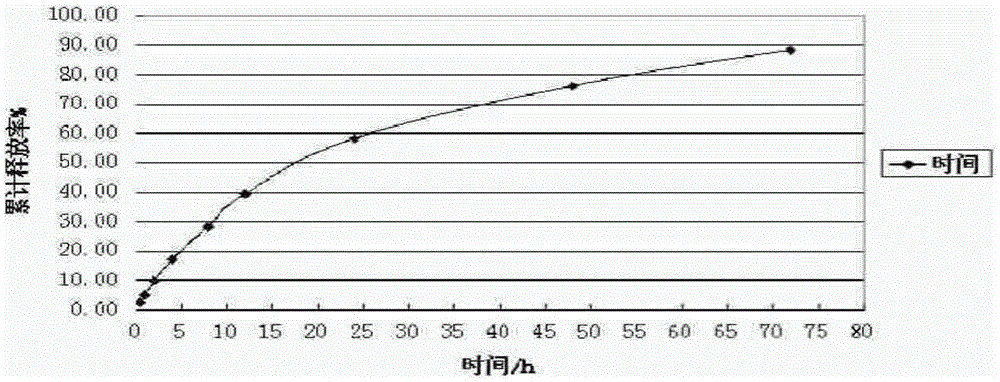
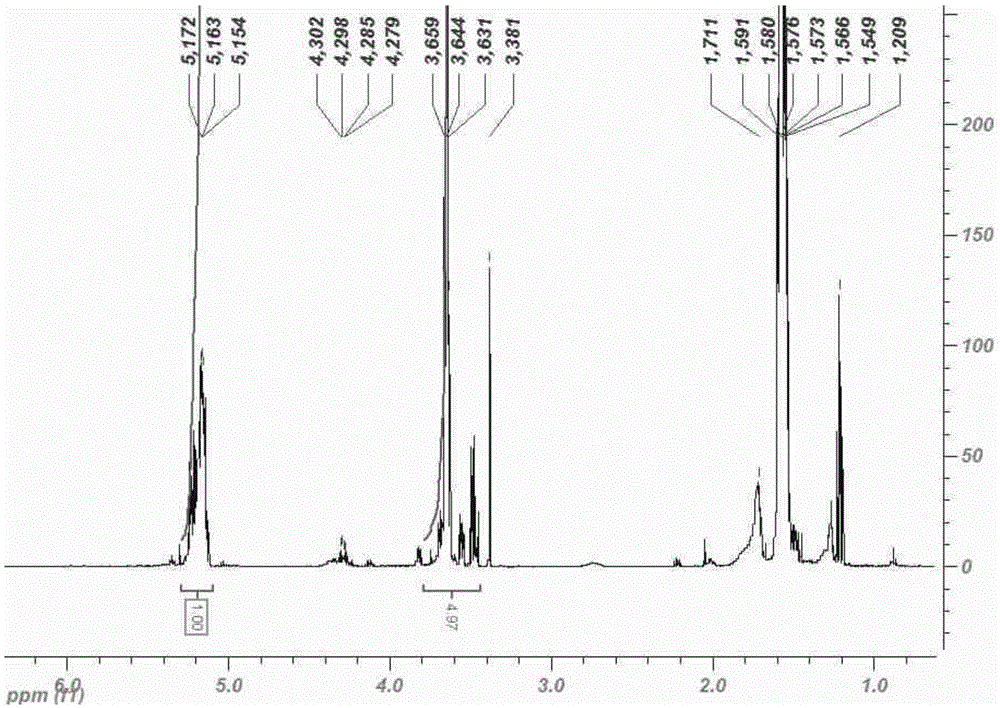
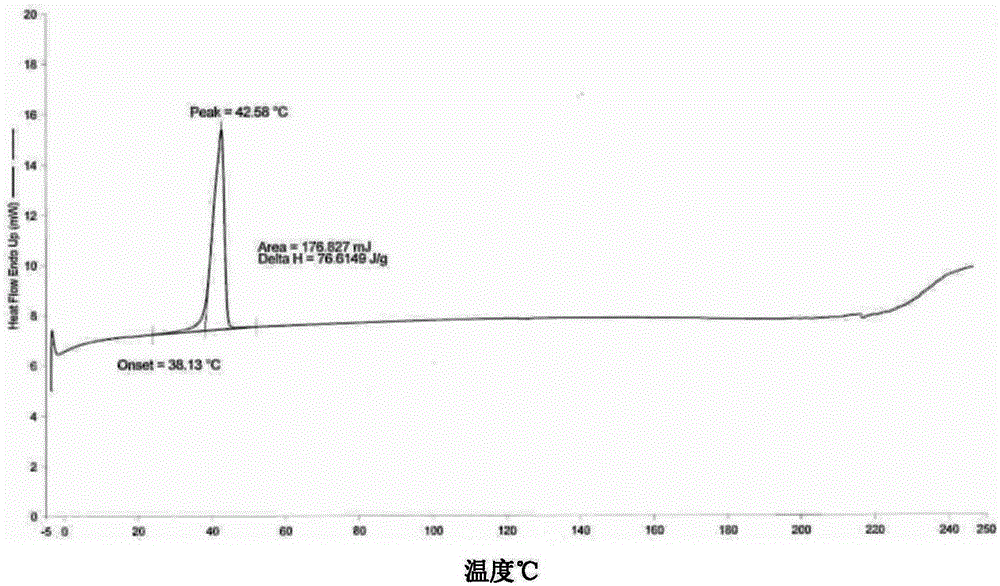
Combretastatin nanometer polymer micelle freeze-dried preparation and preparation method thereof
A technology of nano-polymer and compretidine, which is applied in the field of compretidine nano-polymer micelle freeze-dried preparations and its preparation, can solve the problems of poor stability, poor water solubility and low bioavailability, and achieve stability High, reduced toxicity, good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of amphiphilic biodegradable diblock copolymer carrier material.
[0028] Formula dosage: D, L-lactide 150g, polyethylene glycol monomethyl ether 100g.
[0029] Weigh the formulated amount of D, L-lactide and polyethylene glycol monomethyl ether for later use, vacuum-dry the formulated amount of polyethylene glycol monomethyl ether in the reactor at 110°C for 2 hours, replace with nitrogen, and then add The D of formula quantity, L-lactide, drop into catalyst stannous octoate again, the amount of stannous octoate accounts for D, 0.2% of the total mass of L-lactide and polyethylene glycol monomethyl ether, then vacuumize, wait D, After L-lactide is completely melted, replace with nitrogen three times, then vacuumize the reactor to ensure negative pressure, airtight or nitrogen protection, then raise the temperature to 140°C, react for 6 hours, and after the reaction is completed, a light yellow clear viscous Thick liquid; add 60ml of dichloromethan...
Embodiment 2
[0034] Formula dosage: D, L-lactide 100g, glycolide 100g, polyethylene glycol monomethyl ether 100g.
[0035]Weigh the formulated amount of D, L-lactide, glycolide, and polyethylene glycol monomethyl ether for later use, dry the formulated amount of polyethylene glycol monomethyl ether in a reactor at 100°C for 3 hours under vacuum, nitrogen Replacement, then add the D of formula quantity, L-lactide, glycolide, put into catalyst stannous octoate again, the amount of stannous octoate accounts for D, L-lactide, glycolide and polyethylene glycol monomethyl 0.3% of the total mass of ether, then vacuumize, after D, L-lactide and glycolide are all melted, replace with nitrogen three times, and then vacuumize again to ensure negative pressure in the reactor, airtight or nitrogen protection, and then Raise the temperature to 120°C, react for 8 hours, and after the reaction is completed, a light yellow, clear and viscous liquid is obtained; add 50 ml of dichloromethane to the light yel...
Embodiment 3
[0037] Formula dosage: ε-caprolactone 80g, polyethylene glycol monomethyl ether 100g.
[0038] Weigh the formulated amount of ε- and lactone and polyethylene glycol monomethyl ether for later use, dry the formulated amount of polyethylene glycol monomethyl ether in the reactor at 120°C for 4 hours in vacuum, replace with nitrogen, and then add the formulated amount ε- and lactone, and then put into the catalyst stannous octoate, the amount of stannous octoate accounts for 0.1% of the total mass of ε-caprolactone and polyethylene glycol monomethyl ether, then vacuumize, until ε- and lactone are all After melting, replace with nitrogen for three times, and then vacuumize to ensure negative pressure in the reactor, airtight or nitrogen protection, then raise the temperature to 130°C, react for 10 hours, after the reaction is completed, a light yellow, clear and viscous liquid is obtained; Add 40ml of dichloromethane to the yellow clear viscous liquid to dissolve, stir for 30min, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat | aaaaa | aaaaa |
| heat | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com