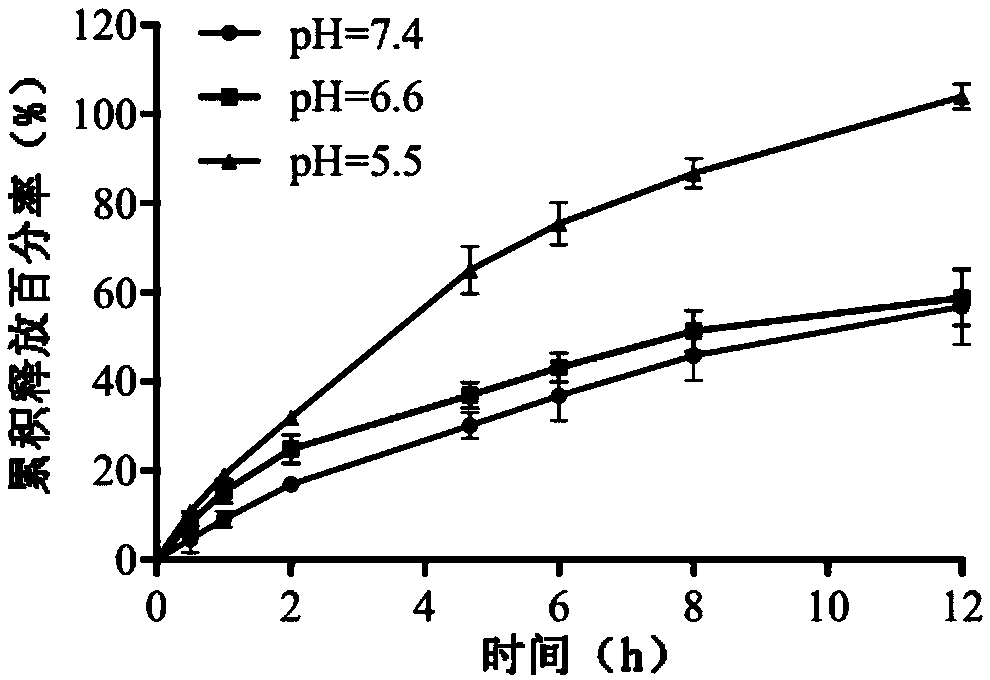
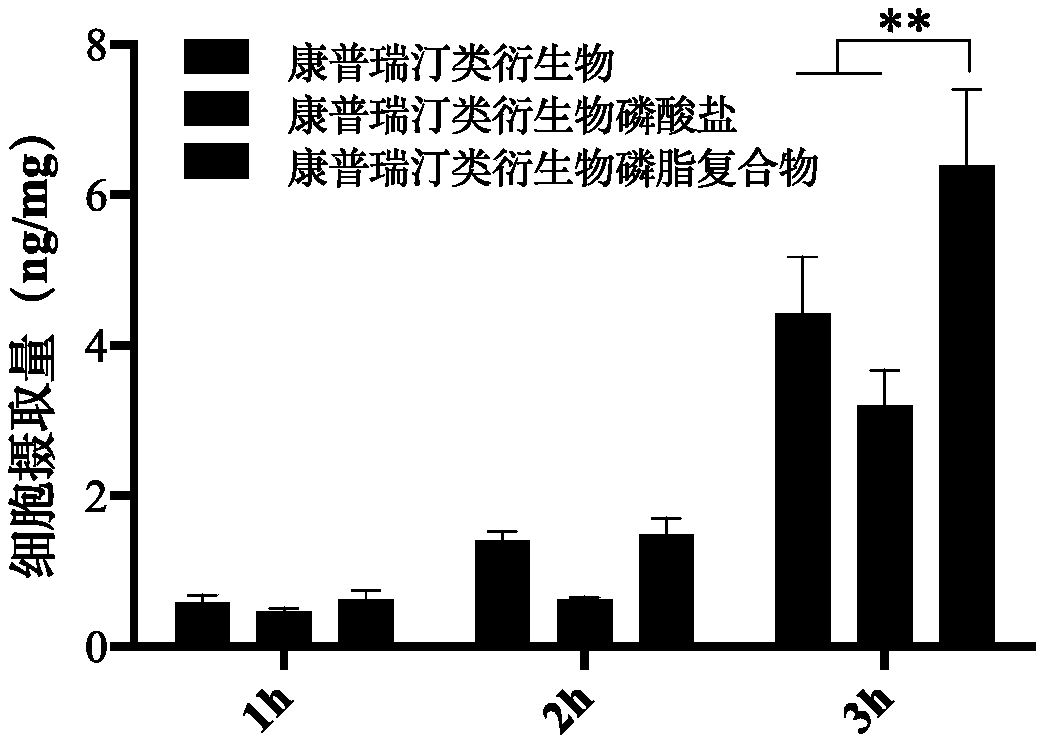
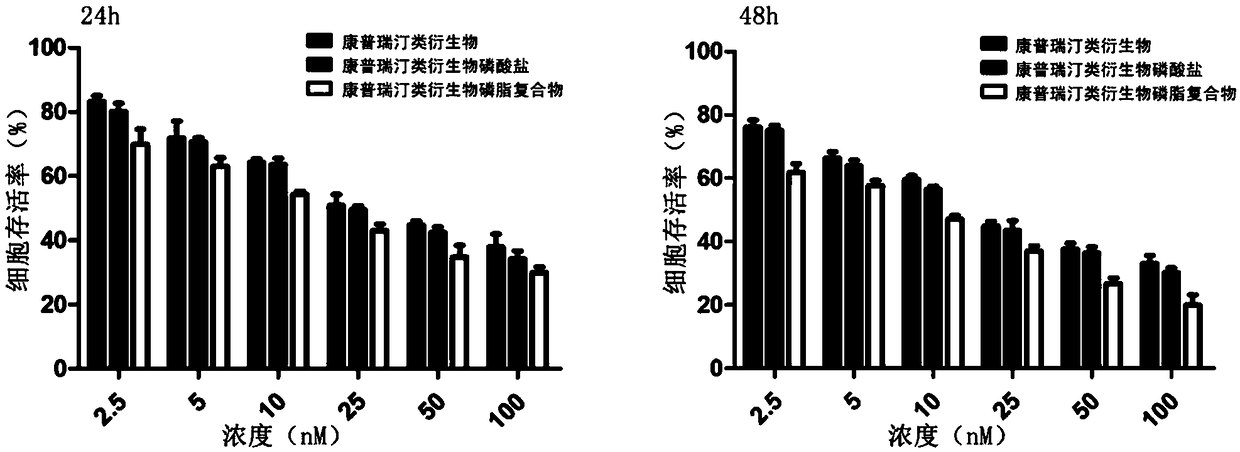
Combretastatin derivative freeze-dried powder injection and preparation method thereof
A technology of compretidines and freeze-dried powder injections, which is applied in the field of compretine derivatives freeze-dried powder injections and its preparation, can solve the problems of preparation process, excipients dosage, excipients safety and insufficient storage, and unseen health issues. The invention of phospholipid complexes of pretinoid derivatives does not have the convenience and compliance of oral administration, so as to improve the oral bioavailability, overcome the high dosage of drugs, and improve the efficacy of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Accurately weigh compretidine derivatives (4-(3,5-dimethoxy)-5-(4-methoxyphenyl) oxazole) and soybean lecithin with a molar mass ratio of 2:1 in a 50mL round In the bottom flask, add 5mL of acetone, control the drug concentration to 20mg / mL, stir until it is completely dissolved, leave it to compound at 30°C for 1 hour, and then vacuum-dry to obtain the compretidine derivative phospholipid complex. The compound rate is determined 50%.
Embodiment 2
[0043] Accurately weigh compretidine derivatives (2-phenyl-4-(3,4,5-trimethoxyphenyl)-5-(4-pyridyl)thiazole) and soybean with a molar mass ratio of 2:1 Put lecithin in a 50mL round bottom flask, add 200mL of acetone, control the drug concentration to 0.5mg / mL, stir until it is completely dissolved, leave it to compound at 35°C for 2 hours, and then vacuum-dry it to obtain compraditin derivatives phospholipid compound substance, the compound rate was determined to be 72%
Embodiment 3
[0045]Accurately weigh compretidine derivatives (4-(3,5-dimethoxyphenyl)-5-(3-hydroxy-4-methoxyphenyl)imidazole) and egg yolk with a molar mass ratio of 1:1 Put lecithin in a 50mL round-bottom flask, control the drug concentration to 3mg / mL, add 30mL of benzene, stir until it is completely dissolved, let it stand at 40°C for 2 hours, and then vacuum-dry to obtain the compretidine derivative phospholipid complex , The measured composite rate was 62%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com