Polymer conjugate of combretastatin
A technology of combretastatin and high molecular weight, which is applied in the field of preparation of high molecular weight conjugates, can solve problems such as no description of combretastatin conjugates, etc., and achieve the effect of effective therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
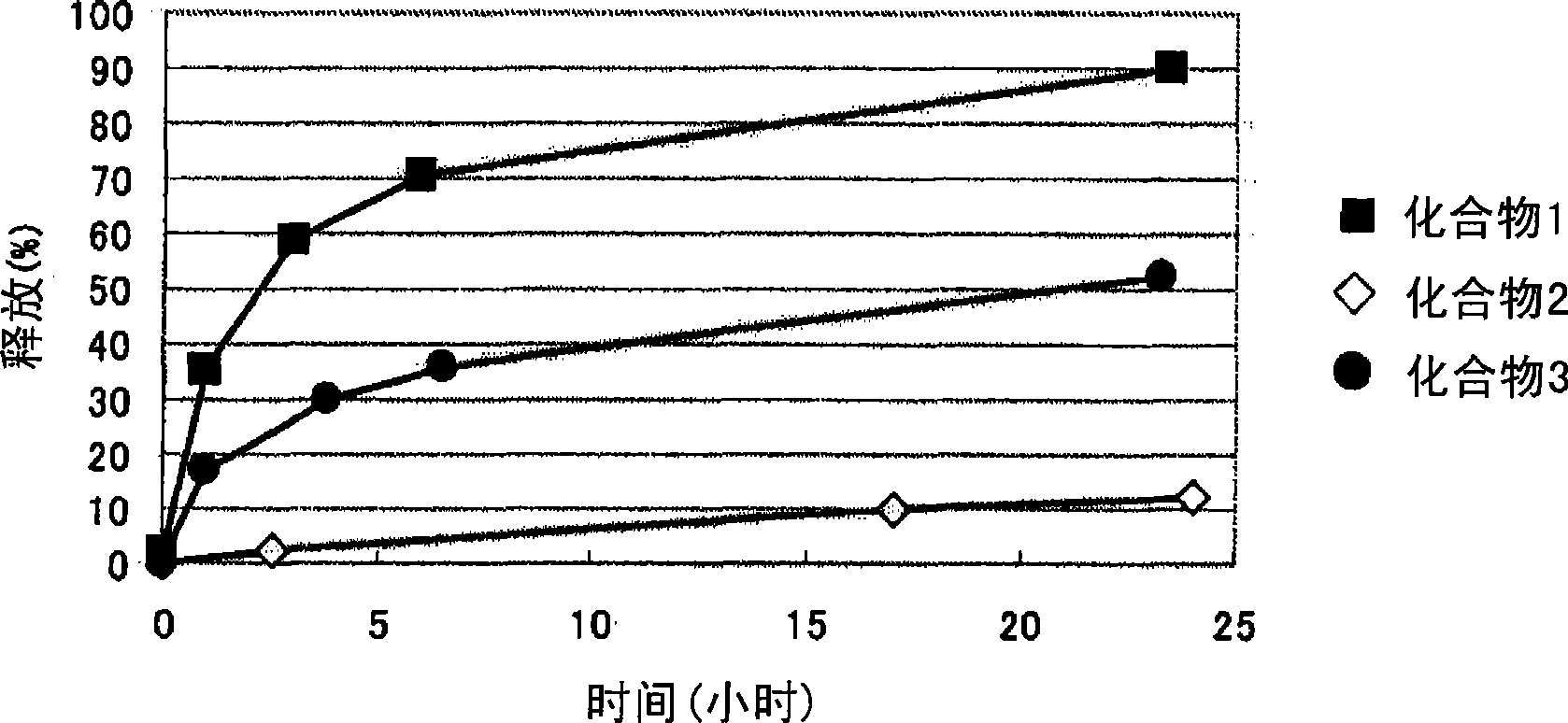
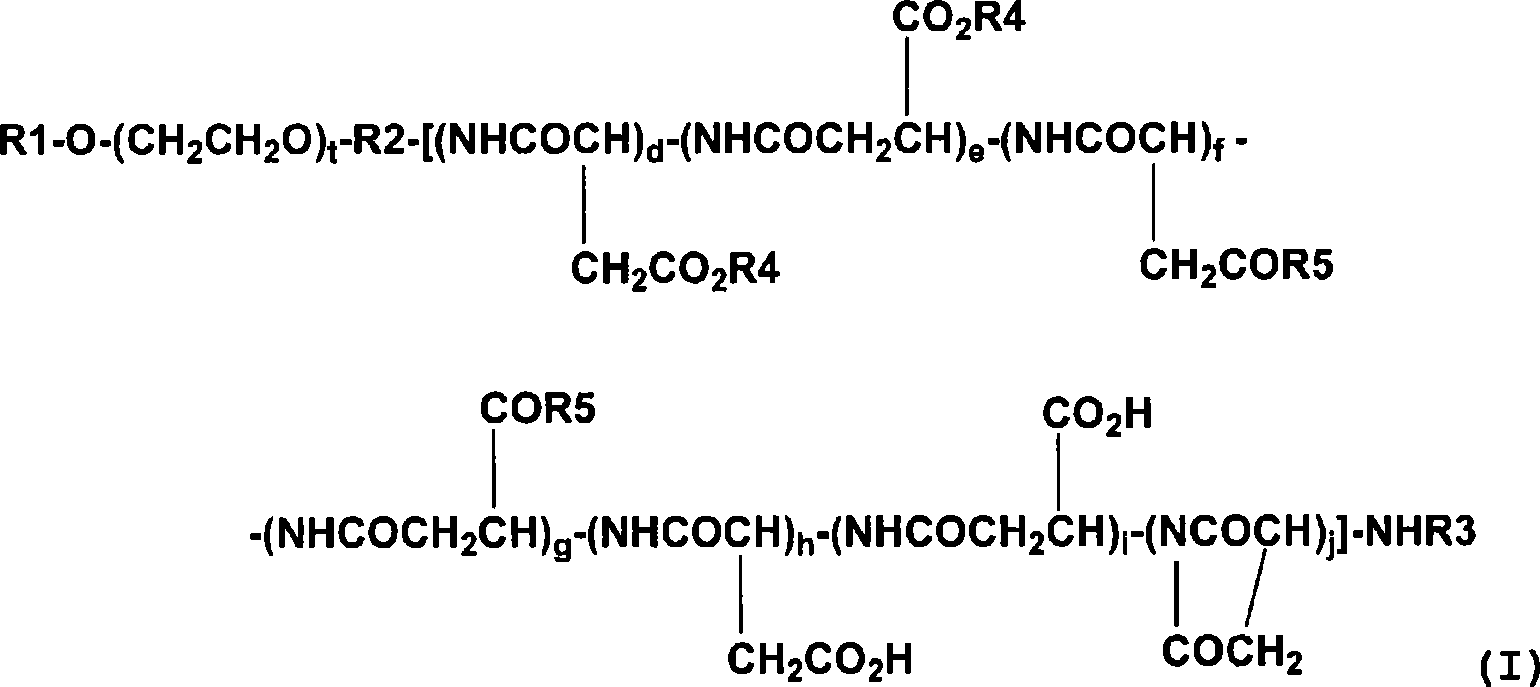
[0086] Embodiment 1: the synthesis of compound 1 (combretastatin A-4 and the methoxypolyethylene glycol part of molecular weight 5000 and the polyaspartic acid part of degree of polymerization 30 constitute the block copolymer conjugate: General Formula (I), wherein R1=Me (methyl), R2=1,3-propylene, R3=Ac (acetyl), R4=combretastatin A-4 residue, R5=isopropylaminocarbonyl isopropylamino, d+e+f+g+h+i+j=30, t=113).
[0087] The methoxypolyethylene glycol-polyaspartic acid block copolymer prepared according to the method described in Patent Document 5 (aspartic acid part: a mixture of α-type and β-type, polymerization degree: 30, 2670 mg) and combretastatin A-4 (600 mg) synthesized according to the method described in Non-Patent Document 1 were dissolved in DMF (60 ml), and DMAP (174 mg) and DIPC (2.97 ml) were added thereto. The mixture was stirred at 25°C for 20 hours. Ethyl acetate (180 ml) and diisopropyl ether (720 ml) were added to the reaction liquid, and the mixture was ...
Embodiment 2
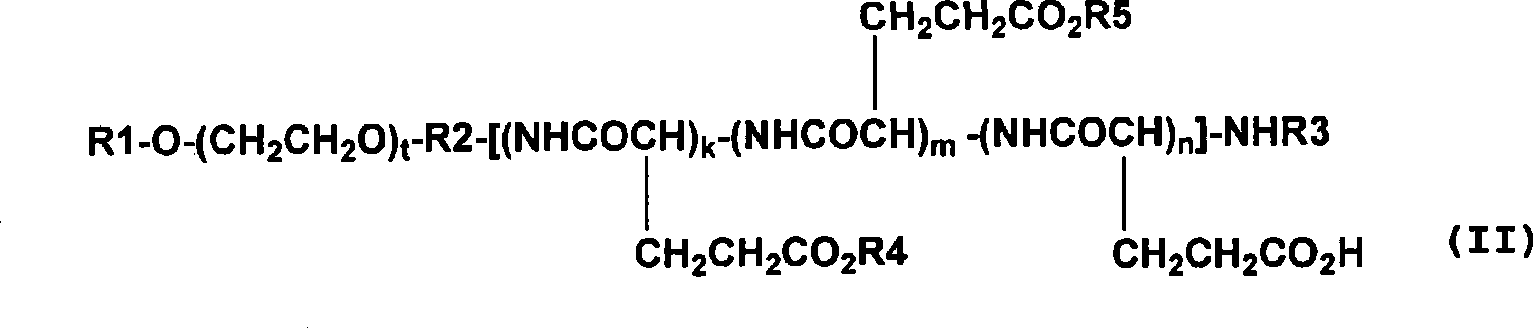
[0090] Embodiment 2: the synthesis of compound 2 (combretastatin A-4 and the methoxypolyethylene glycol part of molecular weight 12000 and the polyglutamic acid part of degree of polymerization 23 constitute the block copolymer conjugate: general formula (II), wherein R1 = Me (methyl), R2 = 1,3-propylene, R3 = Ac (acetyl), R4 = combretastatin A-4 residue, R5 = isopropylaminocarbonyliso Propylamino, k+m+n=23, t=273).
[0091]The methoxypolyethylene glycol-polyglutamic acid block copolymer (581 milligrams) prepared according to the method described in Japanese Patent Application Laid-Open No. 5-955 and the Cobb synthesized according to the method described in Non-Patent Document 1 Statin A-4 (100 mg) was dissolved in DMF (4.5 mL), DMAP (16.5 mg) and DIPC (0.283 mL) were added thereto, and stirred at 20° C. for 40 hours. DIPC (0.070 ml) was added to the reaction solution, and the stirring was continued for 1.5 hours after the temperature reached 25°C. To the reaction solution w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com