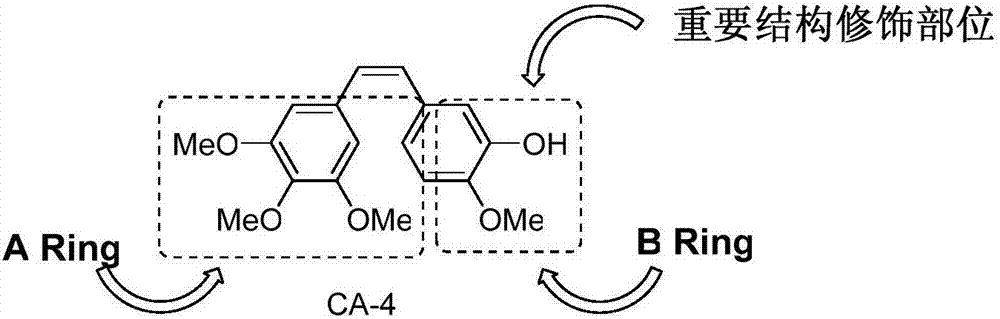
CA-4 antitumor drug, synthesizing method and application thereof
A technology of anti-tumor drugs and synthesis methods, applied in the field of drug synthesis, to achieve the effect of improving tumor blood vessel targeting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
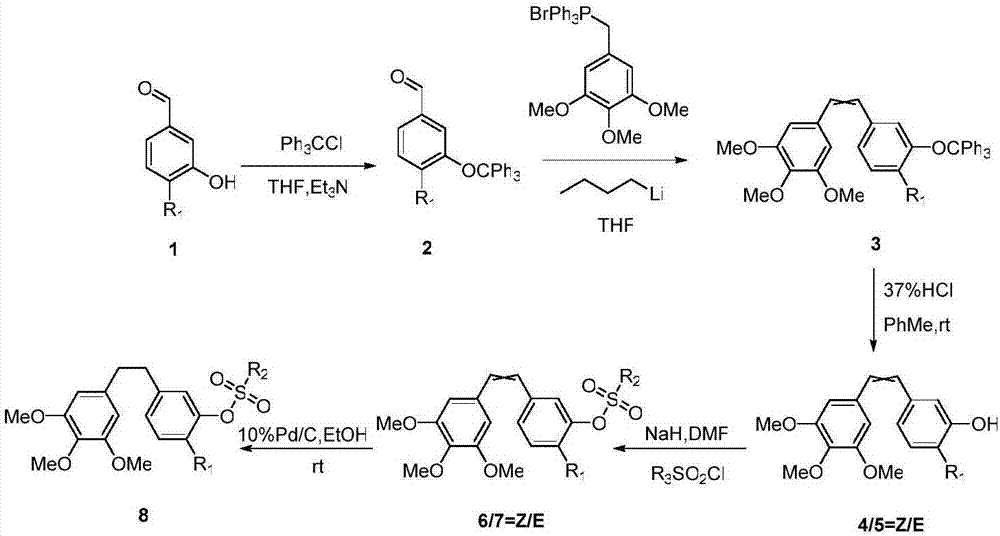
[0047] Embodiment 1: the synthesis of 3-trityloxy-4-methoxybenzaldehyde
[0048] 3-Hydroxy-4-methoxybenzaldehyde (8g, 52.58mmol), trityl chloride (16.8g, 60.47mmol), triethylamine (17.4g, 17.23mmol), dry tetrahydrofuran (30ml) were added to 100ml in the flask. The temperature was raised to reflux, and the reaction was carried out for 5 hours. TLC detects that the reaction is complete. Add an appropriate amount of water to the reaction system to stop the reaction, add ethyl acetate / n-heptane (1:1), a light yellow granular solid is formed, filter, wash the filter cake with distilled water, and dry in vacuo to obtain 3-trityl Oxy-4-methoxybenzaldehyde 15.8g, yield 76.7%.
Embodiment 2
[0049] Example 2: Synthesis of (Z / E)-3,4,5-trimethoxy-4'-methoxy-3'-hydroxyl stilbene
[0050] Under nitrogen protection, trimethoxyphenylmethylenetriphenylphosphonium bromide (13.3g, 25.37mmol) and dry tetrahydrofuran (30ml) were added to a 250ml three-necked flask, cooled to -78°C, and slowly Add n-butyllithium solution (15ml) dropwise, stir for 1 hour after the dropwise addition, slowly add a solution of 3-trityloxy-4-methoxybenzaldehyde (10g, 25.37mmol) in tetrahydrofuran (20ml), rise to room temperature. TLC detected the completion of the reaction, added saturated brine, separated the water layer, washed the organic phase with saturated brine, dried over anhydrous sodium sulfate, and concentrated. Dissolve the residue with toluene and add 37% concentrated hydrochloric acid to react at room temperature for 3 hours. The end of the reaction was detected by TLC, an appropriate amount of water was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and ...
Embodiment 3
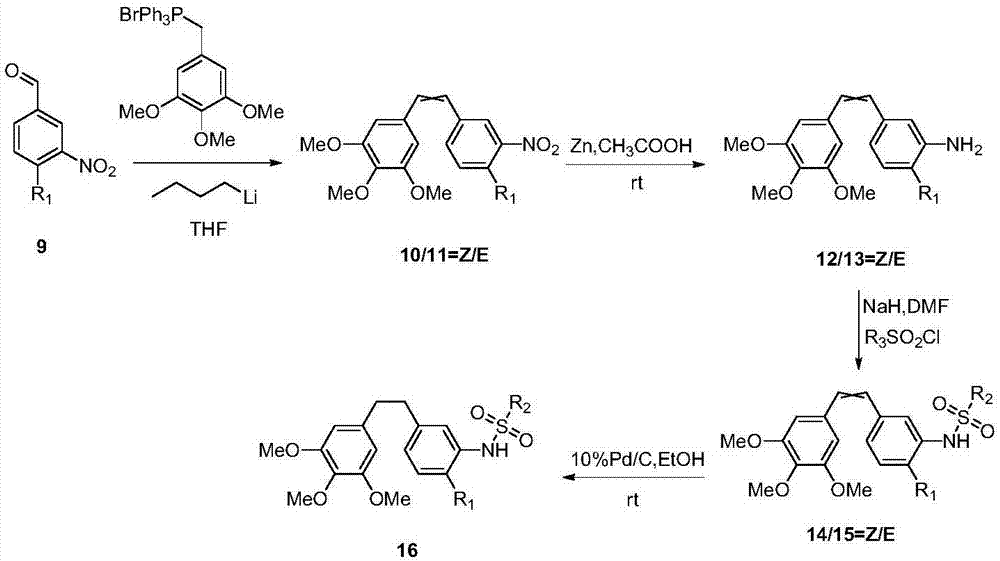
[0051] Example 3: Synthesis of (Z / E)-3,4,5-trimethoxy-4'-methoxy-3'-sulfamate stilbene
[0052] N 2 Under protection, NaH (0.15g, 6.32mmol) was added to a three-necked flask, and (Z / E)-3,4,5-trimethoxy-4'-methoxy-3'-hydroxyl was slowly added dropwise at 0°C A DMF solution (10ml) of stilbene (1g, 3.16mmol) was reacted at this temperature for 1h, and then a DMF solution (2ml) of sulfamoyl chloride (0.82g, 6.32mmol) was slowly added dropwise to the reaction system, rising to React overnight at room temperature. After the reaction was completed, add water to quench, wash with saturated aqueous sodium chloride solution, extract with EA, combine organic phases, anhydrous Na2SO4 4 After drying, filtering, and concentration, petroleum ether / ethyl acetate (3 / 1) column chromatography separated to obtain 0.72 g of light yellow solid with a yield of 72%.
[0053] (Z)-3,4,5-Trimethoxy-4'-methoxy-3'-sulfamate stilbene (A1-1)
[0054]
[0055] 1 H NMR (500MHz, CDCl 3 ): δ7.21(s,1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com