Application of combretastatin
A use and drug technology, applied in the new use field of comproprietine, can solve problems such as targeted drug use and lack of tumor blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
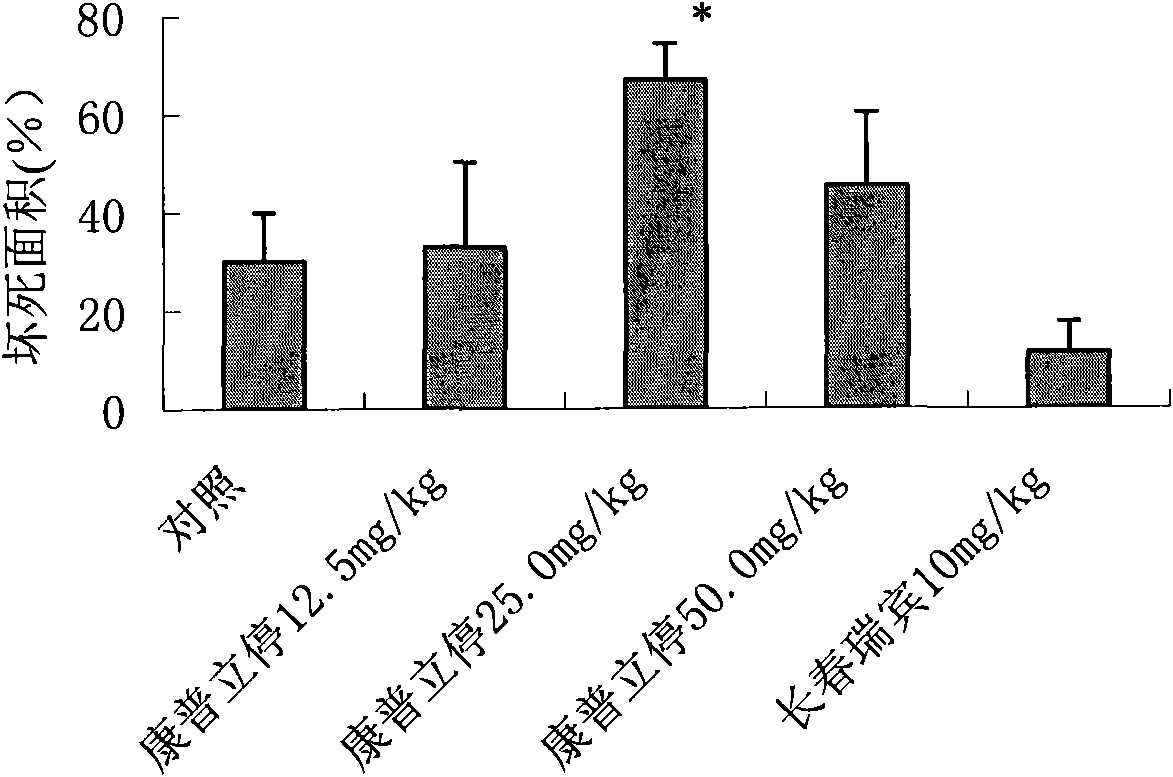
[0061] Efficacy of compridine on human colon cancer Ls174t xenografted tumor in nude mice
[0062] Cell culture: culture human colon cancer cell Ls-174t (purchased from Shanghai Institute of Materia Medica, mouse solid tumor) in vitro, and keep the cells in the logarithmic growth phase.
[0063] Transplantation: The prepared cells were inoculated subcutaneously in nude mice, and passaged 2-3 times after tumor formation.
[0064] Inoculation: Under sterile conditions, the tumor mass was inoculated into the underarm of one side of the animal.
[0065] Grouping and dose setting: Use a vernier caliper to measure the diameter of the transplanted tumor in nude mice, and wait until the tumor grows to 100-300mm 3 Afterwards, the animals were randomly grouped.
[0066] Administration route and time: choose with 80-200mm 3 Nude mice with large and small tumors were randomly divided into groups, and administered after the tumor volume was measured.
[0067] Intravenous administration...
Embodiment 2
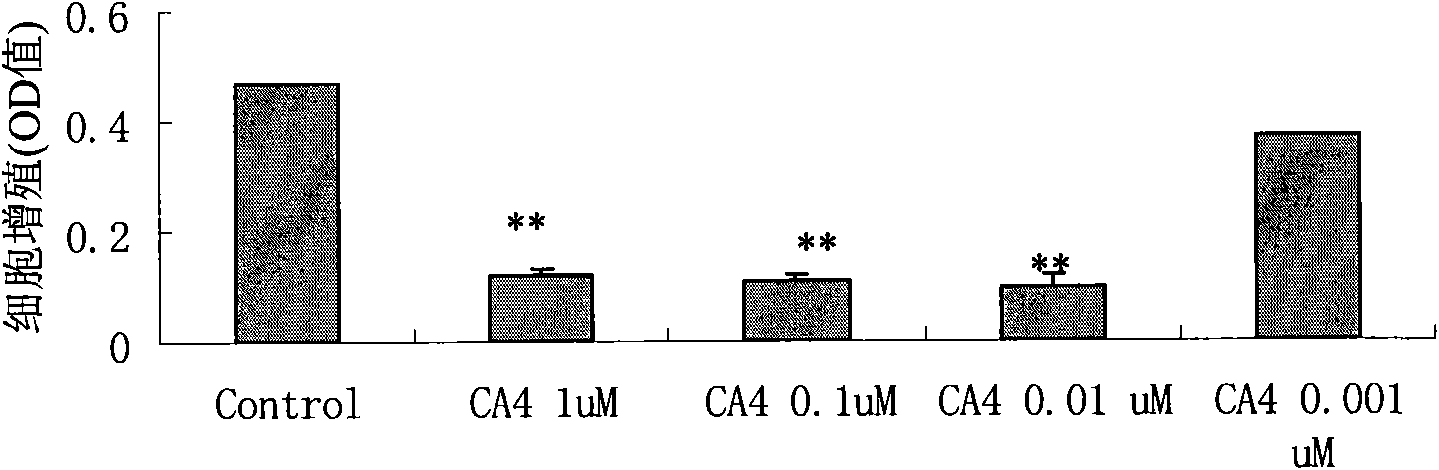
[0088] Effects of compridine on the proliferation of human umbilical vein endothelial cells
[0089] Test reagents, drugs and instruments: HUVEC: human umbilical vein endothelial cells. Cells were purchased from ATCC, USA. The number of cell passages in the experiment did not exceed 6 generations.
[0090] Culture conditions: M199 medium, 20% fetal bovine serum, endothelial cell growth stimulating shadow, VEGF10ng / ml. M199, endothelial cell growth stimulating factor (ECSF) was purchased from GibcoBRL; trypsin was purchased from DIFCO; fetal bovine serum was purchased from Hyclone; microplate reader: POLARstar model, product of BMG, Germany; sultamine B was purchased from Sigma.
[0091] Test Method: Cell Proliferation Analysis: Sultanamine B method, ie SRB method.
[0092] The main test steps of SRB method:
[0093] i) The cells were cultured with 10% fetal bovine serum, and digested with 0.2% trypsin digestion solution during passage, so that the cells were always in the ...
Embodiment 3
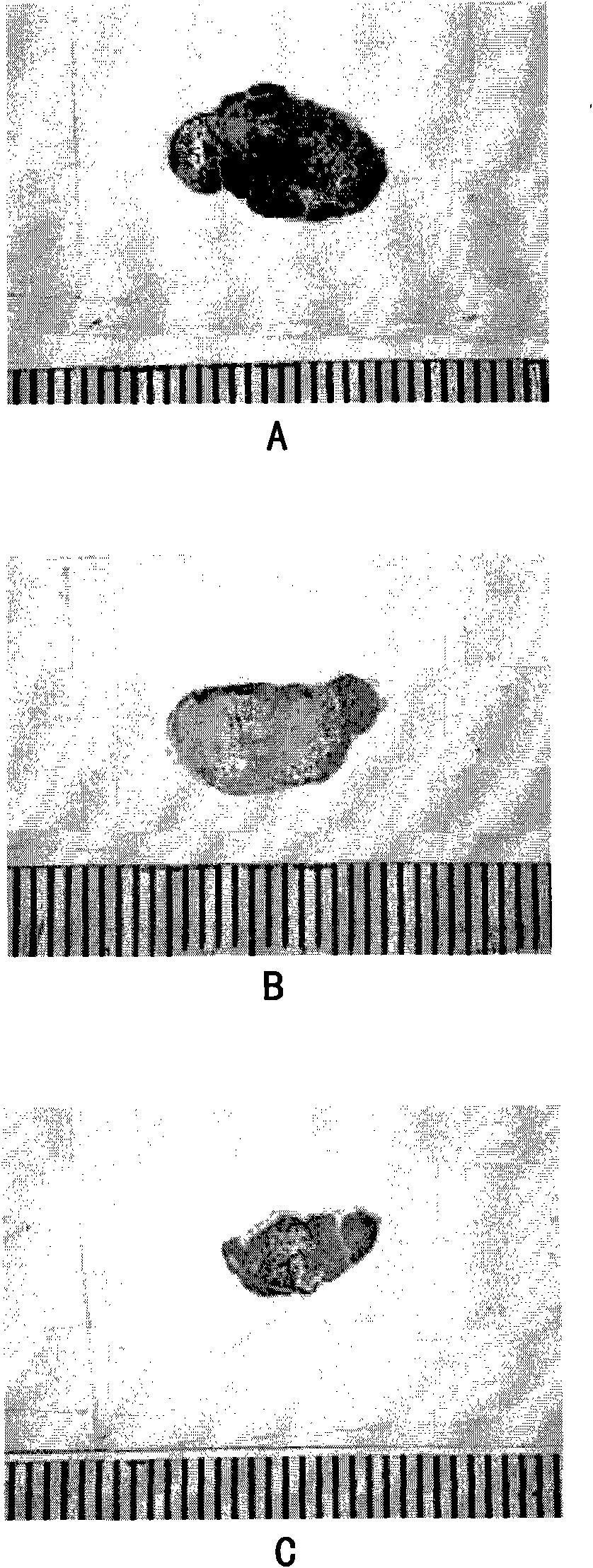
[0108] Effect of compridine on blood flow of tumor tissue
[0109] After one week of adaptation, the nude mice were subcutaneously inoculated with human colon cancer Ls174t cells. After the tumor grew to 0.5 cm3, they were randomly divided into groups, with 5 mice in each group. The mice were intravenously injected with 100 mg / kg of compridine, with an injection volume of 10 ml / kg, and the control group was injected with the same volume of normal saline. 1, 6, and 24 hours after the administration of compridine, the mice were intravenously injected with Hoechst3334 210 mg / kg again. After 1 minute, the mice were killed by neck dislocation, and the tumors were taken out, quickly frozen in liquid nitrogen, and made into cryosections. Three slices were taken from each tumor, respectively in the middle and outer 1 / 3, and the thickness of the slices was 10 μM. The number of stained positive (blue fluorescent) spots was counted under a fluorescent microscope. The excitation wavelen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com