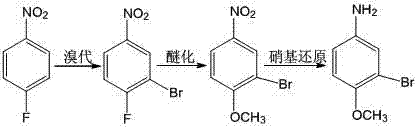
Method for preparing 3-bromo-4-methoxyaniline
A technology of methoxyaniline and methoxynitrobenzene, applied in the production field of pharmaceutical intermediates, can solve the problems of less 3-bromo-4-methoxyaniline, unsuitable for industrial production, and difficult to industrial production. Achieve the effect of convenient post-processing, low price and convenient processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Bromination reaction: Add 7.05 g of p-fluoronitrobenzene and 25 g of acetic acid into the reaction vessel, and control the temperature of the water bath to 15 °C. 9.34 g of N-bromosuccinimide were added slowly with stirring. During the addition, the temperature should not exceed 15°C. After the addition, the reaction was incubated for 10 h, and the reaction ended. After the reaction, the reactant was poured into 500 ml of ice water, and the solid was precipitated, filtered with suction, and dried to obtain 9.95 g of 3-bromo-4-fluoronitrobenzene as a light yellow powder solid, with a yield of 90.5%.
[0024] (2) Etherification reaction: add 11.0 g of 3-bromo-4-fluoronitrobenzene and 175 ml of methanol to the reaction vessel, control the temperature in a water bath at 10 °C, slowly add 12.15 g of sodium methoxide, and keep the system temperature at 10°C. After the addition, the reaction was incubated for 4 h, and the reaction ended. After the reaction was complete...
Embodiment 2
[0027] (1) Bromination reaction: Add 7.05 g of p-fluoronitrobenzene and 25 g of acetic acid to the reaction vessel, and control the temperature of the water bath to 25 °C. 9.34 g of N-bromosuccinimide were added slowly with stirring. During the addition process, the temperature should not exceed 25°C. After the addition, the reaction was incubated for 4.5 h, and the reaction ended. After the reaction was completed, the reactant was poured into 500 ml of ice water to precipitate a solid, which was filtered by suction and dried to obtain 9.98 g of 3-bromo-4-fluoronitrobenzene as a light yellow powder solid, with a yield of 90.7%.
[0028] (2) Etherification reaction: add 11.0 g of 3-bromo-4-fluoronitrobenzene and 175 ml of methanol to the reaction vessel, control the temperature in a water bath at 20 °C, slowly add 12.15 g of sodium methoxide, and keep the system temperature at 20°C. After the addition, the reaction was incubated for 0.8 h, and the reaction ended. After the ...
Embodiment 3
[0031] (1) Bromination reaction: Add 7.05 g of p-fluoronitrobenzene and 25 g of acetic acid into the reaction vessel, and control the temperature of the water bath to 35 °C. 9.34 g of N-bromosuccinimide were added slowly with stirring. During the addition process, the temperature should not exceed 35°C. After the addition, keep the temperature for 2.0 h to complete the reaction. After the reaction, the reactant was poured into 500 ml of ice water, and the solid was precipitated, filtered with suction, and dried to obtain 9.95 g of 3-bromo-4-fluoronitrobenzene as a light yellow powder solid, with a yield of 90.5%.
[0032] (2) Etherification reaction: add 11.0 g of 3-bromo-4-fluoronitrobenzene and 175 ml of methanol to the reaction vessel, control the temperature in a water bath at 40 °C, slowly add 12.15 g of sodium methoxide, and keep the system temperature at 40°C. After the addition, the reaction was incubated for 0.8 h, and the reaction ended. After the reaction was co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com