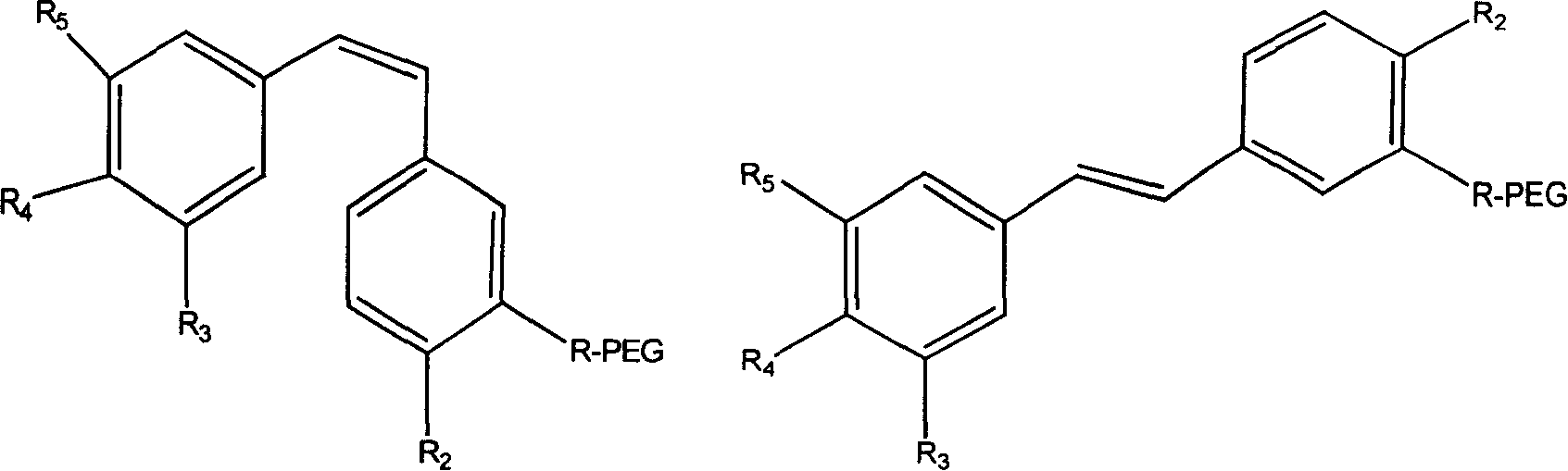
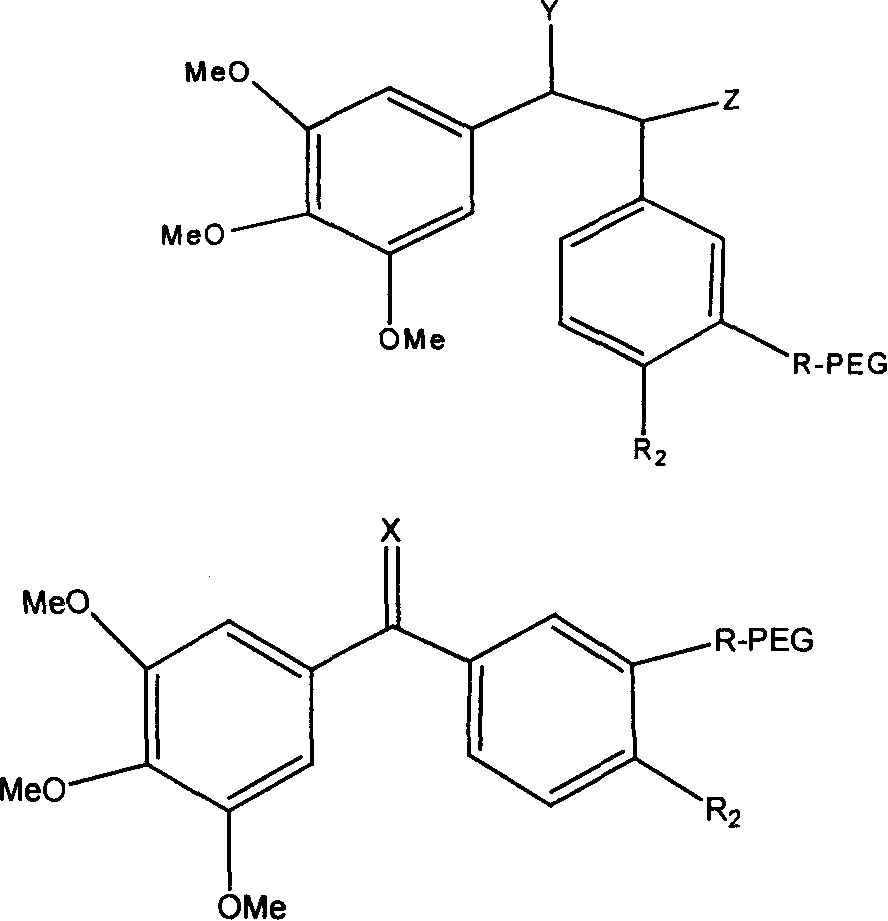
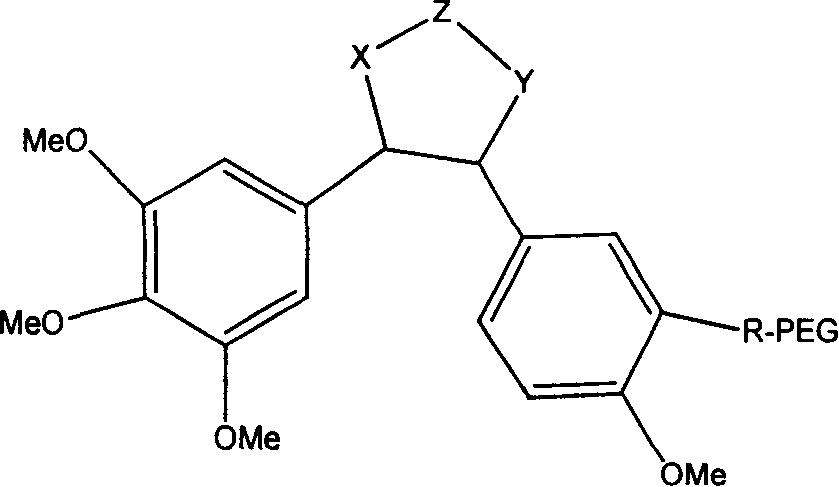
Polyglycol modified antitumor compound and its preparing method
A polyethylene glycol, anti-tumor technology, applied in the field of medicine, can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 50mL three-necked flask equipped with magnetic stirring, add mPEG (2700) (0.54g, 0.2mmol), succinic anhydride (0.05g, 0.24mmol), DMAP (0.0246g, 0.2mmol), triethylamine (0.028mL , 0.2mmol) and 20mL of dioxane, stirred until completely dissolved, then stirred and reacted at room temperature for 24 hours. Filter, remove the solvent dioxane by rotary evaporation, then dissolve in a small amount of dichloromethane to form a thick shape, slowly drop the thick solution into ice anhydrous ether under vigorous stirring, fully analyze the precipitate, filter , to obtain 0.51 g of white solid, which was stored dry.
[0034] In a 100mL three-neck flask equipped with magnetic stirring, add the above white solid (0.135g, 0.05mmol) and 30mL of dichloromethane. -3-Hydroxy-4,3',4',5'-tetramethoxystilbene (0.0316g, 0.1mmol), DIPC (16μL, 0.1mmol) and DMAP (0.0246g, 0.2mmol), stirred All of them were dissolved, slowly raised to room temperature, and continued stirring for 24 hours. ...
Embodiment 2
[0038] In a 100mL three-neck flask equipped with magnetic stirring, add mPEG-COOH (5000+5000) (0.5g, 0.05mmol), (Z)-3-hydroxy-4,3',4',5'-tetramethoxy Diphenylethylene (0.0316g, 0.1mmol), triethylamine (0.042mL, 0.3mmol) and 30mL of dry tetrahydrofuran were reacted at room temperature for 4 hours, and then refluxed for 72 hours. After cooling, filter, remove most of the solvent by rotary evaporation, slowly drop the concentrated mPEG conjugated tetrahydrofuran solution into ice anhydrous ether under vigorous stirring, so that the mPEG-Combretastatin A-4 conjugate is fully precipitated, filtered, 0.46 g of white solid was obtained, which was stored dry. The chemical structure is shown in Formula 2, 1H NMR (CDCl3): δ 3.65 (s, 1483H, CH2), 3.49 (d, 2.1H, OMe), and the calculated connection rate is about 35%. Silica gel column separation, ethanol:dichloromethane ratio of 1:90. Compared with water solubility, it is 560 times higher.
[0039]
[0040] F...
Embodiment 3
[0042]In a 100mL three-neck flask equipped with magnetic stirring, add mPEG-COOH (5000+5000) (0.5g, 0.05mmol), (Z)-3-amino-4,3',4',5'-tetramethoxy Diphenylethylene (0.0315g, 0.1mmol), triethylamine (0.042mL, 0.3mmol) and 30mL of dry tetrahydrofuran were reacted at room temperature for 4 hours, and then refluxed for 72 hours. After cooling, filter, remove most of the solvent by rotary evaporation, slowly drop the concentrated mPEG conjugate solution in tetrahydrofuran into ice anhydrous ether under vigorous stirring, so that the mPEG-Combretastatin conjugate is fully precipitated, and filter to obtain a white solid 0.41g, dry storage. The chemical structure is shown in Formula 3, 1H NMR (CDCl3): δ 3.65 (s, 1483H, CH2), 3.49 (d, 2.1H, OMe), and the calculated connection rate is about 35%. Silica gel column separation, ethanol:dichloromethane ratio of 1:90. Compared with water solubility, it is 270 times higher.
[0043]
[0044] Formula 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com