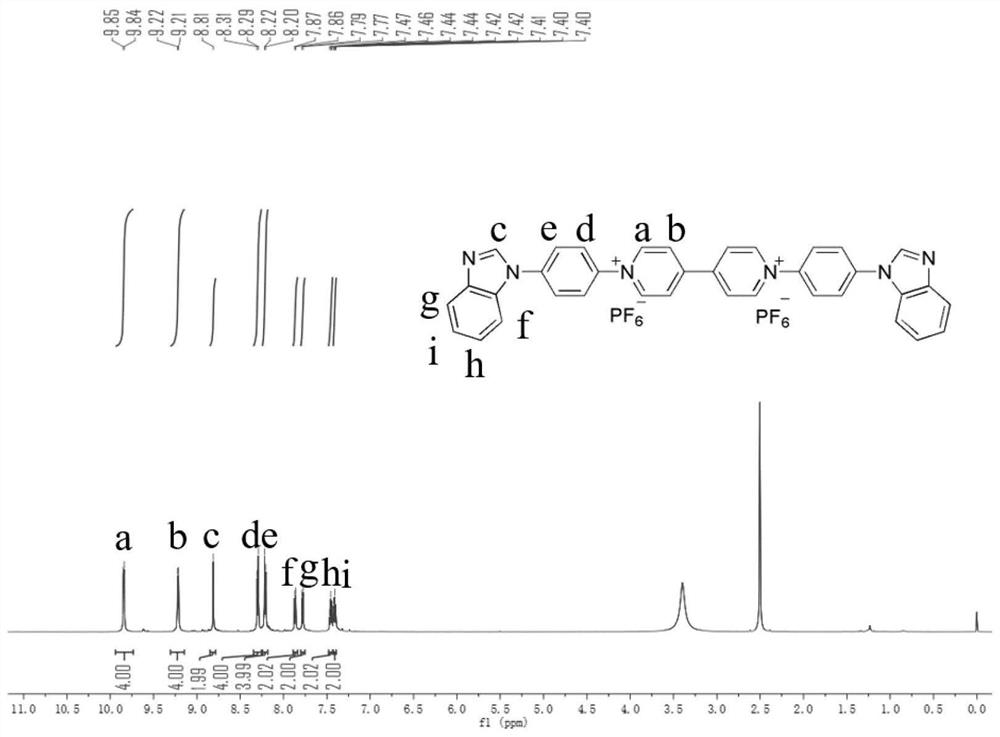
[0044] Preparation of Viologen Derivative Electrochromic and Electrofluorochromic Bifunctional Material (A) 1,1'-(4-Benzimidazolylphenyl)-4,4'-Bipyridyl Dihexafluorophosphate method, comprising the steps of:
[0045] 1) Add 2.5g benzimidazole, 3.3g p-fluoronitrobenzene, 3.3g potassium carbonate, 60mlDMF in a 100ml three-necked flask, 2 Stir at room temperature for 30 minutes under an atmosphere, then rise to 110°C, heat to reflux for 24 hours; after the reaction is completed, cool the solution to room temperature, add cold water, precipitate, filter, and obtain a precipitate; wash with deionized water and petroleum ether successively Precipitate 3 times; cross silica gel chromatography column with ethyl acetate-petroleum ether (1:2, v / v) eluent, obtain yellow powder product N-(4-nitrophenyl) benzimidazole, yield 96%;
[0046] MS: m / z=240.1
[0047] 1 H NMR (DMSO-d 6 ,600MHz,ppm)δ8.75(s,1H),8.47(d,2H),8.04(d,2H),7.80(dd,2H),7.39(dt,2H).
[0048] 2) Add 3.0g N-(4-nitrophen...
Embodiment 2
[0066] Preparation of Viologen Derivative Electrochromic and Electrofluorochromic Bifunctional Material (A) 1,1'-(4-Benzimidazolylphenyl)-4,4'-Bipyridyl Dihexafluorophosphate method, comprising the steps of:
[0067] 1) Add 2.0g benzimidazole, 3.0g p-fluoronitrobenzene, 3.0g potassium carbonate, 60mlDMF in a 100ml three-necked flask, 2 Stir at room temperature for 30 minutes under an atmosphere, then rise to 110°C, heat to reflux for 24 hours; after the reaction is completed, cool the solution to room temperature, add cold water, precipitate, filter, and obtain a precipitate; wash with deionized water and petroleum ether successively Precipitate 3 times; cross silica gel chromatography column with ethyl acetate-petroleum ether (1:2, v / v) eluent, obtain yellow powder product N-(4-nitrophenyl) benzimidazole, yield 90%;
[0068] MS: m / z=240.1
[0069] 1 H NMR (DMSO-d 6 ,600MHz,ppm)δ8.75(s,1H),8.47(d,2H),8.04(d,2H),7.80(dd,2H),7.39(dt,2H).
[0070] 2) Add 1.5g N-(4-nitrophen...
Embodiment 3
[0087] Preparation of Viologen Derivative Electrochromic and Electrofluorochromic Bifunctional Material (A) 1,1'-(4-Benzimidazolylphenyl)-4,4'-Bipyridyl Dihexafluorophosphate method, including the following steps:
[0088] 1) Add 4.0g benzimidazole, 6.0g p-fluoronitrobenzene, 6.0g potassium carbonate, 80mlDMSO in a 100ml three-necked flask, 2 Stir at room temperature for 30 minutes under an atmosphere, then rise to 110°C, heat to reflux for 24 hours; after the reaction is completed, cool the solution to room temperature, add cold water, precipitate, filter, and obtain a precipitate; wash with deionized water and petroleum ether successively Precipitate 3 times; cross silica gel chromatography column with ethyl acetate-petroleum ether (1:5, v / v) eluent, obtain yellow powder product N-(4-nitrophenyl) benzimidazole, yield 85%;
[0089] MS: m / z=240.1
[0090] 1 H NMR (DMSO-d 6 ,600MHz,ppm)δ8.75(s,1H),8.47(d,2H),8.04(d,2H),7.80(dd,2H),7.39(dt,2H).
[0091] 2) Add 2.0g N-(4-ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com