Compretastatin derivative and antibody drug conjugate thereof
A technology of compretin and antibody drugs, which can be used in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., and can solve problems such as poor water solubility and difficulty in direct preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation of antibody-drug conjugate
[0034] (1) Synthesis of Compound 50-5
[0035]
[0036] Synthesis of Compound 50-1
[0037] Comprestatin (316.35 mg, 1 mmol) was dissolved in DCM (10 mL), followed by the addition of p-nitrophenyl chloroformate (403.12 mg, 2 mmol) and pyridine (237.3 mg, 3 mmol). Reaction at room temperature for 4h. The reaction was checked for completion by TLC thin layer chromatography. The crude product was obtained after direct decompression rotary evaporation to remove the solvent, and 5 mL of CH was added 2 Cl 2 and 1g of 60-100 mesh silica gel, mix well and spin dry. The crude product was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain white solid 50-1 (480 mg, 0.997 mmol, 99%). ESI-HRMS calculation result: C 25 h 24 NO 9 [M+H] + :482.1451, actual result: 482.1410.
[0038] Synthesis of Compound 50-2
[0039] Compound 50-1 (0.480 g, 1 mmol), N-Boc-N,N'-dimethylethy...
Embodiment 2
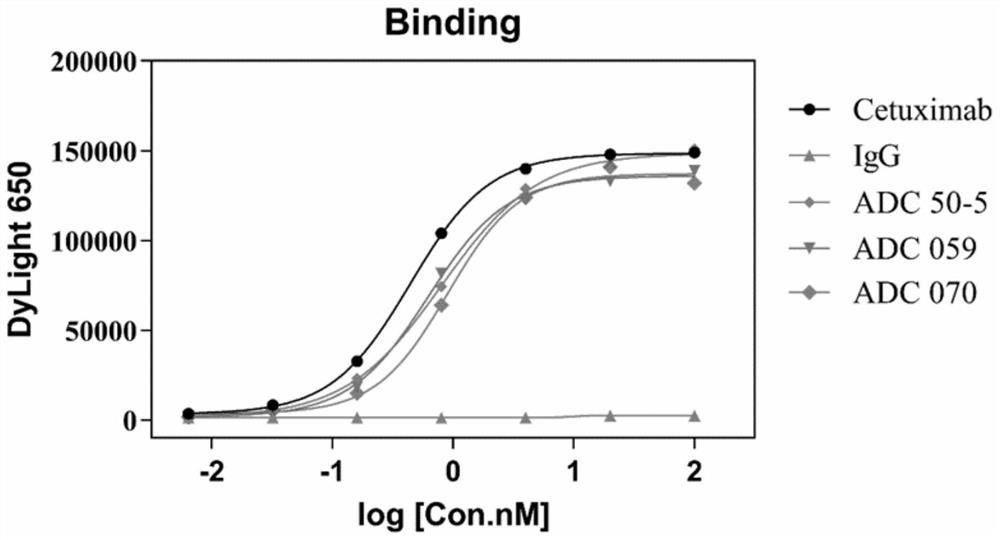
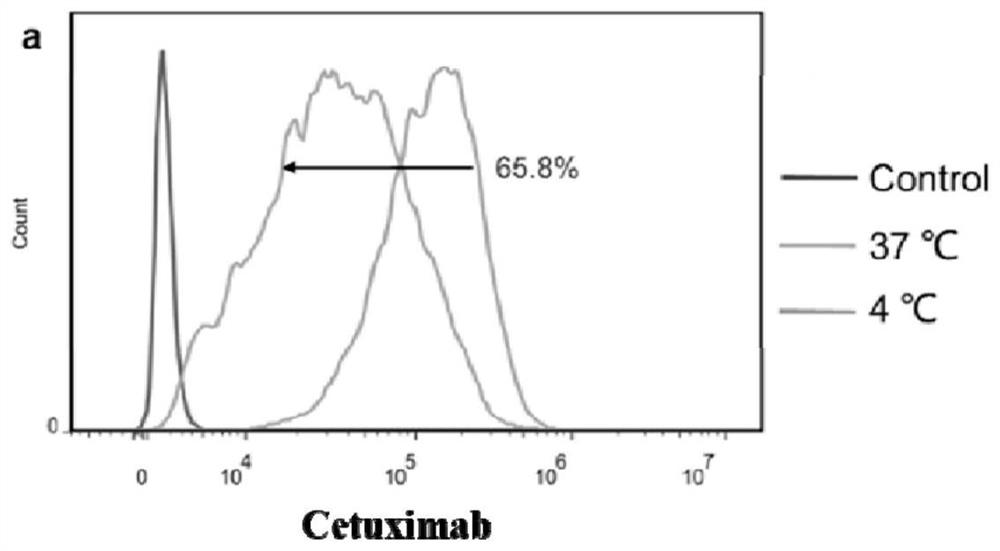
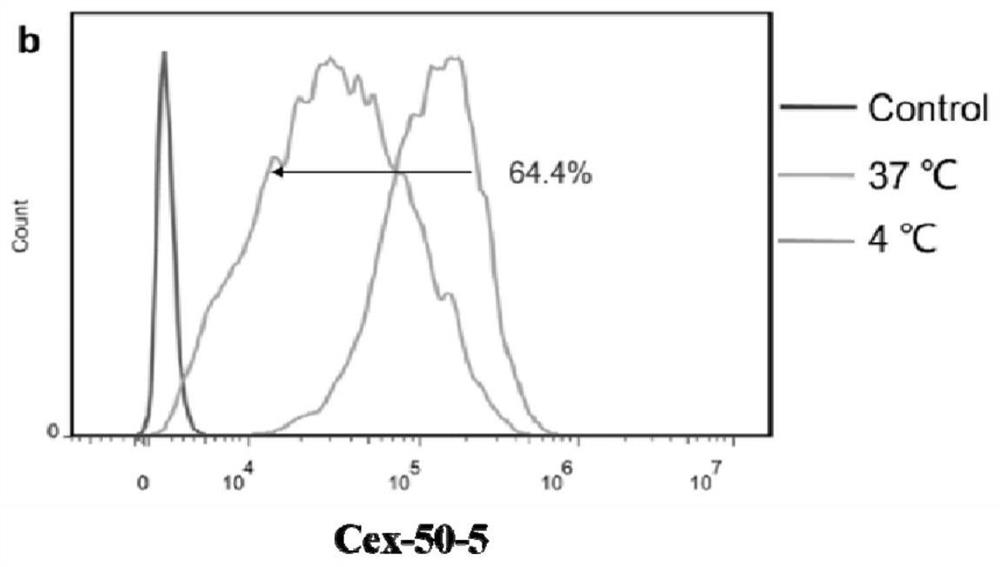
[0110] Example 2: Affinity and Endocytosis Assays of Antibody-Drug Conjugates
[0111] In this example, the affinity and endocytosis of Cetuximab, Cex-50-5, Cex-59, and Cex-70 on tumor cell lines were studied. The cells can be endocytized normally at 37°C, and most of the physiological activities of the cells are inhibited at 4°C, which is regarded as non-endocytosis. The test substance and the cells were incubated at 4°C and 37°C for a period of time, and the internal endocytosis during this time period = (4°C cell surface antibody amount - 37°C cell surface antibody amount) / 4°C cell surface antibody amount × 100% .
[0112] The reagents and consumables used in the following experiments come from: Cetuximab (Shanghai Huanyao Biotechnology Co., Ltd., article number: 205923-56-4), Goat Anti-Human IgG H&L (FITC) was purchased from Abcam Company , DAPI was purchased from Cell Signaling Company, face washing solution (PBS+1%BSA). Instrument: CytoFLEX Flow Cytometer.
[0113] I...
Embodiment 3
[0121] Example 3: In Vitro Cell Proliferation Biological Activity Determination of Antibody-Drug Conjugates
[0122] In this example, the effect of Cetuximab, ADCs Cex-50-5, Cex-59, Cex-70 on the proliferation of tumor cell lines was investigated.
[0123] The reagents and consumables used in the following experiments come from: RPMI1640 medium, trypsin-EDTA, fetal calf serum, recombinant human insulin, 100×penicillin, 1×PBS (pH7.4), CCK8 color reagent purchased from Gibco Company; 10cm petri dish (Corning) and 96-well cell culture plate (Corning); multifunctional microplate reader (SpectraMax i3).
[0124] In this example, the CCK8 colorimetric method was used to evaluate the anti-proliferation effect of the test substance. Cell Counting Kit-8 (referred to as CCK-8) reagent contains WST-8 [chemical name: 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5 -(2,4-disulfonic acid benzene)-2H-tetrazole monosodium salt]. It is reduced to a highly water-soluble yellow formazan produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com