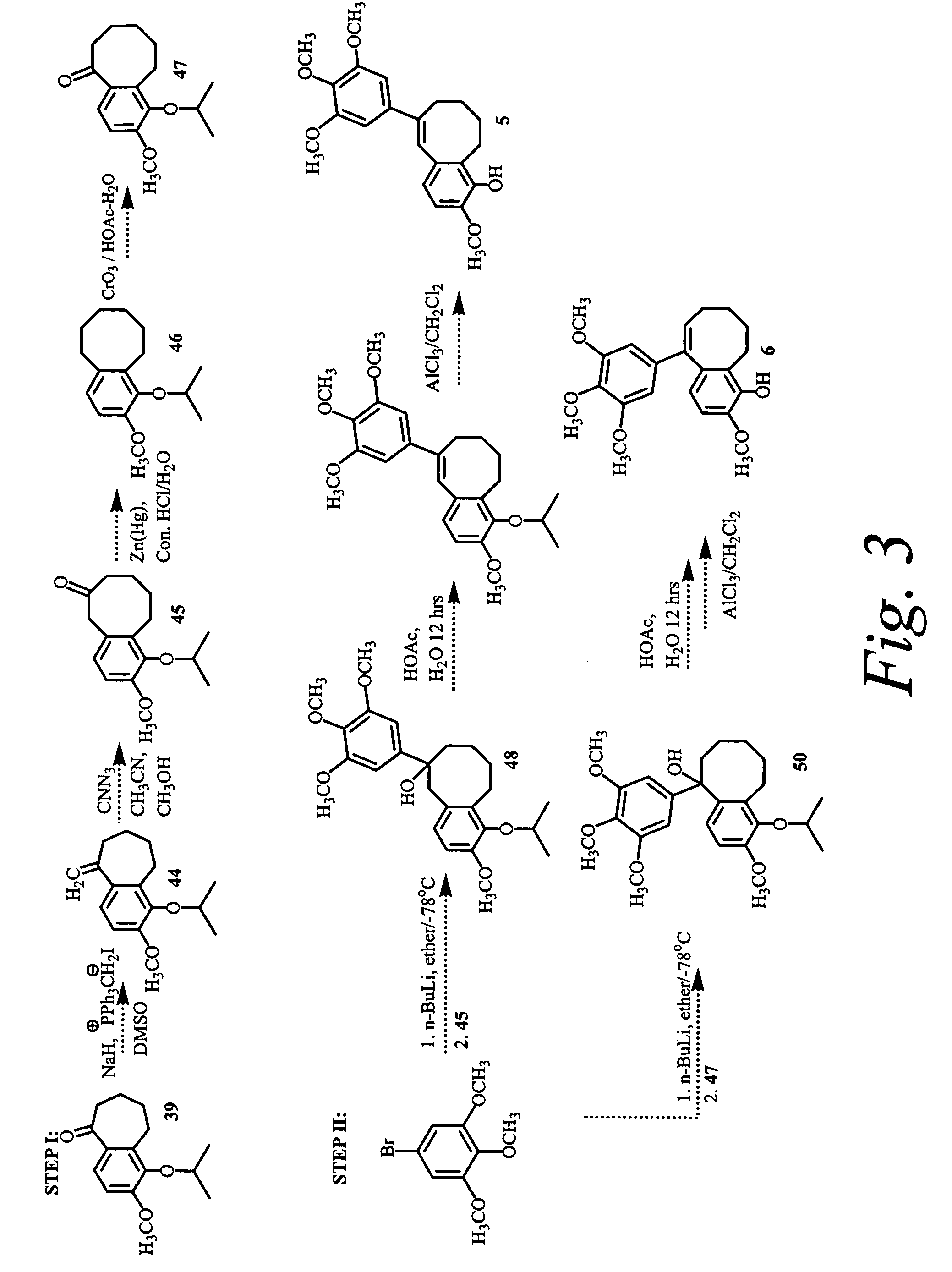
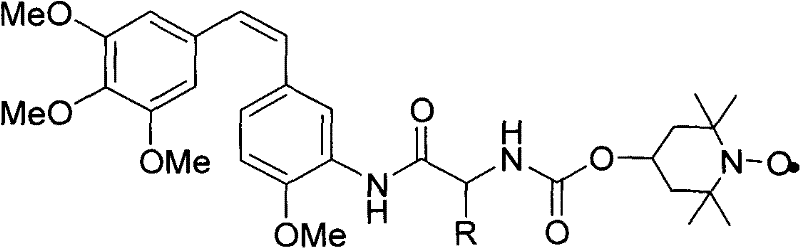
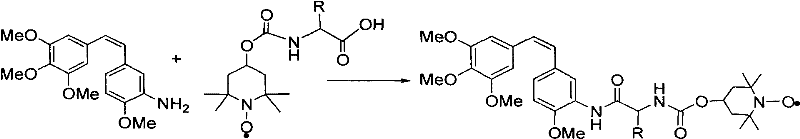
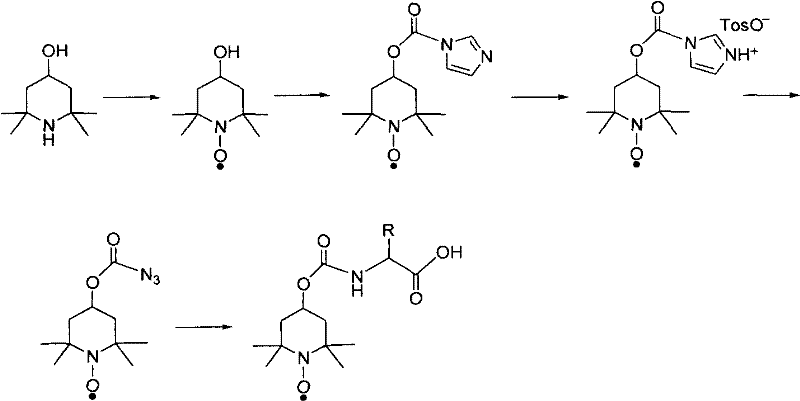
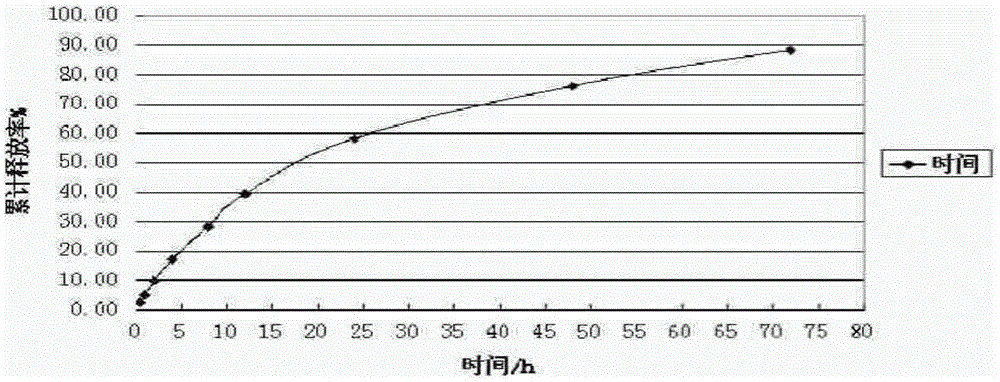
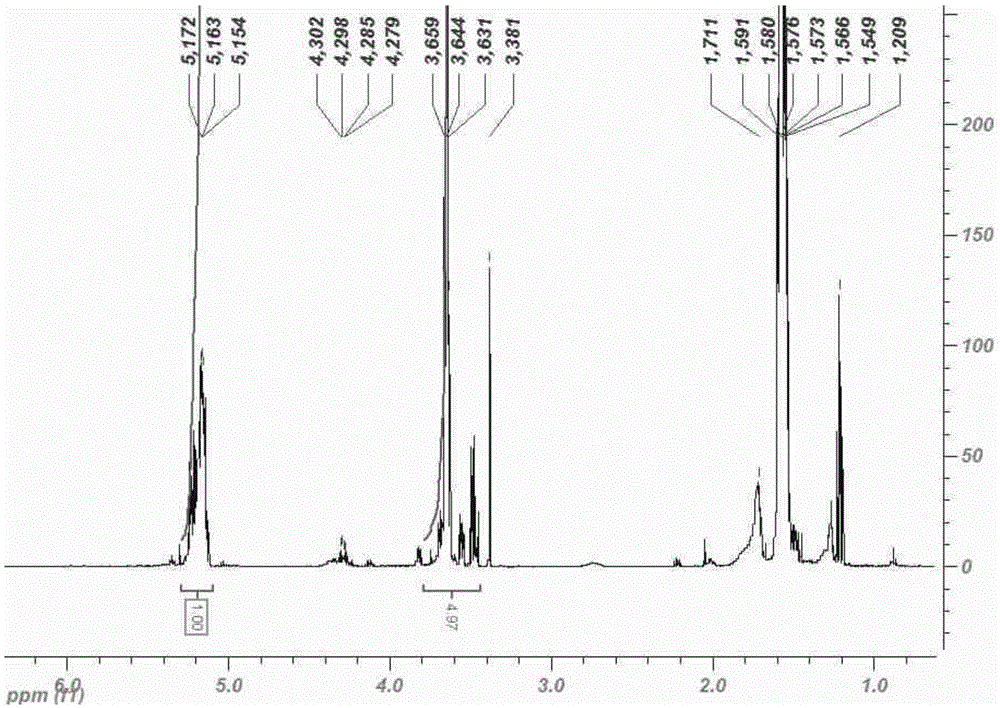
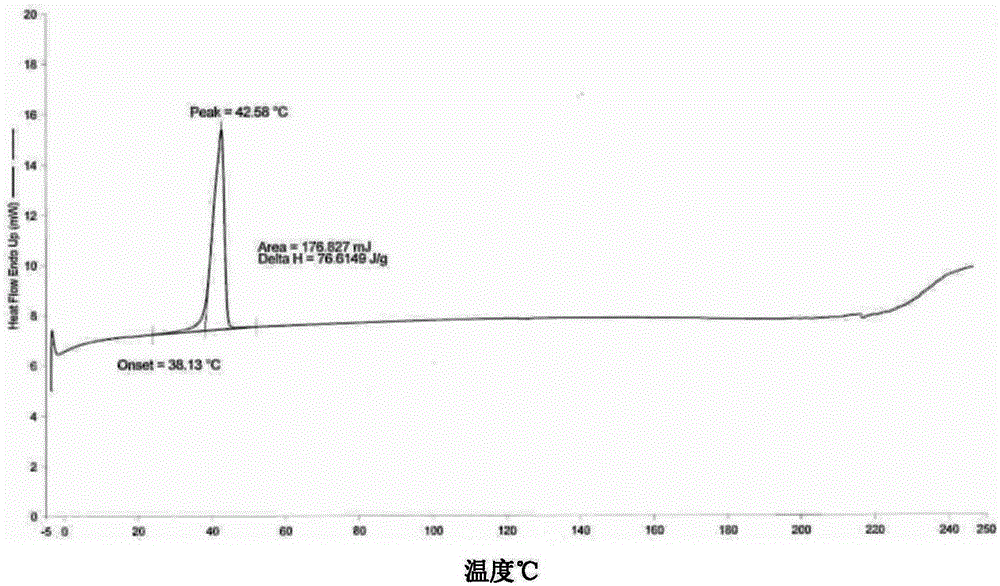
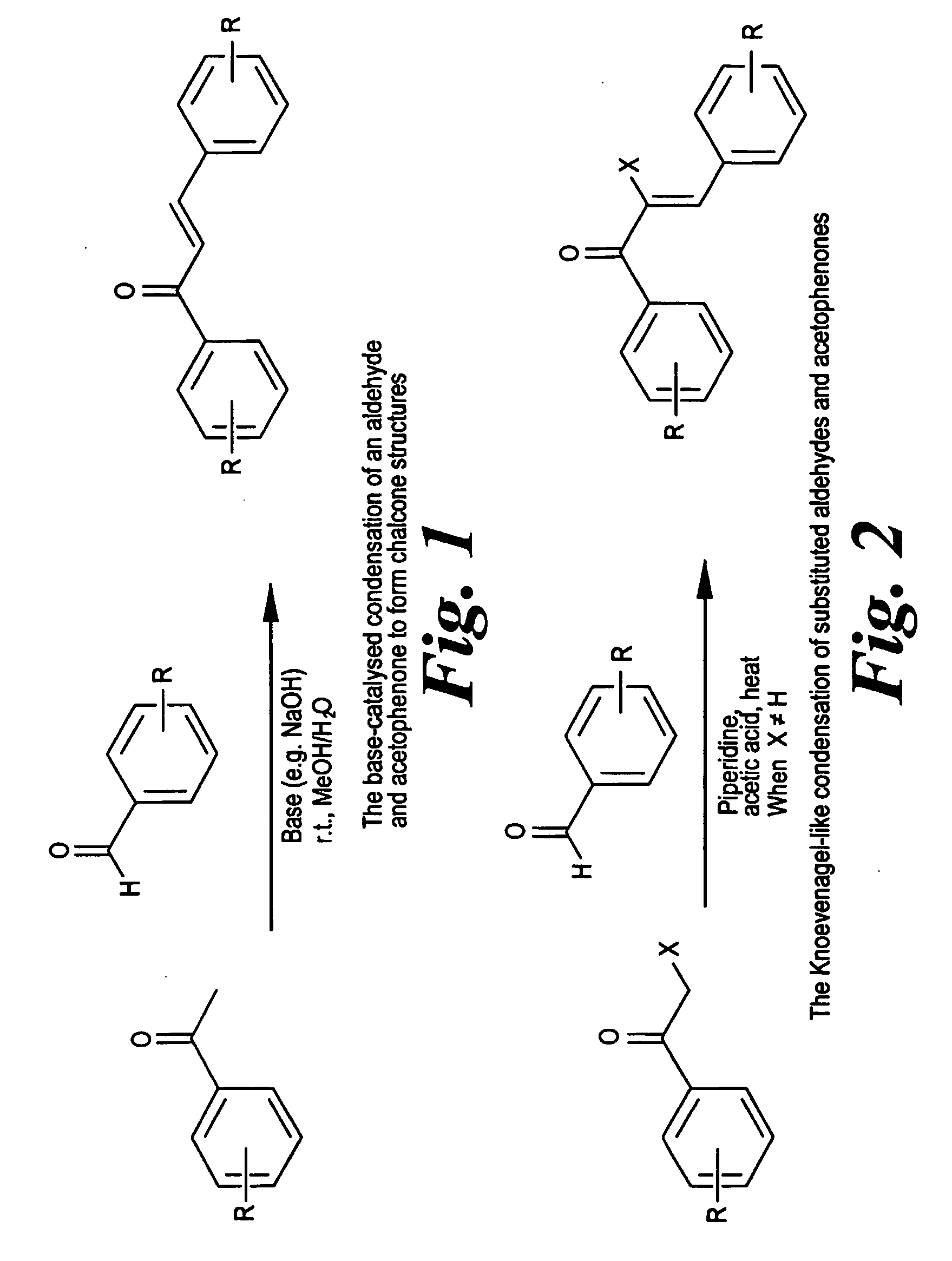
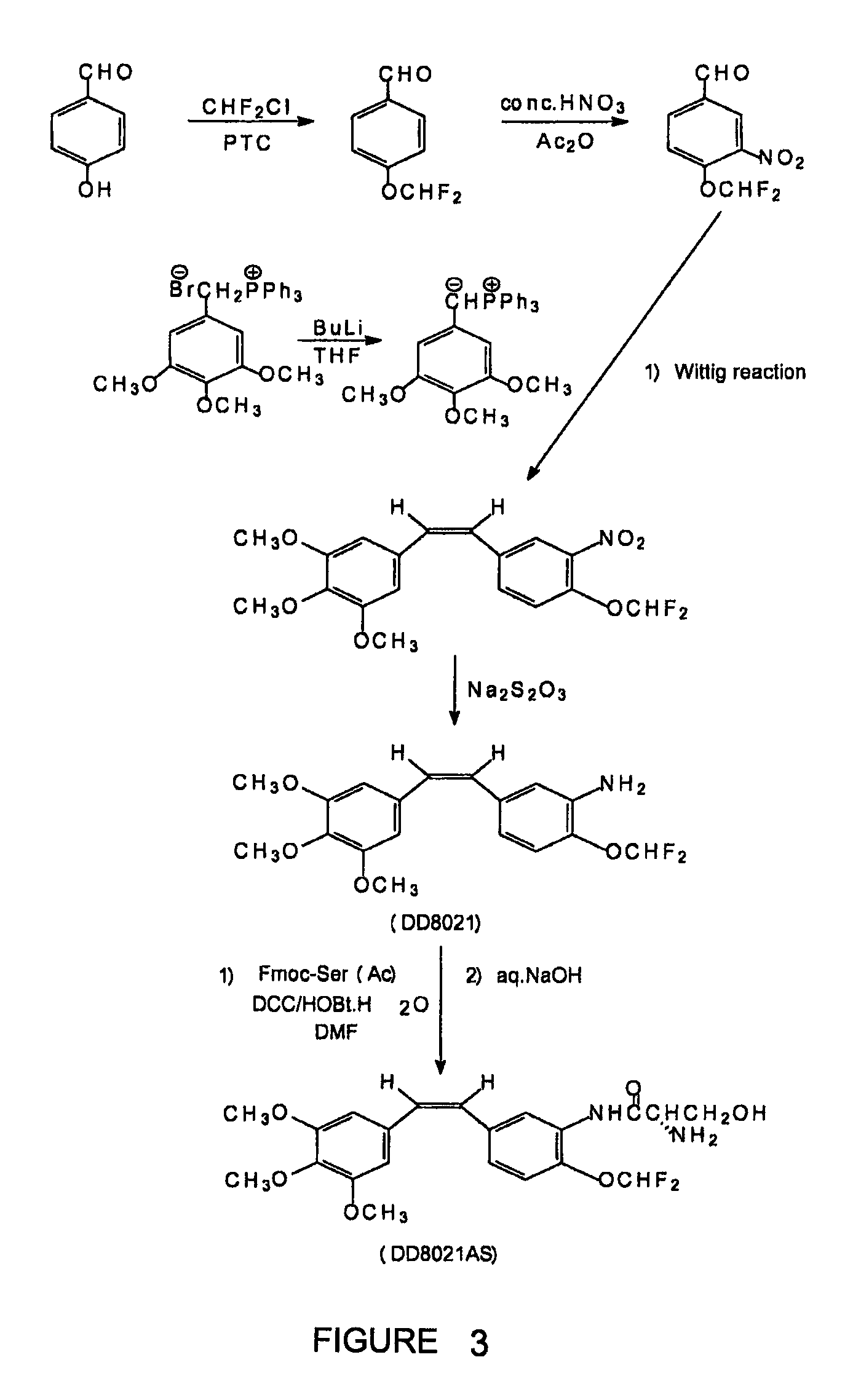
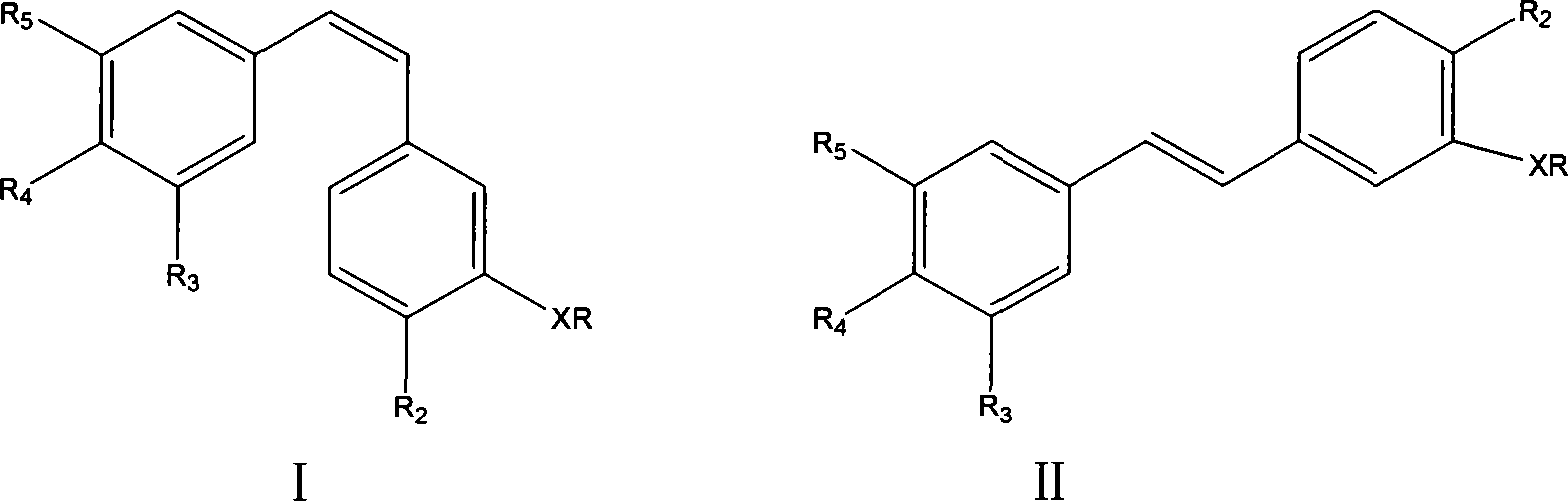
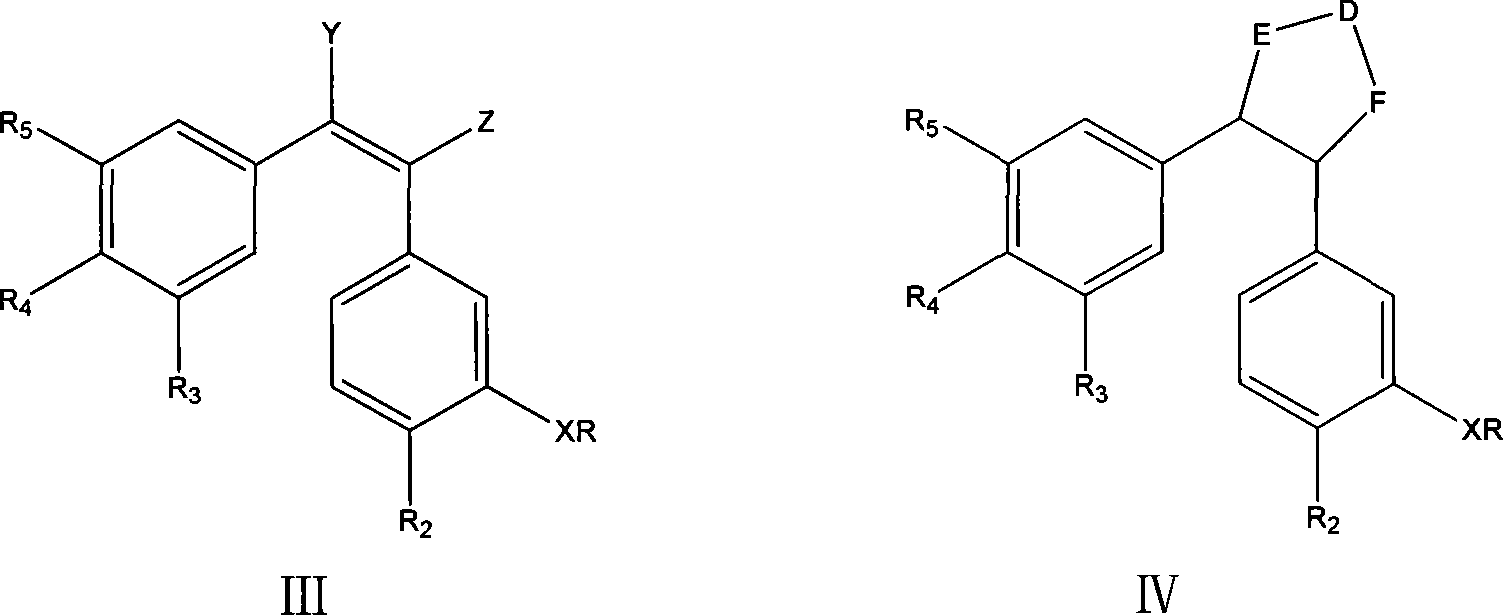
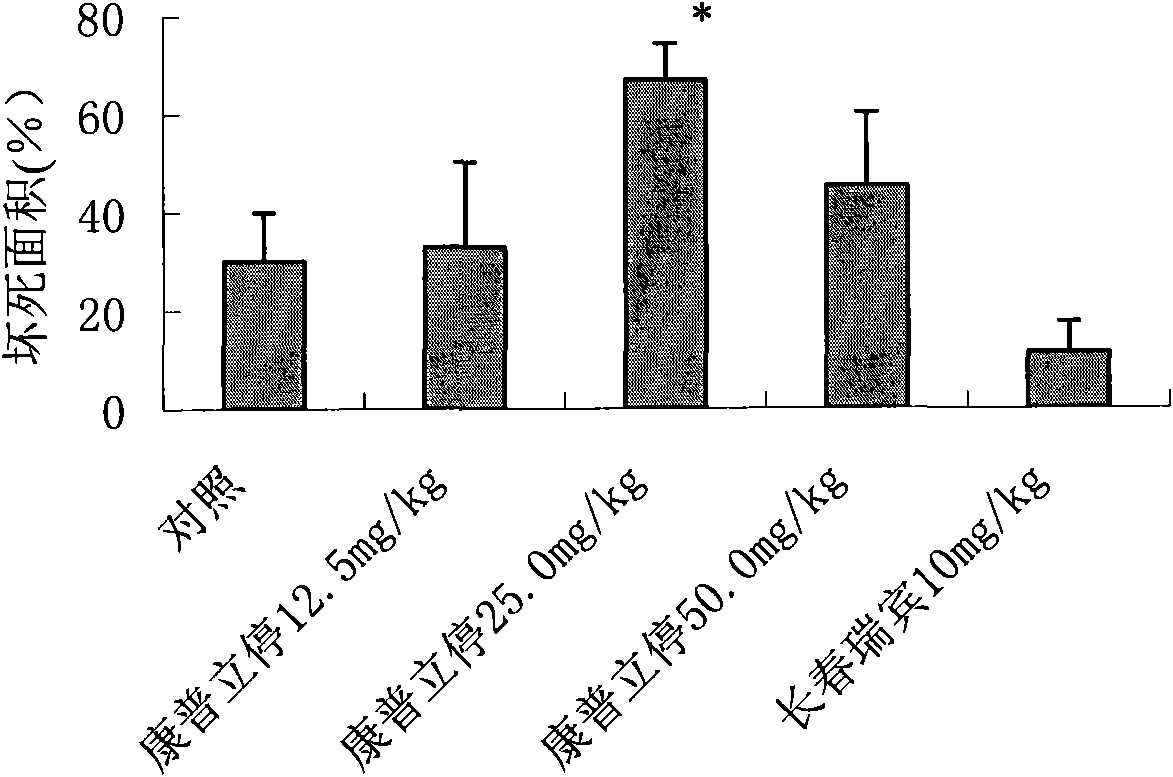
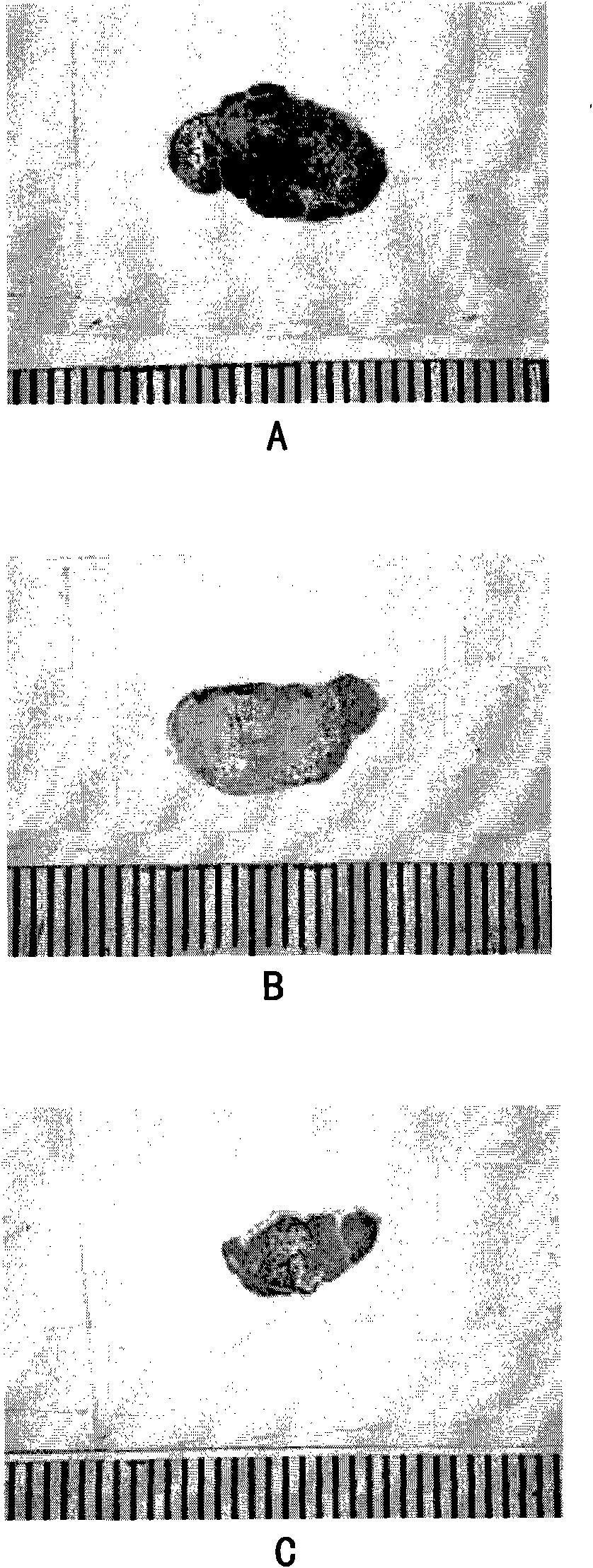
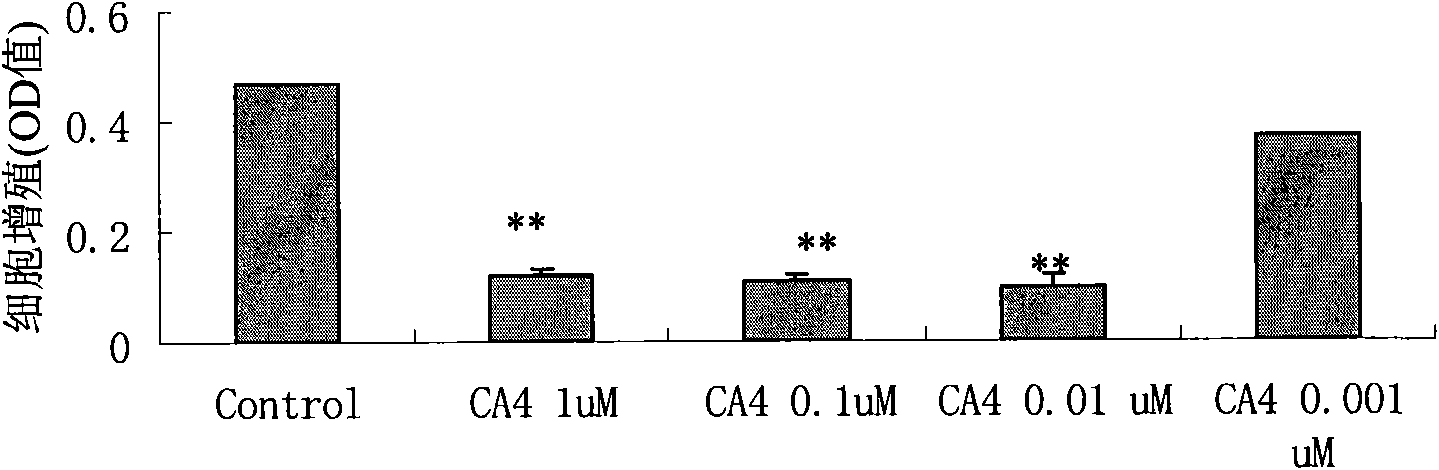
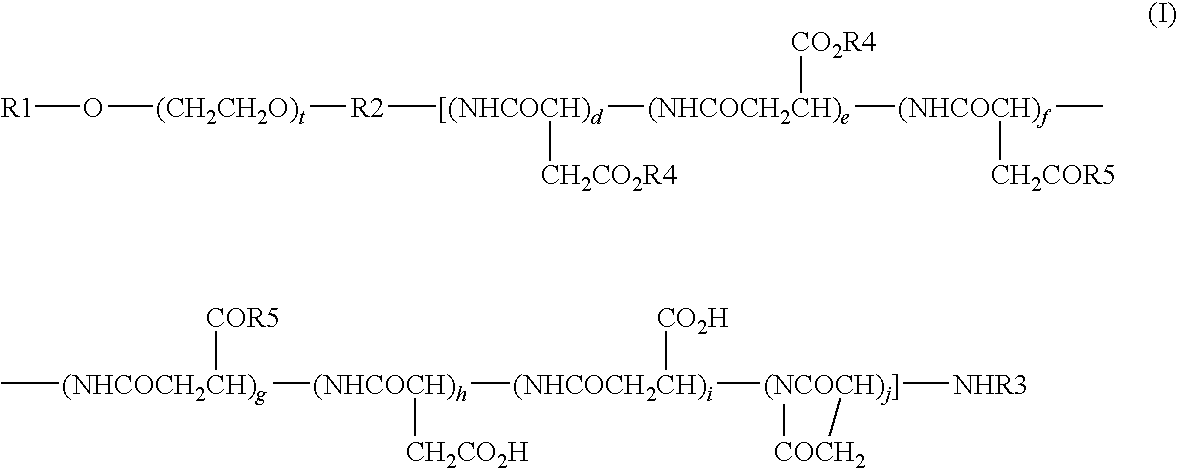Patents
Literature
58 results about "Combretastatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
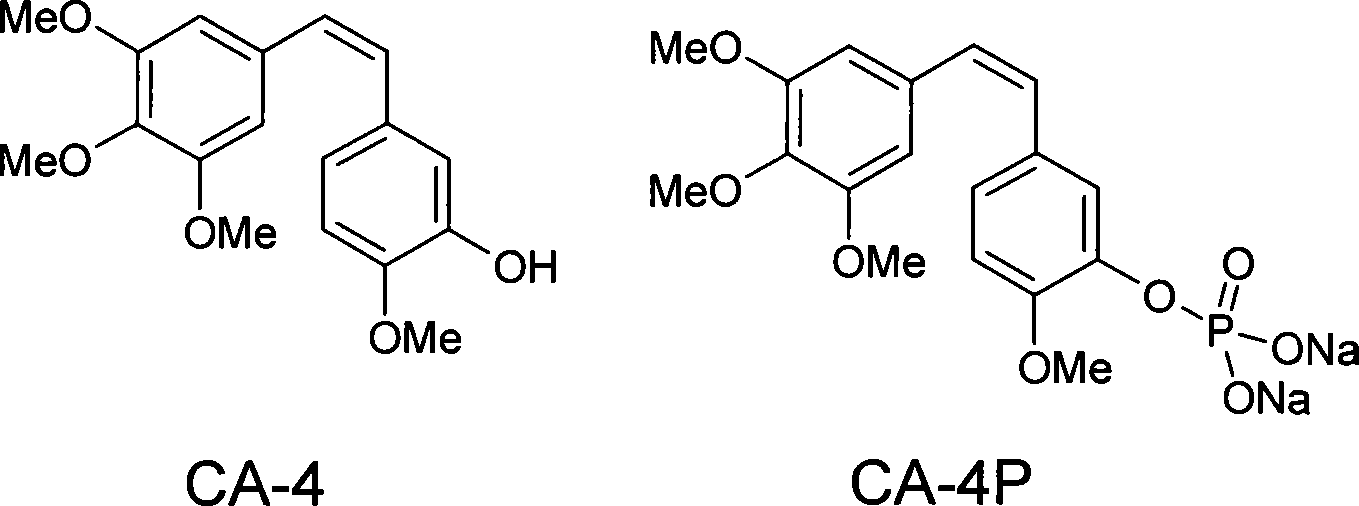
Combretastatin is a dihydrostilbenoid found in Combretum caffrum.
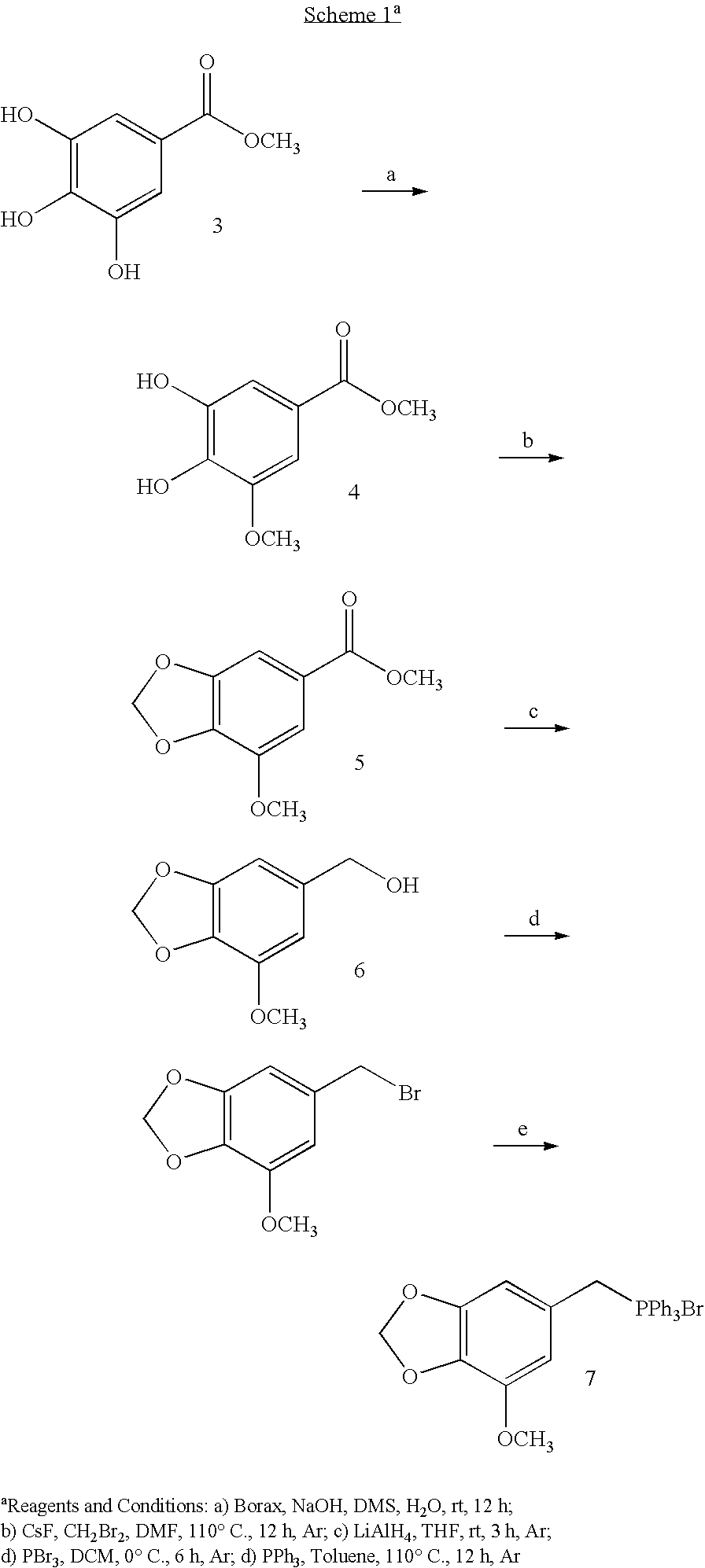
Functionalized stilbene derivatives as improved vascular targeting agents
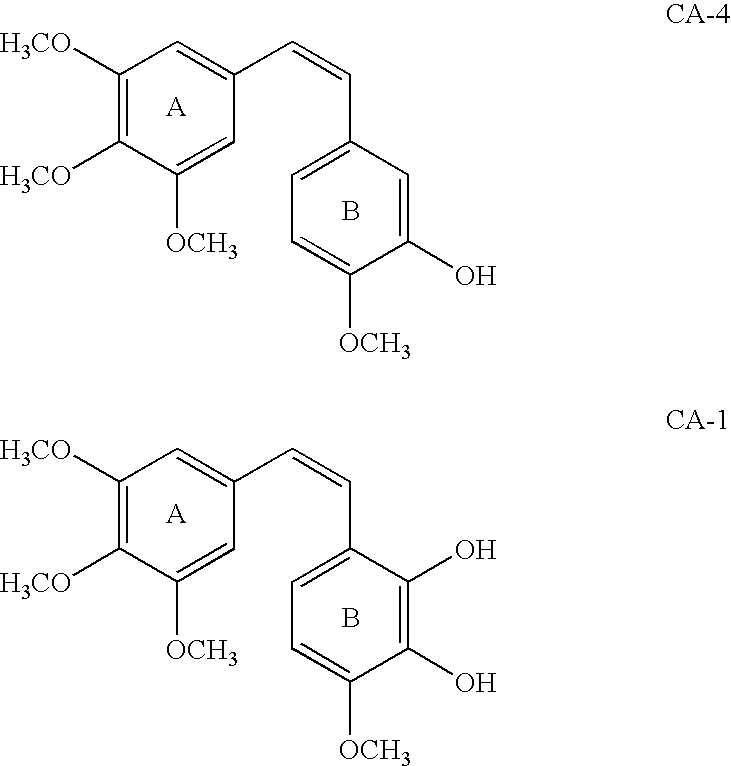
Novel stilbenoid compounds and their prodrug forms are disclosed, which serve as potent vascular targeting agents useful for the treatment of solid tumor cancers and other diseases associated with unwanted neovascularization. The novel stilbenoid compounds are tubulin-binding stilbenoid analogs structurally related to combretastatin A-1 and combretastatin A-4. The prodrug forms serve as potent vascular targeting agents (VTAs) useful for the treatment of solid tumor cancers and diseases associated with retinal neovascularization.
Owner:BAYLOR UNIVERSITY +1
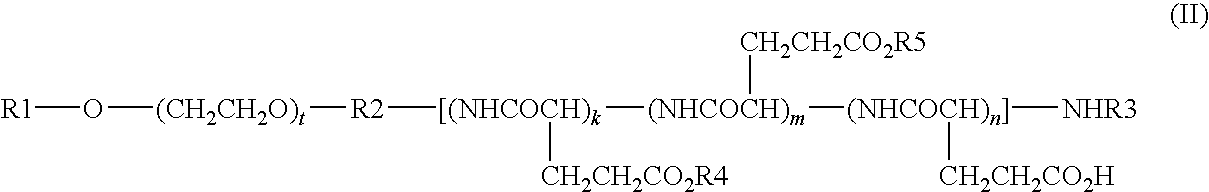
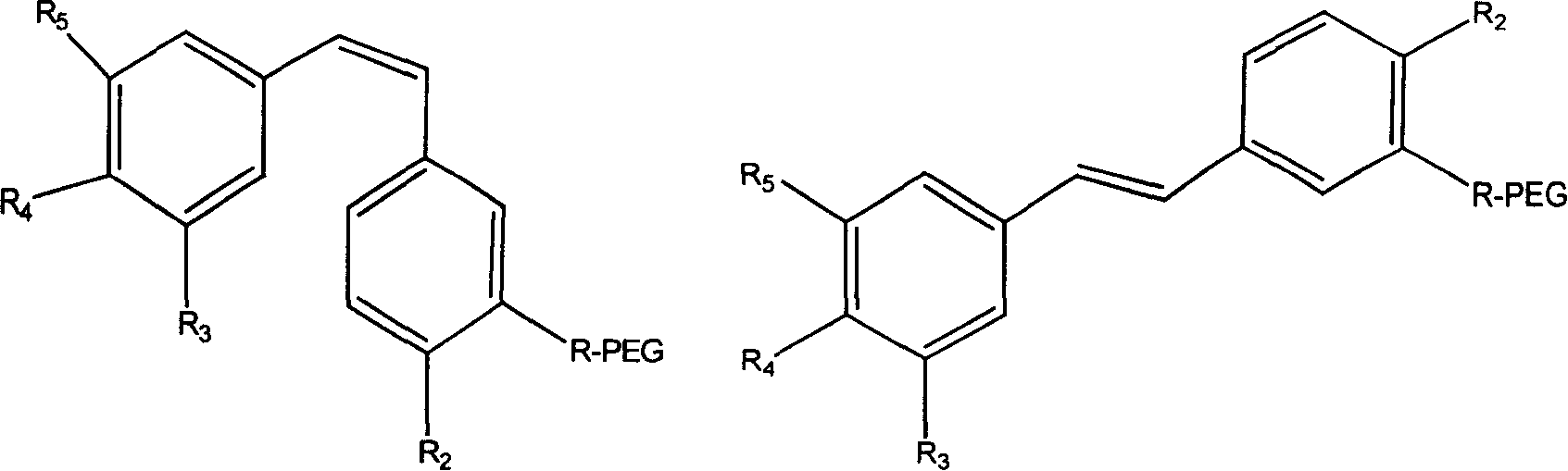
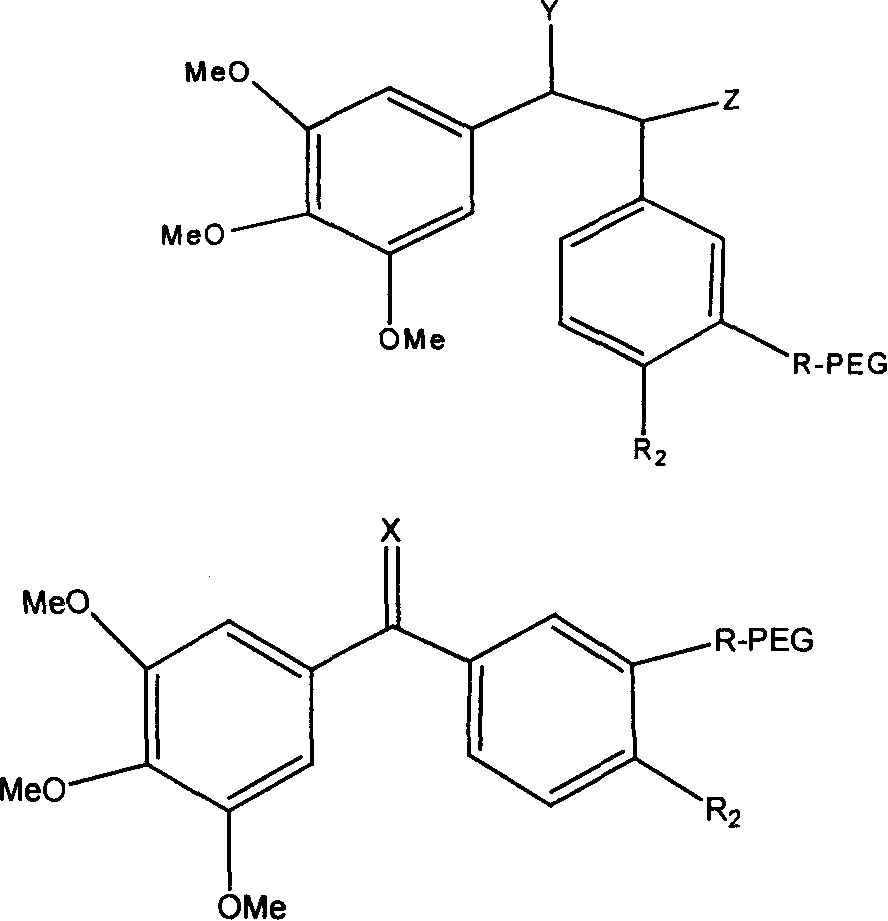
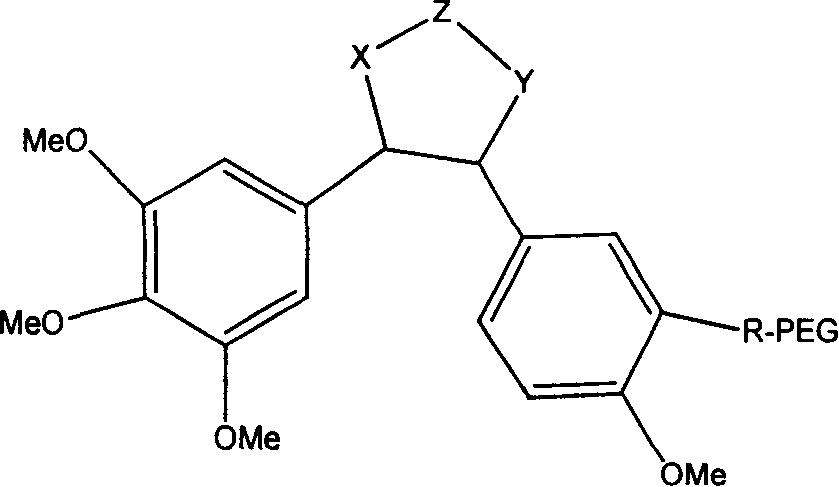
High-Molecular Weight Conjugate of Combretastatins
InactiveUS20100004403A1Good treatment effectHardly affected by individual differencesPharmaceutical non-active ingredientsAntineoplastic agentsSolubilityTreatment effect
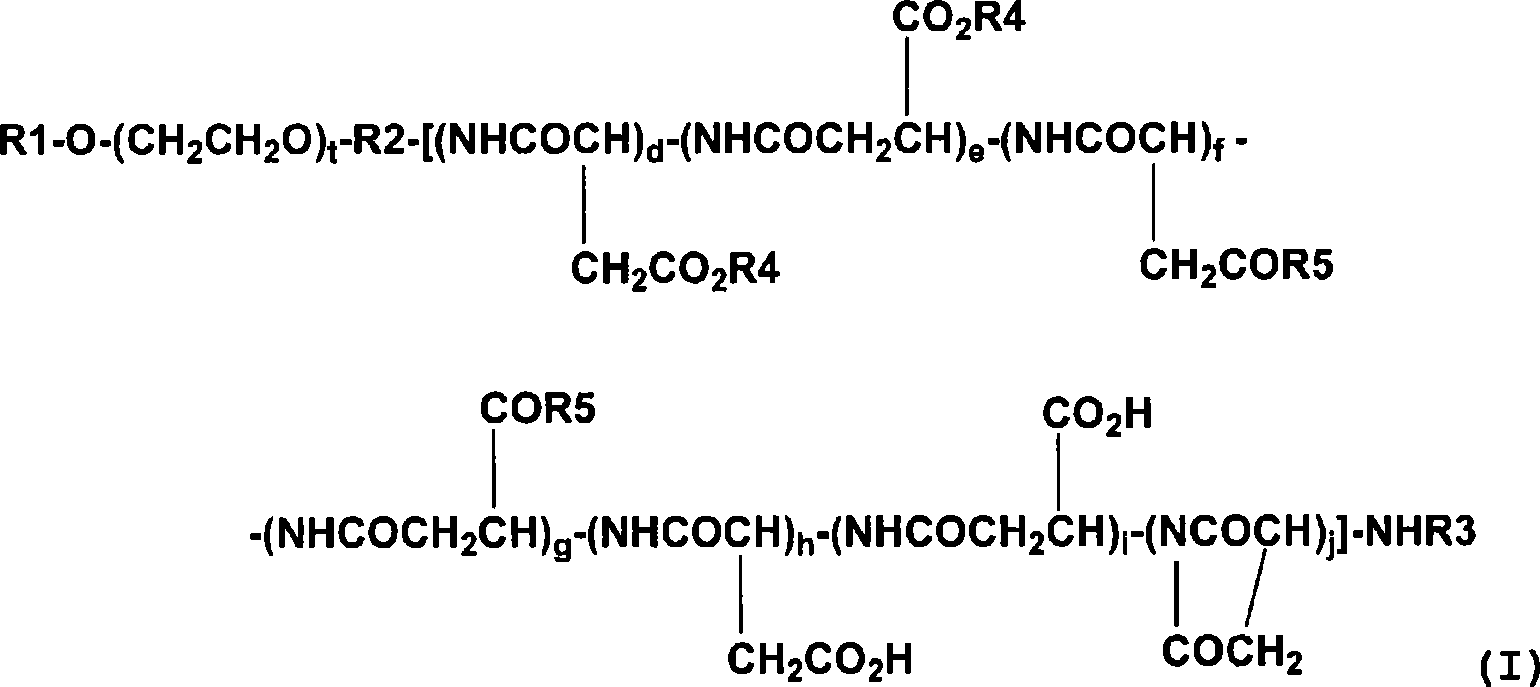
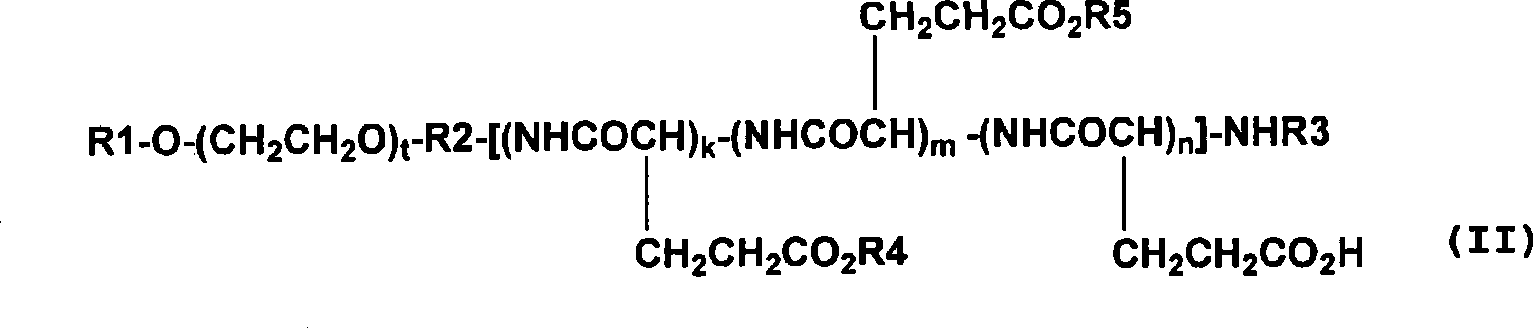
A novel derivative of combretastatins which has water solubility and is capable of releasing the drug independent of biological enzymes likely to cause individual differences and whose effective therapeutic effect can be expected has been demanded. A high-molecular weight conjugate of combretastatins, characterized by having a structure in which a hydroxyl group of a combretastatin is linked via an ester bond to a carboxylic acid group of the polymer moiety in a block copolymer of a polyethylene glycol moiety with the polymer moiety having two or more carboxylic acid groups such as polyaspartic acid or polyglutamic acid is provided.
Owner:NIPPON KAYAKU CO LTD
Combretastatin analogs with tubulin binding activity
Analogs of combretastatin have been discovered which demonstrate impressive cytotoxicity as well as a remarkable ability to inhibit tubulin polymerization. Such compounds are excellent clinical candidates for the treatment of cancer in humans. In addition, certain of these ligands, as pro-drugs, may well prove to be tumor selective vascular targeting chemotherapeutic agents or to have vascular targeting activity resulting in the selective prevention and / or destruction of nonmalignant proliferating vasculature.
Owner:BAYLOR UNIVERSITY
Combretastatin compound and preparation method and application thereof
InactiveCN102249987ANovel structureThe synthesis process is simpleOrganic chemistryAntineoplastic agentsDistillationEthyl acetate
The invention discloses a combretastatin compound and a preparation method. The preparation method of the combretastatin compound of the invention comprises the following steps: dissolving 3'-amino combretastatin and N-(1-oxyl-2,2,6,6,-tetramethyl-oxygen-carbonyl)-L-amino acid in dried dichloromethane, uniformly stirring under argon protection, adding dicyclohexylcarbodiimide and 1-hydroxybenzotriazole, stirring and reacting under argon protection, filtering and removing white precipitates after the reaction, removing the solvent by distillation to obtain a crude product, purifying the crude product by column chromatography, and eluting the product by petroleum ether and ethyl acetate liquid with a volume ratio of 10:1-5:1 to obtain the target product. The combretastatin compound of the invention is applicable to the preparation of anticancer medicaments, and is especially applicable to the preparation of medicaments for treating leukemia, liver cancer, gastric cancer, and cervical cancer.
Owner:LANZHOU UNIVERSITY
Polyglycol modified antitumor compound and its preparing method
InactiveCN1857736ASolve some key problems in clinical applicationHydroxy compound active ingredientsSulfur/selenium/tellurium active ingredientsSolubilityPolyethylene glycol
The present invention discloses a kind of polyglycol modified antitumor compound and its preparation process. The polyglycol modified antitumor compound has Combretastatin and its derivative as matrix and linear chain polyglycol and dendritic polyglycol for modifying to improve water solubility. It has water solubility 200-2500 times that of Combretastatin and its derivative before modification. The present invention solves the difficult problem of applying Combretastatin and its derivative clinically.
Owner:SHANGHAI JIAO TONG UNIV
Synthesis of combretastatin A-4 prodrugs and trans-isomers thereof
InactiveUS7279466B2Increased phosphorylationHigh yieldAntibacterial agentsBiocideWater solublePre-clinical development
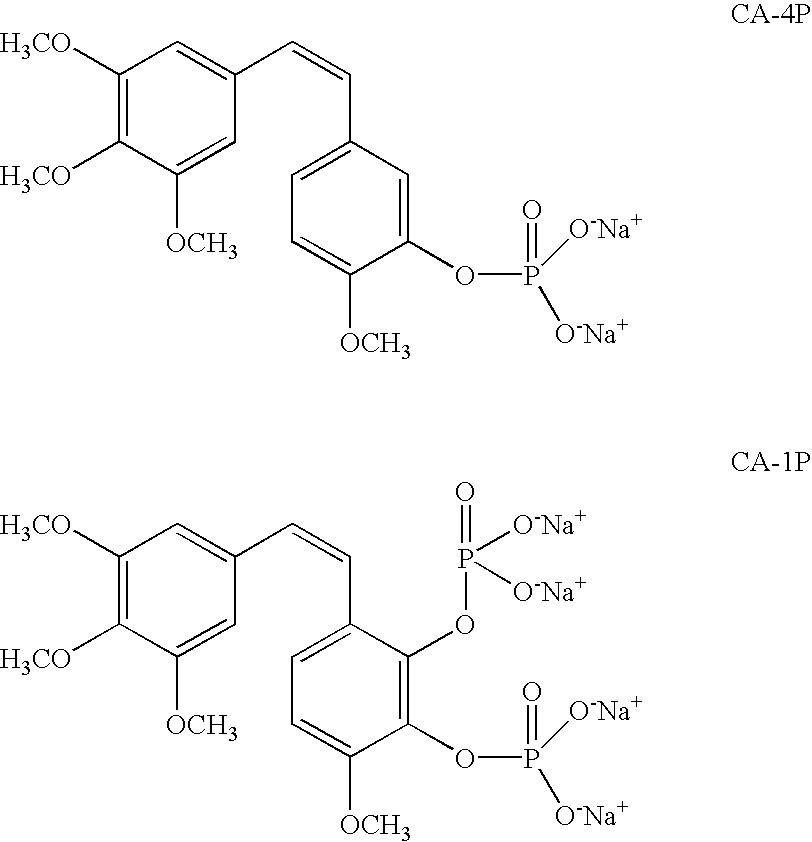
Combretastatin A-4 has been previously selected for pre-clinical development as antineoplastic agent. However, it is essentially insoluble in water. New water soluble derivatives of combretastatin A-4 and its qualified analogs have been discovered and synthesized through a multistage process using other derivatives of combretastatin A-4 as intermediates. These water soluble derivatives are herein denominated as “Combretastatin A-4 Prodrugs”.
Owner:ARIZONA STATE UNIVERSITY
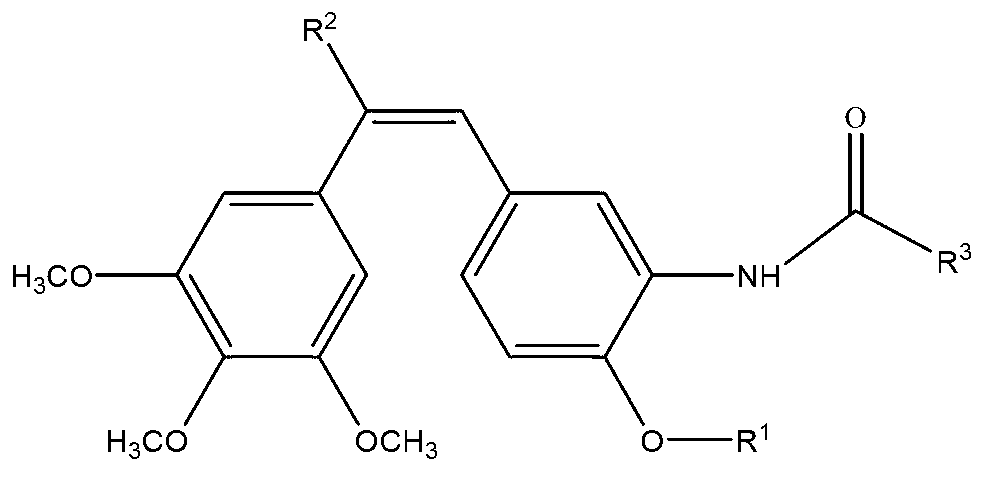
Synthesis of amino combretastatin derivative and application of amino combretastatin derivative as oral antitumour drug
ActiveCN103012248AVerify absorptionVerify suppression capabilityAntibacterial agentsSenses disorderNeovascularizationTubulin Inhibitors
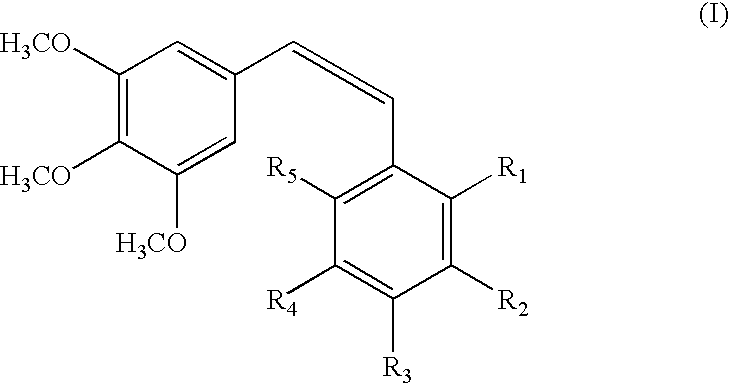
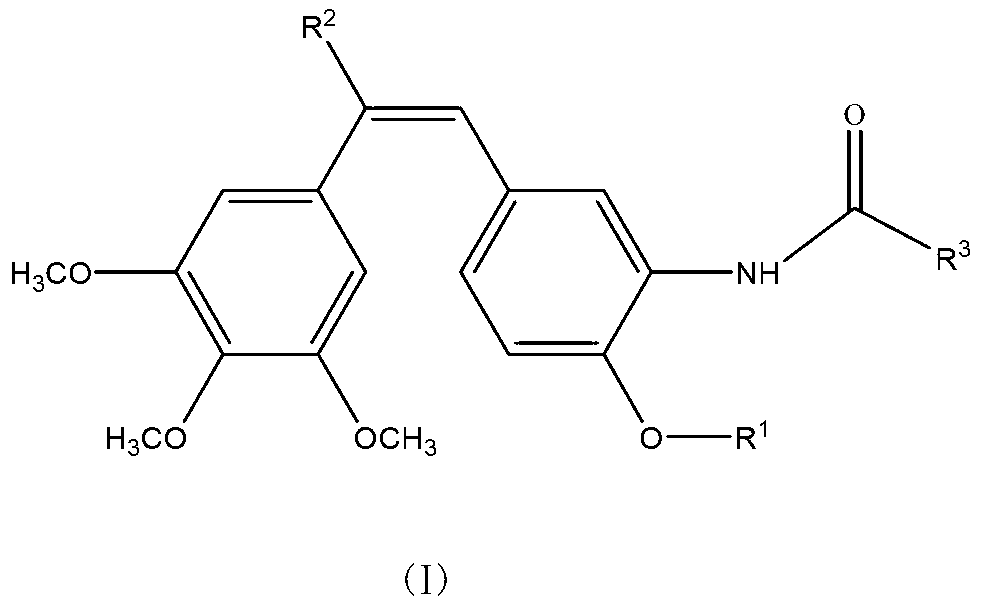
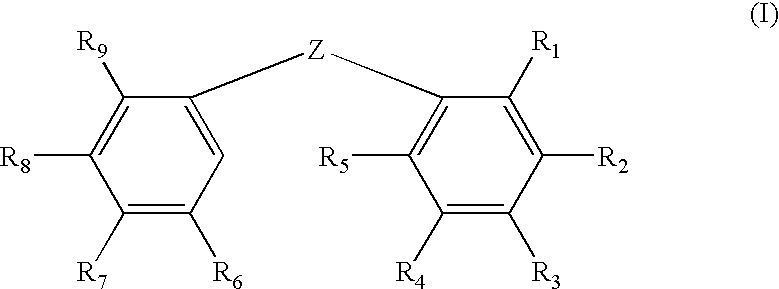
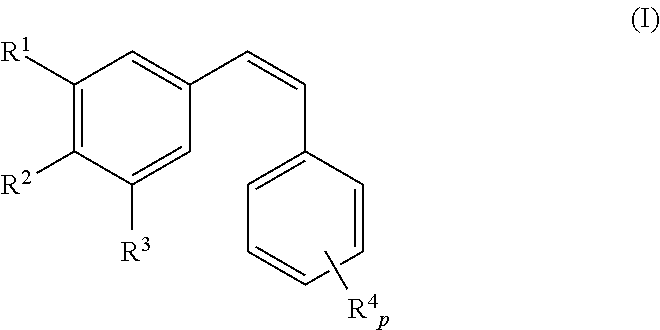
The invention discloses a compound which is shown as a formula (I) and used for inhibiting generation of tumour neovascularization as well as officinal salt or a configurational isomer thereof, wherein substituent groups R1, R2 and R3 are defined in the specification. The invention also discloses a preparation method of the compound in the formula (I), a pharmaceutical composition and an application of the compound as a microtubulin inhibitor in the aspect of inhibiting the tumour neovascularization, especially an application as an oral preparation.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
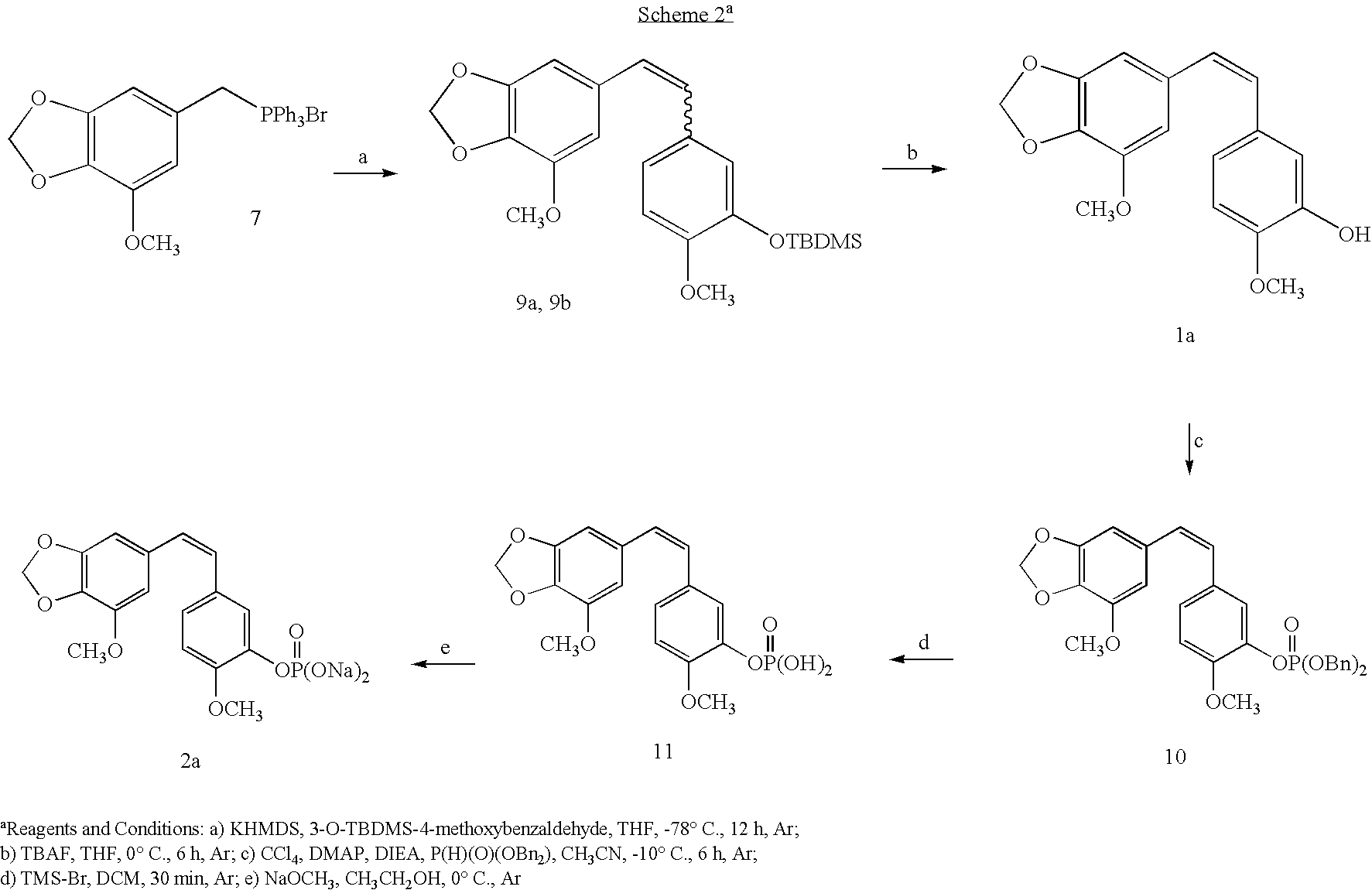
Synthesis of combretastatin A-2 prodrugs
InactiveUS7105695B2Organic compound preparationPhosphorus organic compoundsSodium methoxideSolubility
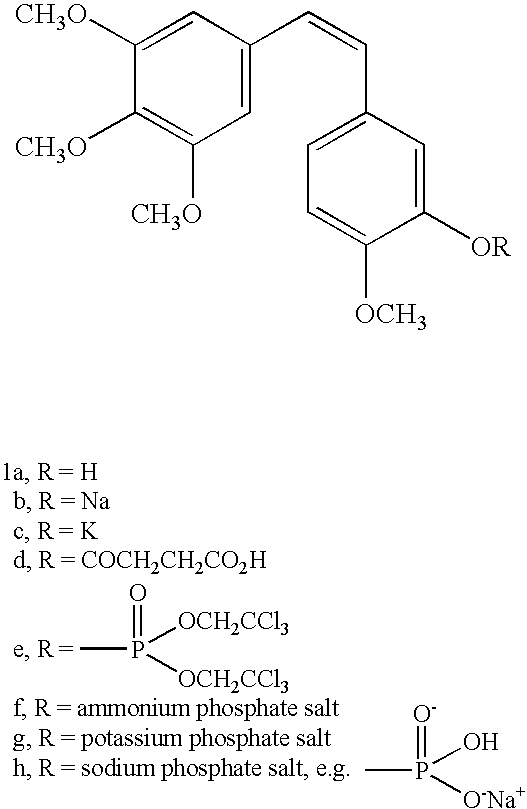
The original synthesis of combretastatin A-2 (1a) was modified to provide an efficient scale-up procedure for obtaining this antineoplastic stilbene. Subsequent conversion to a useful prodrug was accomplished by phosphorylation employing in situ formation of dibenzylchlorophosphite followed by cleavage of the benzyl ester protective groups with bromotrimethylsilane to afford phosphoric acid intermediate 11. The latter was immediately treated with sodium methoxide to complete a practical route to the disodium phosphate prodrug (2a). The phosphoric acid precursor (11) of phosphate 2a was employed in a parallel series of reactions to produce a selection of metal and ammonium cation prodrug candidates. Each of the phosphate salts (2a–q) was evaluated with respect to relative solubility behavior, cancer cell growth inhibition, and antimicrobial activity.
Owner:ARIZONA STATE UNIVERSITY
Combination comprising combretastatin and anticancer agents
An antitumor combination comprising a stilbene derivative and an anticancer compound selected from the group consisting of taxanes, alkylating agents, antimetabolites, vinca alkaloids, platinum compounds, epidophylloptoxins, and antibiotics as the active ingredients is provided. Methods of using these pharmaceutical preparations for the treatment of solid carcinomas and the like are also provided.
Owner:AVENTIS PHARMA SA
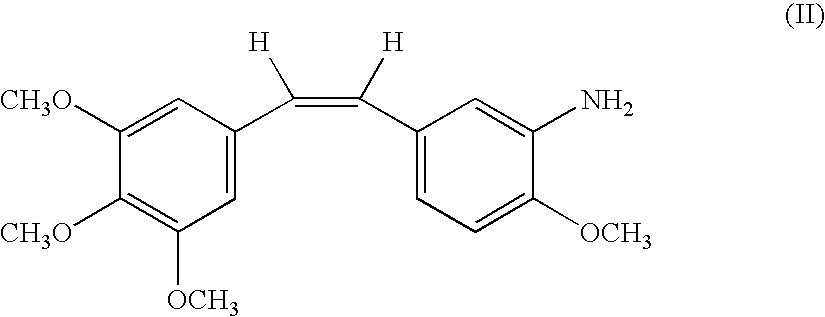
Methods for monitoring combretastatin resistance
Owner:PINNEY KEVIN G +2
CA-4 antitumor drug, synthesizing method and application thereof
ActiveCN107382796AImprove targeting activityAntibacterial agentsSenses disorderNatural productAlkoxy group
The invention discloses a CA-4 antitumor drug, a synthesizing method and an application thereof. The CA-4 antitumor drug is formed in the manner of introducing the alkoxy or fluorine-containing alkoxy into 4' site of natural product combretastatin and functionally chemical modifying 3' site thereof. The CA-4 antitumor drug disclosed by the invention has higher capacity of restraining tubulin gathering and can be used for anti-tumor therapy.
Owner:浙江华理生物制药有限公司
Combretastatin nanometer polymer micelle freeze-dried preparation and preparation method thereof
InactiveCN105250225AImprove stabilityGood compatibilityPowder deliveryEther/acetal active ingredientsPolyesterPolymer science
The invention discloses a combretastatin nanometer polymer micelle freeze-dried preparation. The combretastatin nanometer polymer micelle freeze-dried preparation comprises a methoxy polyethylene glycol-polyester block copolymer carrier material and combretastatin. Combretastatin is wrapped by the copolymer carrier material. A mass ratio of combretastatin to the copolymer carrier material is 0.01-0.15. The copolymer carrier material is a block copolymer prepared from a mixture of one or more polyester monomers and methoxy polyethylene glycol by ring opening polymerization. A mass ratio of methoxy polyethylene glycol to polyester is 1: 0.55-2. The polyester monomer is one of D, L-lactide, glycolide, caprolactone and trimethylcarbonate. Through optimization of a mass ratio of polyester to polyether of the block copolymer, a mass ratio of combretastatin to the carrier is further optimized. After water redissolution, greater than 90% entrapment rate lasting time of the combretastatin micelle preparation is 12h or more, accords with clinical medicine application physical truth and satisfies clinical requirements.
Owner:SUZHOU HAITE BIAO BIOLOGICAL TECH
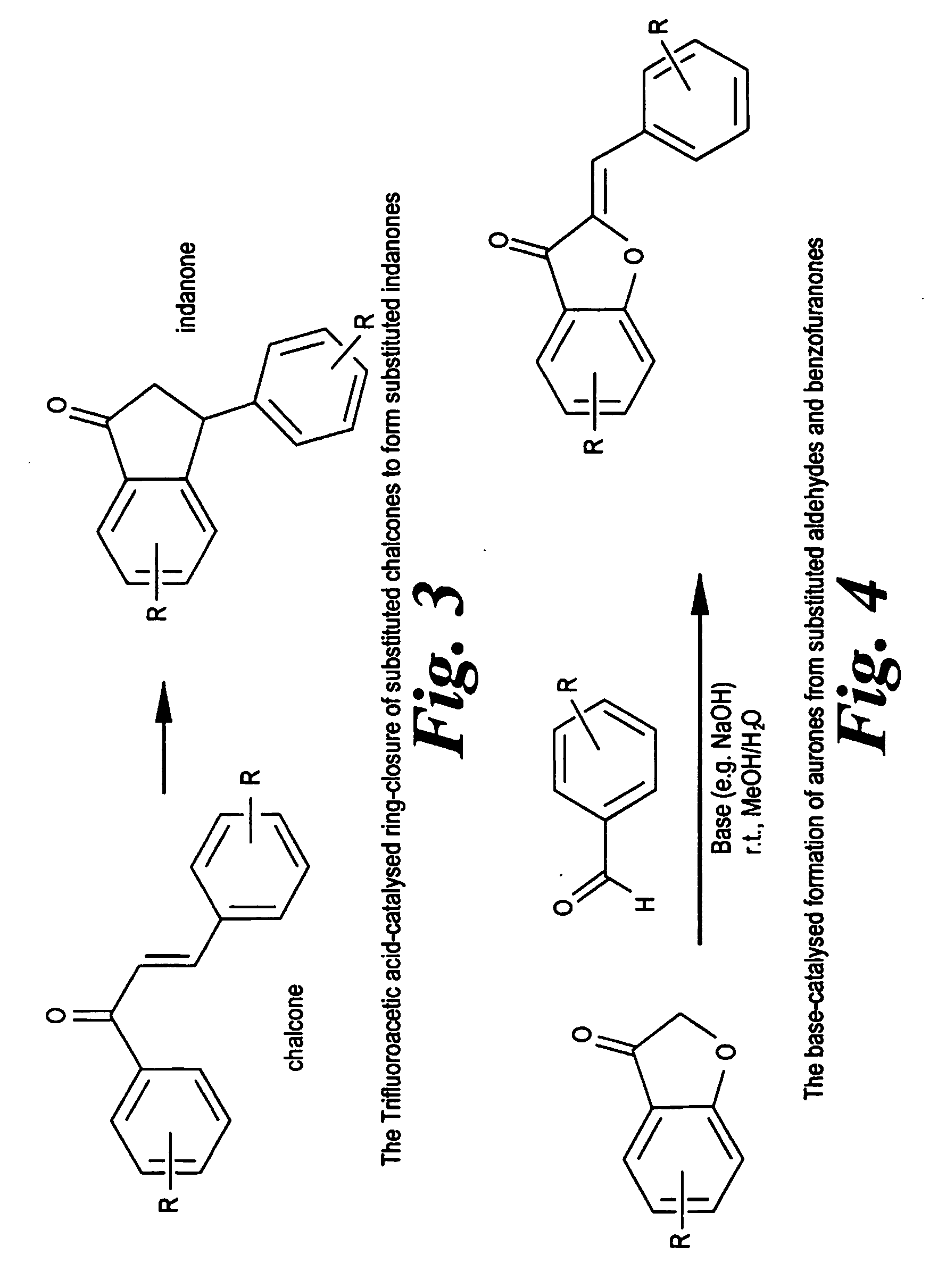
Combretastatin a-4 derivatives having antineoplastic activity
Compounds are disclosed that are designed to mimic the activity of combretastatin A-4 based on chalcone, aurone, or indanone structures, or involving benzoquinone or quinone rings. The anti-cancer activity of exemplified compounds is demonstrated in a range of in vitro and in vivo assays.
Owner:LAWRENCE NICHOLAS JAMES +6
Combretastatin derivatives and uses therefor
InactiveUS20120309734A1Low toxicityInhibit angiogenesisBiocideOrganic active ingredientsCause of deathNitrogen
Cancer is one of the major causes of death worldwide. Although many advances have been made in the treatment and management of the disease, the existence of chemotherapy-resistance means there is still a great need to develop new strategies and drugs for its treatment. Provided herein are synthetic derivatives of combretastatin A-4, in particular those in which the aromatic rings are locked into a non-isomerisable active conformation, thus resulting in improved, stable compounds. The novel compounds are structurally related to combretastatin A-4 (CA-4) and lock the rings into the known active conformation by means of a four membered nitrogen containing heterocyclic ring, such as a beta-lactam ring, incorporated into the standard CA-4 structure. The compounds exhibit potent anti-cancer activity.
Owner:TRINITY COLLEGE DUBLIN
Compretastatin derivative and antibody drug conjugate thereof
The invention discloses a combretastatin derivative and an antibody drug conjugate thereof, and a preparation method and application thereof. The combretastatin derivative and the antibody drug conjugate thereof comprise a compound shown in a formula I or a pharmaceutically acceptable salt or solvate thereof, wherein in the formula I, Ab represents a targeting ligand; L represents a linker for connecting an antibody with CA4 or a derivative thereof; n represents a ratio of a drug to the targeting ligand; and X is NR, wherein R is an alkane chain or a PEG chain. The invention provides preparation of the antibody drug conjugate by taking CA4 as a drug molecule and the derivative thereof, and application of the antibody drug conjugate in treatment of high-proliferative diseases or lesions. The diseases are characterized in that cells generate an antigen or target, and the antigen can be specifically combined with the antibody.
Owner:SHANGHAI TECH UNIV
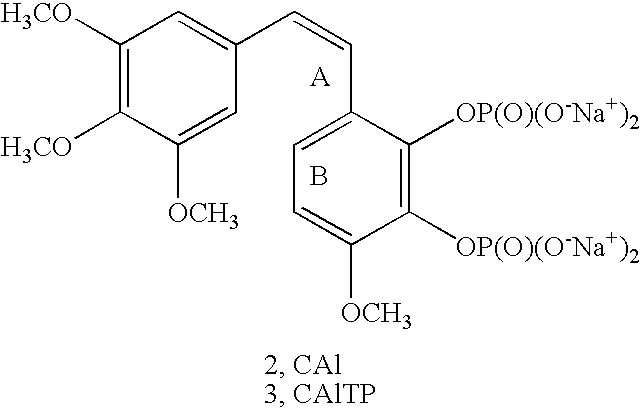
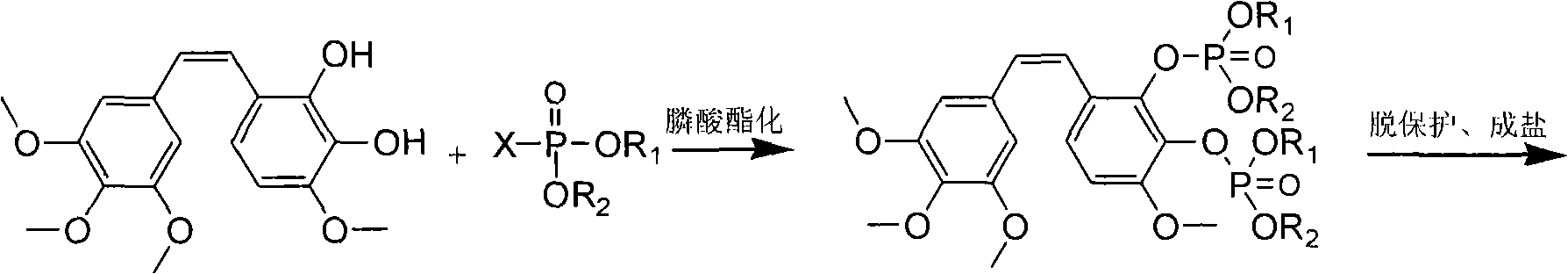
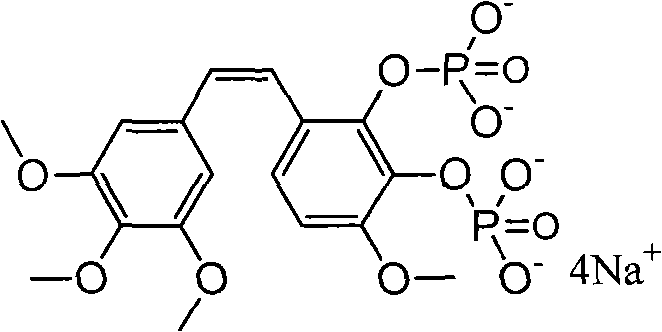
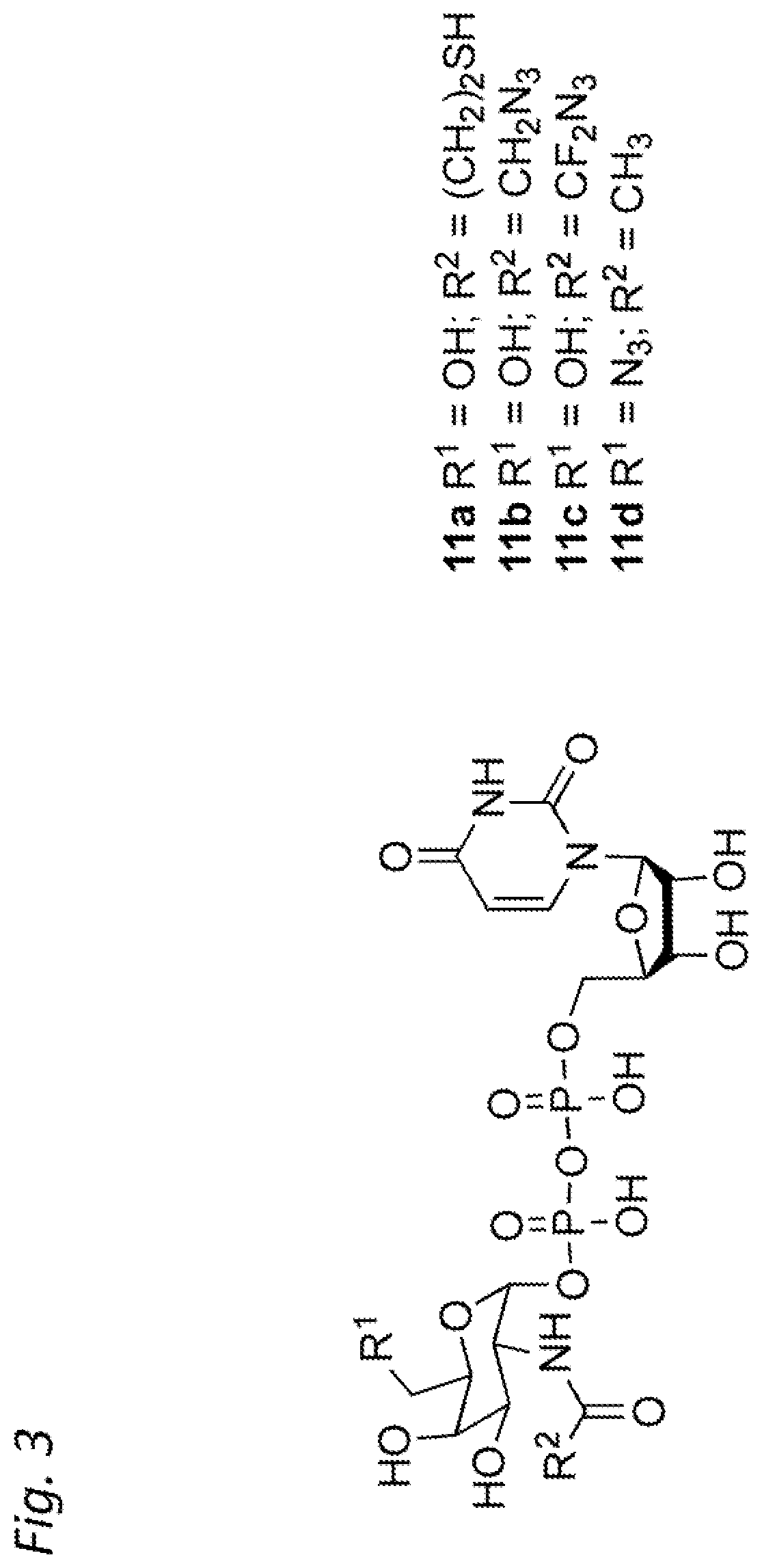
Method for preparing Combretastatin A-1 phosphate ester tetrasodium salt
ActiveCN101514213AReduce usageMild responseGroup 5/15 element organic compoundsSodium methoxidePhosphoric acid
The invention relates to a method for synthesizing and preparing antineoplastic Combretastatin A-1 phosphate ester tetrasodium salt, comprising the steps: under the anhydrous condition, Combretastatin A-1 is dissolved in aprotic polar solvent, and dialkyl oxyl phosphoryl chloride / bromine (chloride / bromine phosphoric acid when R1 and R2 are hydrogen atoms) is dipped into the solution when acid-binding agent exists for reaction, and water is added into the reaction solution for stopping the reaction when the reaction is finished; products are extracted by the aprotic polar solvent, and then is decompressed and concentrated until being dried, so that the Combretastatin A-1 phosphate ester is obtained; (2) the obtained Combretastatin A-1 phosphate ester is dissolved into polar organic solvent to remove R1 and R2 radicals, and then obtained product is salified with sodium methoxide, finally, the Combretastatin A-1 phosphate ester tetrasodium salt is obtained. The method has simple and convenient technique, high yield coefficient and utilization rate of the Combretastatin A-1, and low production cost.
Owner:NANJING CHENGONG PHARM CO LTD
Therapeutic anti-tumor compound
InactiveCN101735265ATherapeutic valueOrganic active ingredientsPhosphorus organic compoundsSolubilityWater soluble
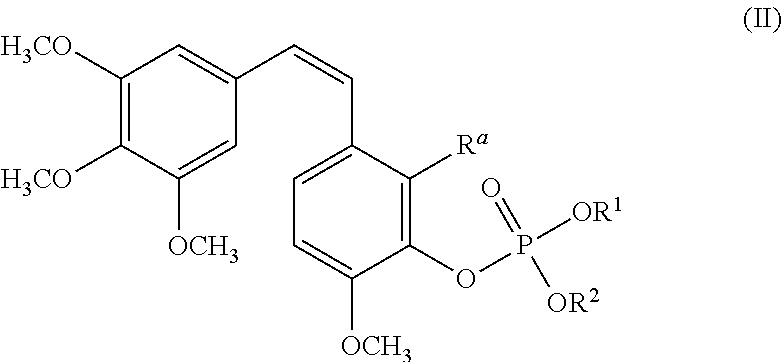
The invention provides a compound or a pharmaceutical salt thereof of formula (I) R1-O-(CH2)nO-L, wherein R1 represents a combretastatin type compound containing non-hydroxyl part, n is 1 to 10, and L represents CO-alkyl carboxylic acid, amino acid residues, phosphoryl or phosphoryl derivatives. The compound of the invention is used for in treatment of anti-tumor medicaments and has high water solubility.
Owner:HC SYNTHETIC PHARMA CO LTD
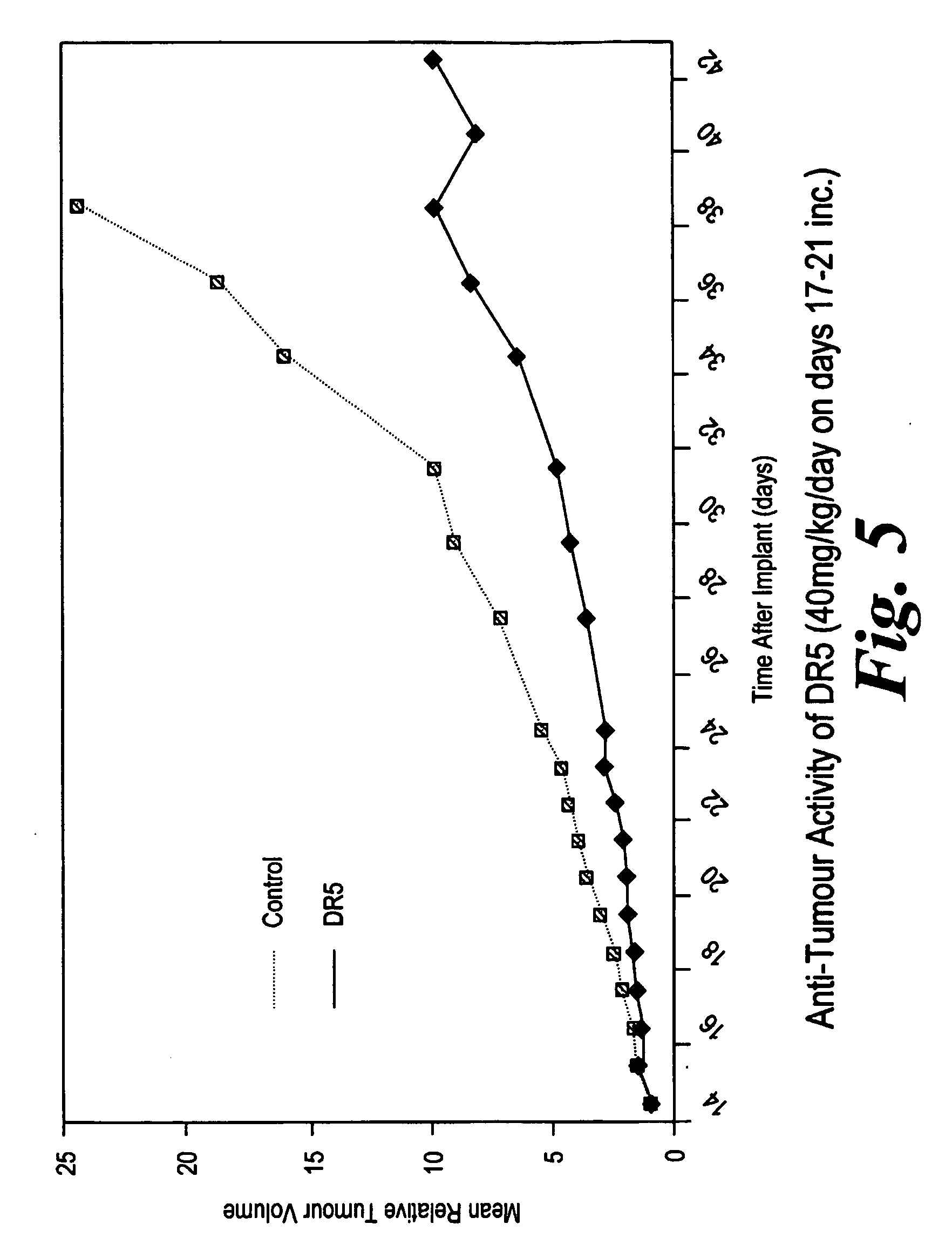
Method for treating hematopoietic neoplasms
InactiveUS20090192098A1Improve the immunityReduce apoptosis rateBiocidePhosphorous compound active ingredientsQuinoneIn vivo
This invention relates to methods for treating a hematopoietic neoplasm comprising administering a therapeutically effective amount of a combretastatin compound, or a pharmaceutically acceptable salt thereof, to a subject having a hematological malignancy, wherein the combretastatin compound comprises a catechol or quinone moiety and is capable of forming a reactive oxygen species (ROS) in vivo. The method may further comprise co-administering a second chemotherapeutic agent.
Owner:OXIGENE
Fluoroalkoxycombretastatin derivatives, method for producing the same and use thereof
Combretastatin derivatives of formula (I), preparation and use thereof are disclosed, wherein: Rf is alkyl with 1-8 carbon atoms and 1-17 fluorine atoms, R is amino, substituted amino, hydroxyl, nitro, halo, alkyloxy, phosphate or amino acid side chain. Said derivatives have a capability to inhibit the polymerization of microtubules and are useful in treatment against tumor and neovascularization.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
Antineoplastic drug Combretastatin water-soluble derivative and method for making same
InactiveCN101157600AGood water solubilityHigh selectivityEther/acetal active ingredientsPharmaceutical non-active ingredientsChemical industryLipid formation
The invention discloses water-solubility derivate of the antineoplastic medicine Combretastatin and the corresponding preparing method, and belongs to the technical field of medical and chemical industry. The compound of the invention takes substituted phenylacetic acid and isovanillin or the substitutional isovanillin as the initial material, and by condensation obtains (E)-substituted styrene acid; after that decorates a target group by amphotericity multi-polymer micromolecule with ether linkage, and finally, obtains water-solubility antineoplastic medicine Combretastatin derivate decorated by amphotericity multi-polymer micromolecule through decarboxylation or further decorating alkene linkage. The water-solubility is remarkably improved compared with the Combretastatin and the derivates before decoration, and basically maintains the intrinsic lipid solubility. Apart from easy material obtaining, the invention has the advantages of short synthesis line, simple operation and high yield as well as possessing strong selectivity to the cis-product with activity.
Owner:SHANGHAI JIAO TONG UNIV
Combretastatin derivative freeze-dried powder injection and preparation method thereof
ActiveCN109453123ALow toxicitySimple preparation processPowder deliveryEther/acetal active ingredientsSolubilitySide effect
The invention discloses Combretastatin derivative freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection is prepared from a Combretastatin derivative phospholipid complex, a stabilizer and a freeze-drying protecting agent. The preparation method comprises the following steps of: preparing the Combretastatin derivative phospholipid complex; homogenizing ina high pressure; and freeze-drying. The freeze-dried powder injection is dissolved in 5% of glucose or 0.9% of a normal saline injection solution for using, and can be used for intravenous injection,instillation or direct oral administration so as to treat non-small cell lung cancer, liver cancer and colon cancer. The prepared freeze-dried powder injection has the advantages of being high in ratio of drug to lipid, small in toxic and side effect, good in solubility, and capable of improving hydrophilicity and lipotropy of a Combretastatin derivative, improving oral administration bioavailability, reducing an intravenous administration dosage, and reducing toxicity and enhancing efficacy, and has a certain slow release effect and passive targeting. In addition, the preparation method is simple in technological process, and easy in industrial production.
Owner:CHINA PHARM UNIV
Ophthalmic Formulations
The present disclosure provides novel ophthalmic formulations for ocular administration comprising a pharmaceutically effective amount of a combretastatin, from 60% to 95% w / w pre-gelatinized starch, from 1% to 10% w / w hydrophilic matrix forming polymer, and from 0.2% to 5% lubricant.
Owner:OXIGENE
Antibody-conjugates for targeting of tumours expressing trop-2
PendingUS20210393792A1Increase polarityGood water solubilityPharmaceutical non-active ingredientsAntineoplastic agentsDimerAntibody conjugate
The present invention concerns antibody-conjugate having general structure (2):AB-[(L6)-{Z-(L1)n-(L2)o-(L3)p-(L4)q-D}xx]yy (2)wherein AB is an antibody capable of targeting Trop-2-expressing tumours and D is selected from the group consisting of taxanes, anthracyclines, camptothecins, epothilones, mytomycins, combretastatins, vinca alkaloids, maytansinoids, enediynes such as calicheamicins, duocarmycins, tubulysins, amatoxins, bleomycins, dolastatins and auristatins, pyrrolobenzodiazepine dimers, indolinobenzodiazepine dimers, radioisotopes, therapeutic proteins and peptides (or fragments thereof), kinase inhibitors, MEK inhibitors, KSP inhibitors, and analogues or prodrugs thereof. These antibody-conjugates exhibit an improved therapeutic index. The invention further concerns a process for preparing the antibody-conjugate according to the invention, a method for targeting Trop-2-expressing cells, medical uses of the antibody-conjugates according to the invention.
Owner:SYNAFFIX
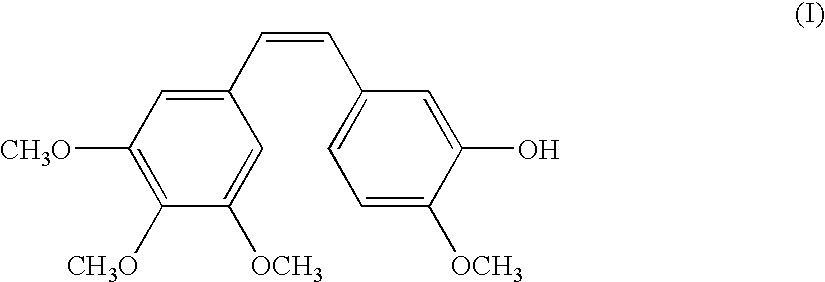
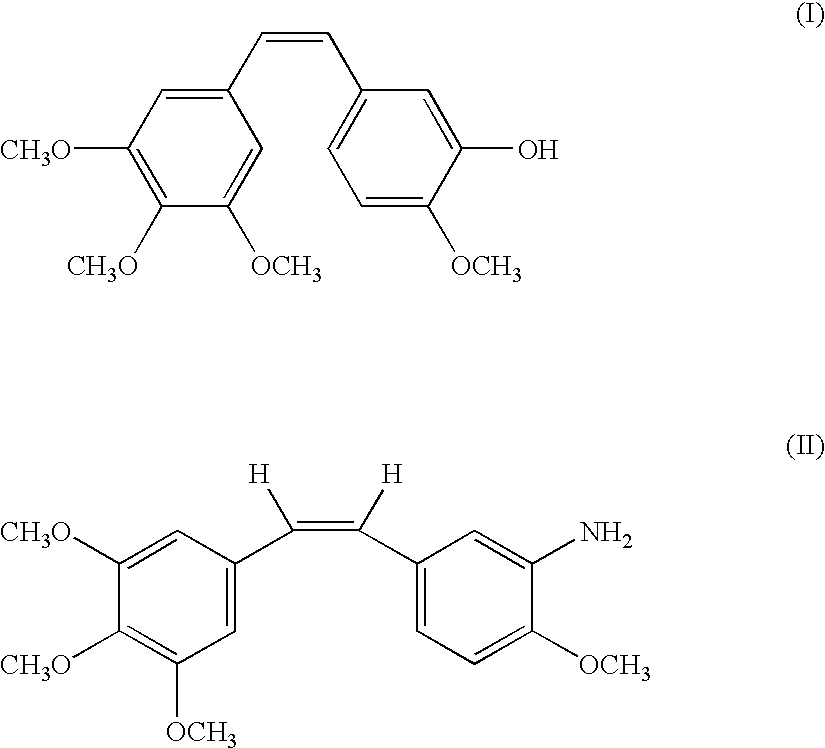
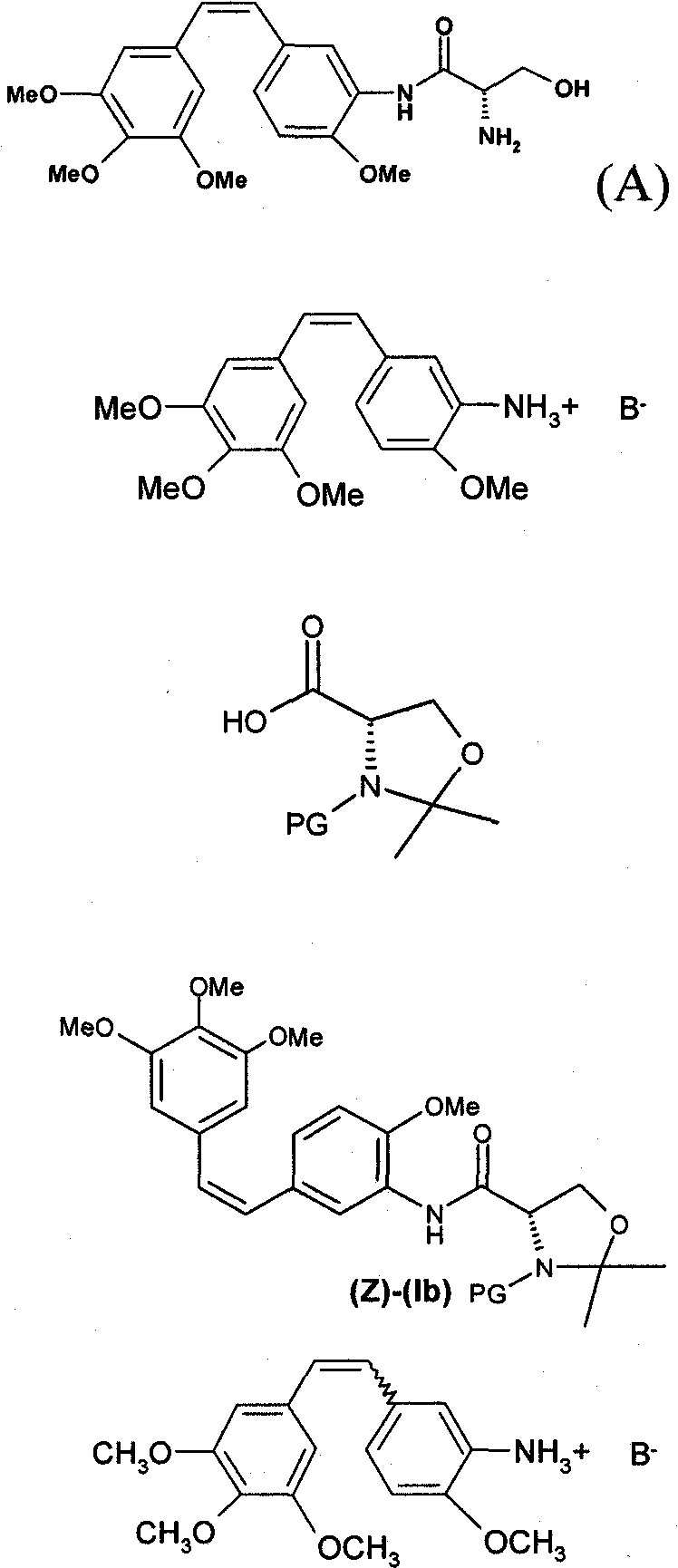
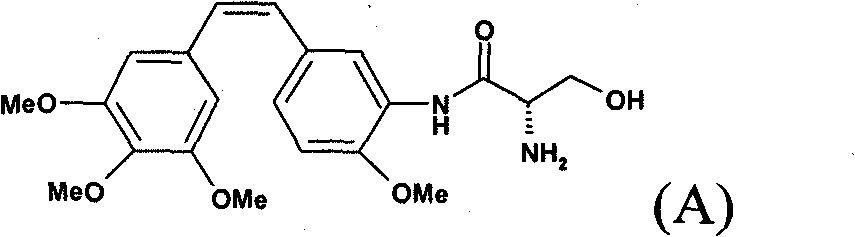
Method for preparing combretastatin
InactiveCN102015620AOrganic compound preparationOrganic chemistry methodsL serineMedicinal chemistry
The invention relates to a method for preparing a combretastatin (A): formula (I) in the form of a base or of an addition salt with an acid, which comprises coupling, in the presence of a base and of T3P, the salt of the (Z)-amino compound of formula (II) with a doubly protected L-serine derivative of formula (III) in which PG denotes a group protecting the amine function, so as to obtain the compound of formula (Z)-(Ib): formula (IV), then deprotecting and opening the ring of (Z)-(Ib) in the presence of an acid, so as to obtain the combretastatin (A) in the form of a salt; and, optionally, adding a base, so as to obtain the combretastatin (A) in the form of a base, the salt of the (Z)-amino compound having been obtained by enrichment of the salt of the amino compound of formula (V) in (Z) isomer.
Owner:SANOFI AVENTIS SA
Application of combretastatin
InactiveCN101579328AGrowth inhibitionDecreased blood flowEther/acetal active ingredientsAntineoplastic agentsMedicineTumor vessel
The invention discloses the application of a compound shown as a formula I or a formula II. The application is characterized in that the compound is used for preparing medicine for destroying tumor vessels.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
Drug combinations to treat hyperproliferative disorders
InactiveUS20100035911A1High anticancer activitySubstantial anticancer activityBiocideCarbohydrate active ingredientsDocetaxel-PNPCis-Retinoic Acid
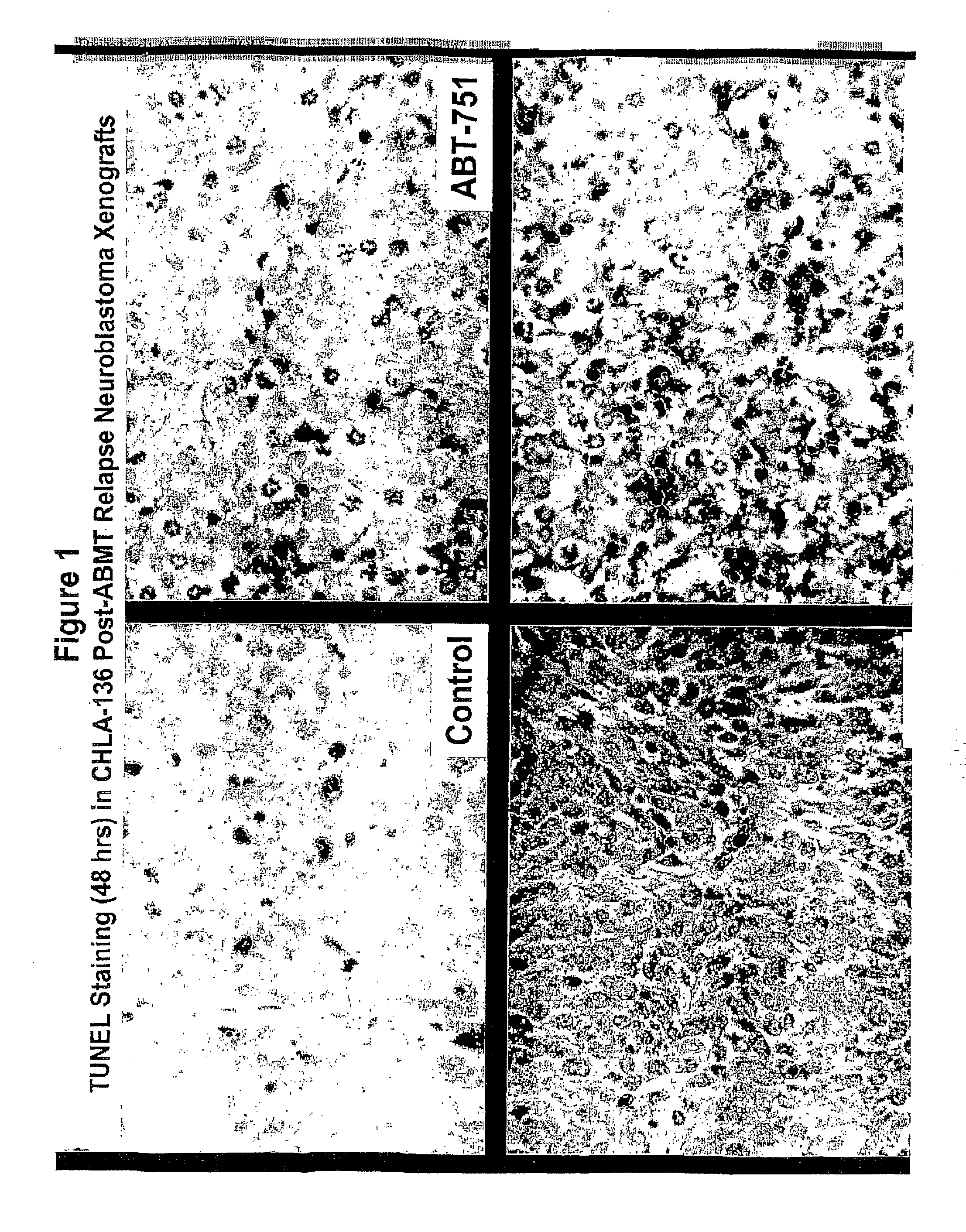
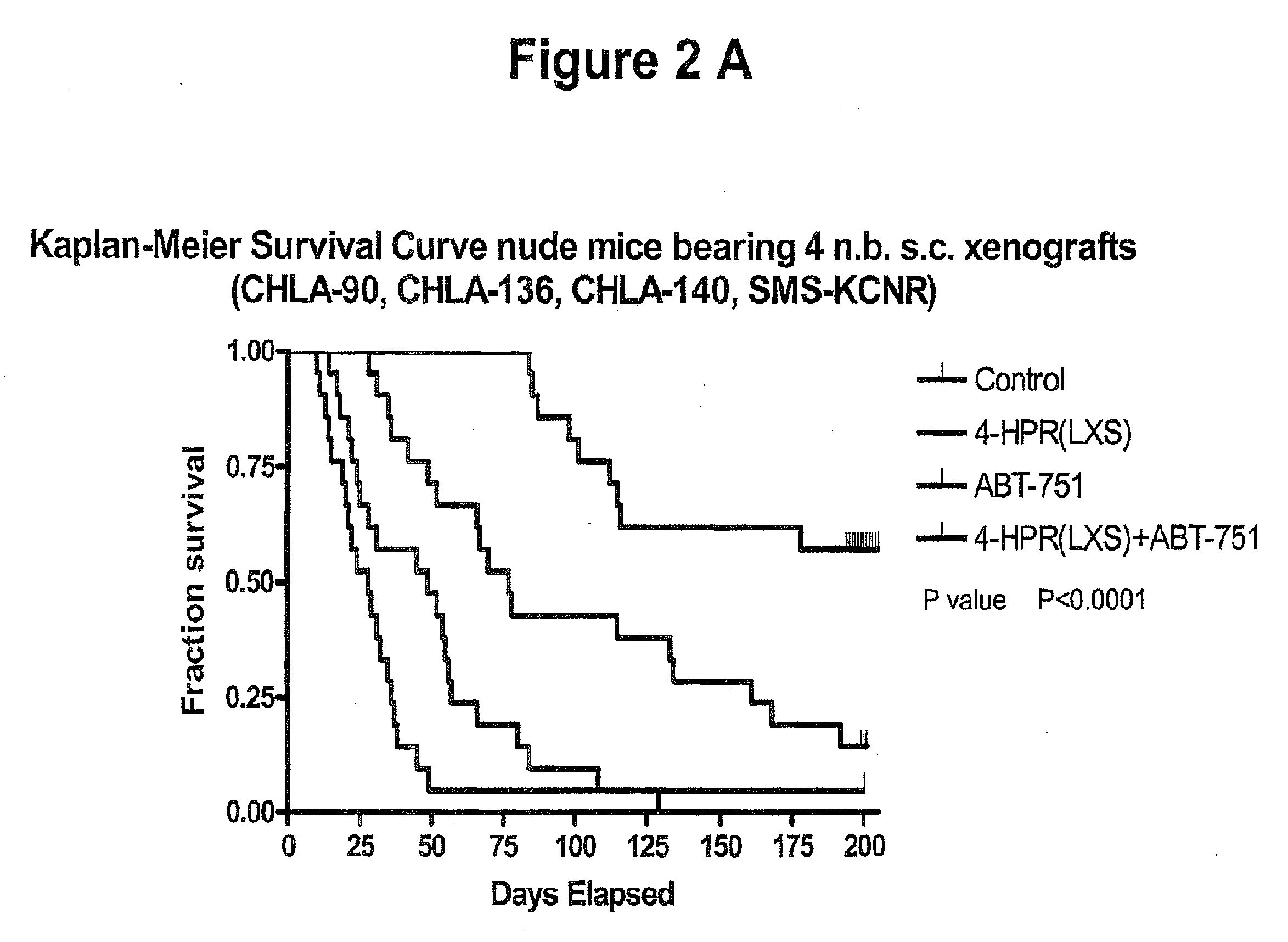
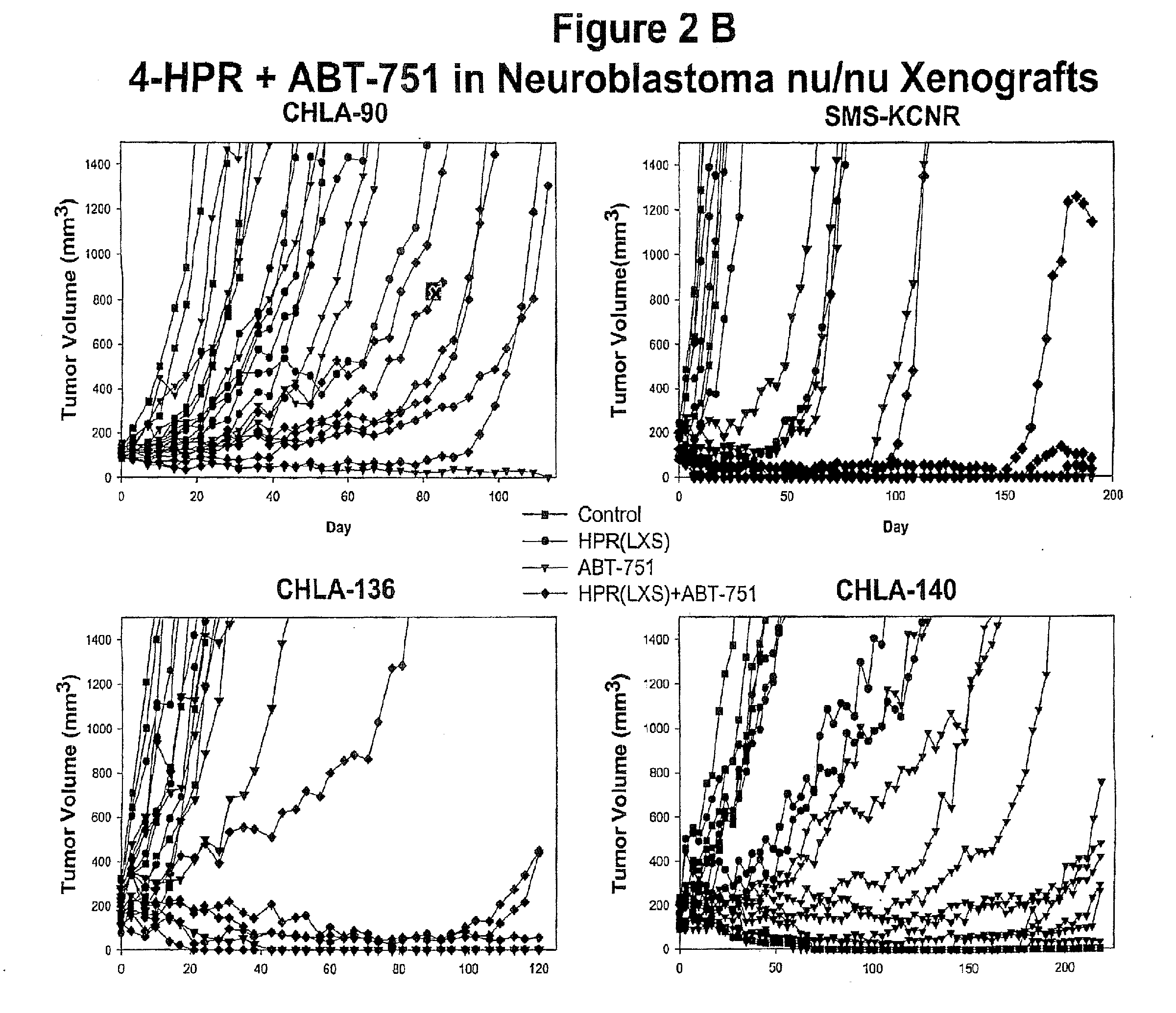
A method of treating a hyperproliferative disorder, including a cancer, in a subject in need of such treatment, comprising administering to said subject a pharmaceutical combination containing a treatment effective amount of: (a) a vitamin A derivative (i.e., a retinoid), or a pharmaceutically acceptable salt thereof, and an inhibitor of microtubule structure or function; or (b) a combination containing fenretinide (i.e., N-(4-hydrophenyl) retinamide, 4-HPR) and ABT-751 (i.e., N-[2-[(4-hydroxyphenyl)amino]-3-pyridinyl]-4-methoxybenzenesulfonamide). Vitamin A derivatives that may be useful for this invention according to (a) include, but are not limited to, all-trans-retinoic acid, 13-cis-retinoic acid, and fenretinide. Microtubule inhibitors that may be useful for this invention according to (a) include, but are not limited to, inhibitors of the Vinca binding domain (e.g., vincristine, vinblastine, vinorelbine, and cryptophycin 52), inhibitors of the Taxane domain (e.g., paclitaxel, docetaxel, and epothilones), and inhibitors of the colchicine site (e.g., colchicine, ABT-751, CI-980, and combretastatin). A preferred retinoid according to (a) is fenretinide. A preferred microtubule inhibitor according to (b) is ABT-751.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES
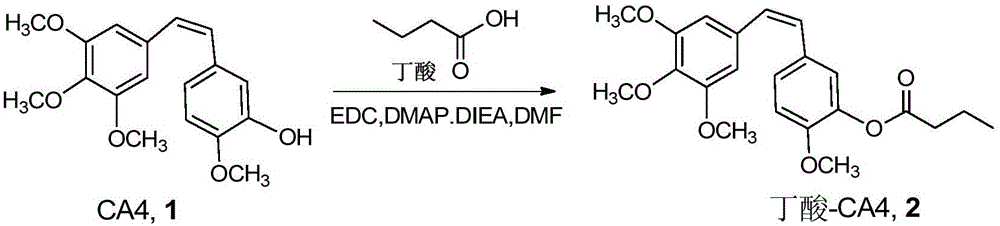
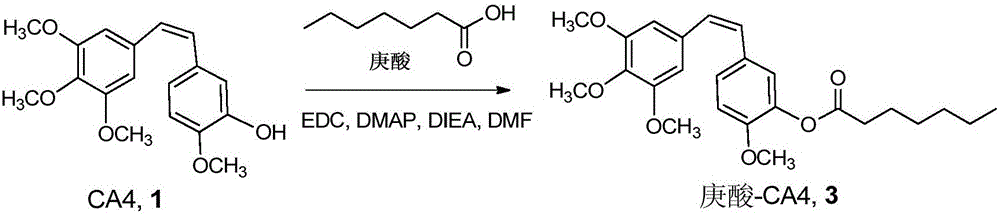
Combretastatin prodrug, pharmaceutical preparation and preparation method
InactiveCN106242972AIncrease loopIncrease generation curative effectOrganic active ingredientsPreparation from carboxylic acid halidesIn vivoLipophilic drug
The invention discloses a combretastatin prodrug, and discloses a preparation method thereof. The invention also discloses a preparation containing the combretastatin prodrug and a preparation method of the preparation. The combretastatin lipophilic prodrug provided by the invention can hydrolyze rapidly to release active pharmaceutical ingredients and play its efficacy. And the nano-preparation composed of the combretastatin prodrug can slowly release internal drugs, is conducive to prolonging the drug circulation in vivo and improving the drug efficacy, thus having good clinical application prospect and application value.
Owner:ZHEJIANG UNIV
Preparation method of combretastatin furan type analogues
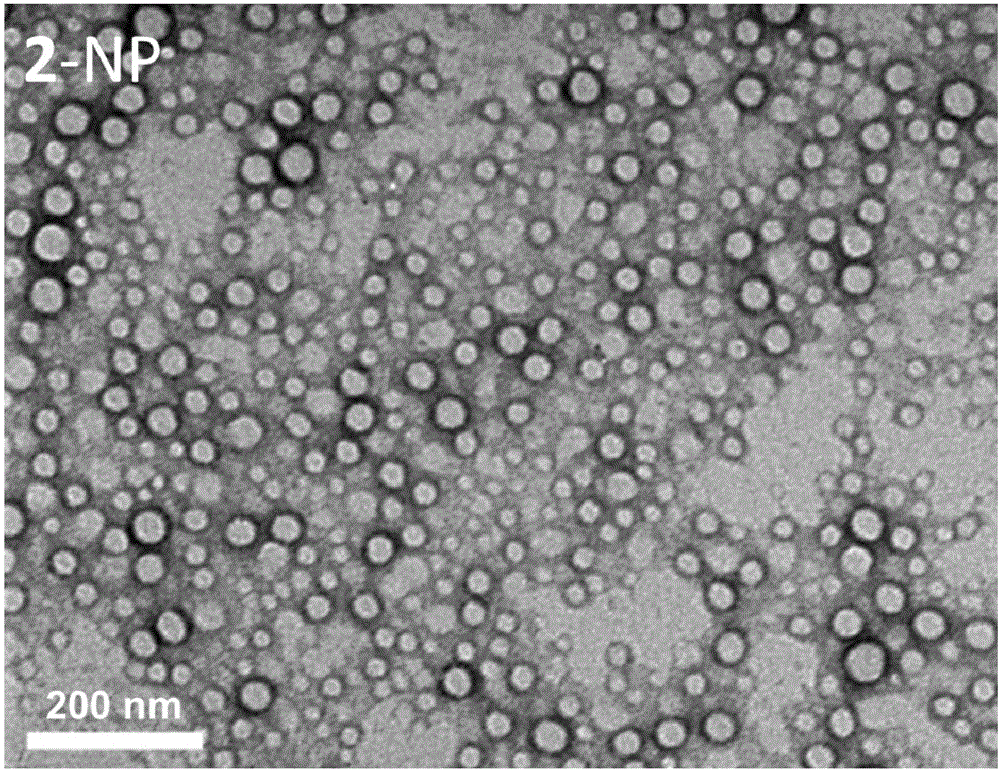
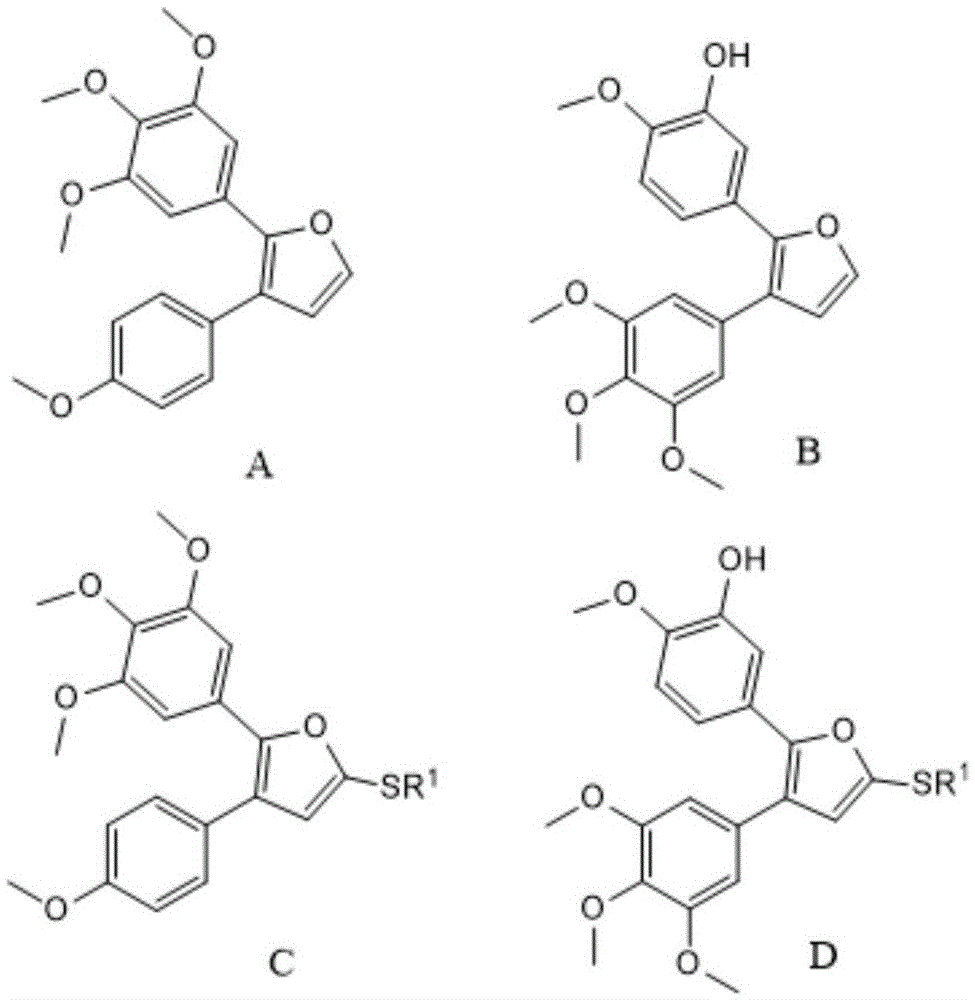
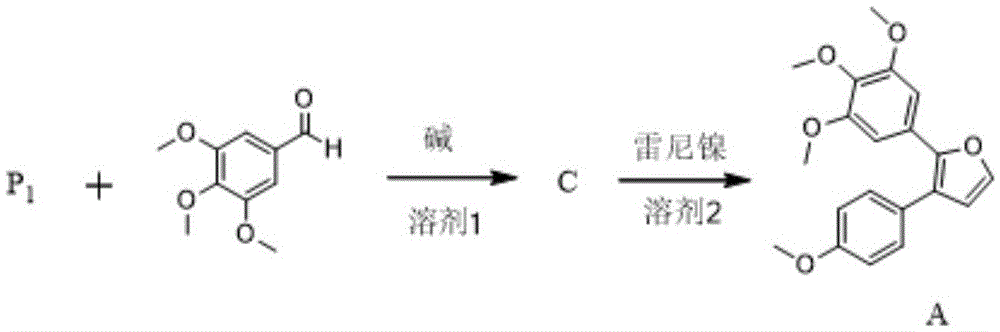
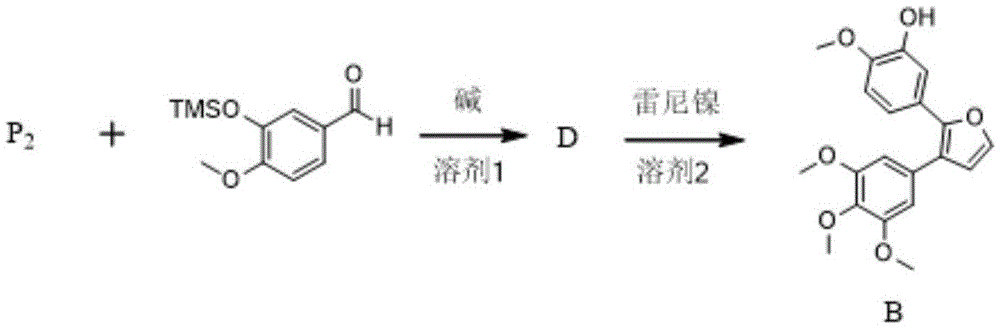
The invention provides a preparation method of combretastatin furan type analogues. The preparation method of the combretastatin furan type analogues (A and C) comprises the following steps of adding a solvent 1, a compound P1 and 3,4,5-triethoxy benzaldehyde into a reaction flask; adding alkali while stirring; reacting for 10 minutes at room temperature; adding diluted acid to regulate pH (Potential of Hydrogen) to be neutral; obtaining the combretastatin furan type analogue C through separation and purification; obtaining a compound A by reducing the C in a solvent 2 by using Raney Ni. The preparation method of combretastatin furan type analogues (B and D) comprises the following steps of adding the solvent 1, a compound P2 and 3-trimethylsilyl ether-4-methoxybenzaldehyde into the reaction flask; adding alkali while stirring; reacting for 10 minutes at the room temperature; adding the diluted acid to regulate pH (Potential of Hydrogen) to be neutral; obtaining the combretastatin furan type analogue D through separation and purification; obtaining a compound B by reducing the D in the solvent 2 by using the Raney Ni. The preparation method of the combretastatin furan type analogues, provided by the invention, has the advantages that the yield is high, the operation is simple, the reaction conditions are moderate, the used solvents are cheap and easy to get, industrial production is easy to realize, and the like.
Owner:LANZHOU UNIVERSITY
Fluoroalkoxycombretastatin Derivatives, Method For Producing the Same and Use Thereof
InactiveUS20080306027A1Improve its targeting activityHigh activityAntibacterial agentsBiocideAmino acid side chainPhosphate
Combretastatin derivatives of formula (I), preparation and use thereof are disclosed, wherein: Rf is alkyl with 1-8 carbon atoms and 1-17 fluorine atoms, R is amino, substituted amino, hydroxyl, nitro, halo, alkyloxy, phosphate or amino acid side chain. Said derivatives have a capability to inhibit the polymerization of microtubules and are useful in treatment against tumor and neovascularization.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
Polymer conjugate of combretastatin
InactiveCN101489592ABest practicePharmaceutical non-active ingredientsAntineoplastic agentsSolubilityPolyethylene glycol
The invention relates to a novel derivative of a combretastatin which has water solubility and is capable of releasing a drug independent of biological enzymes in which individual differences are likely caused and whose effective therapeutic effect can be expected has been demanded. A polymer conjugate of a combretastatin, characterized by having a structure in which a hydroxy group of a combretastatin is linked via an ester bond to a carboxylic acid group of the following polymer moiety in a block copolymer of a polyethylene glycol structure moiety with a polymer moiety having two of more carboxylic acid groups such as polyaspartic acid or polyglutamic acid is provided.
Owner:NIPPON KAYAKU CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com