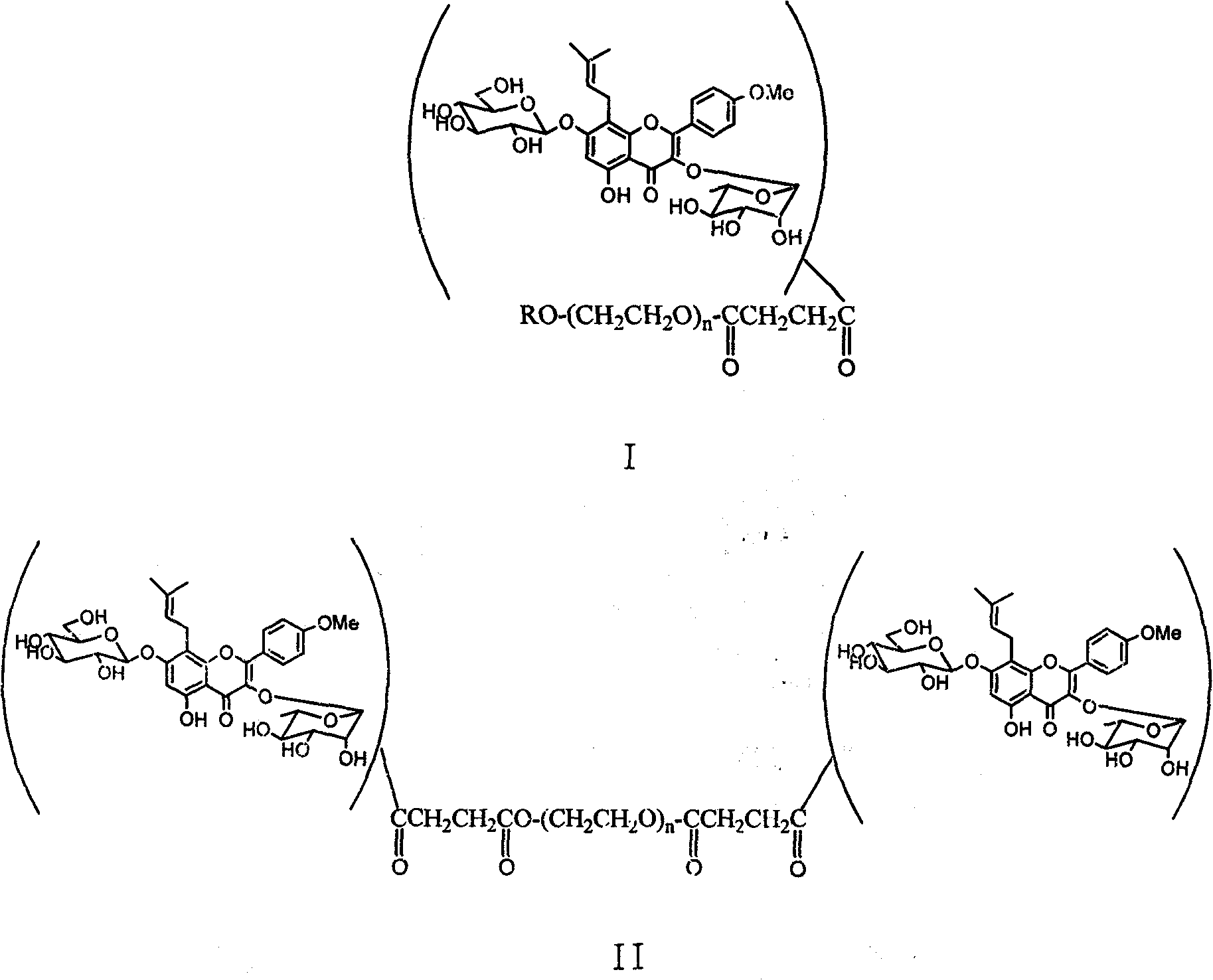
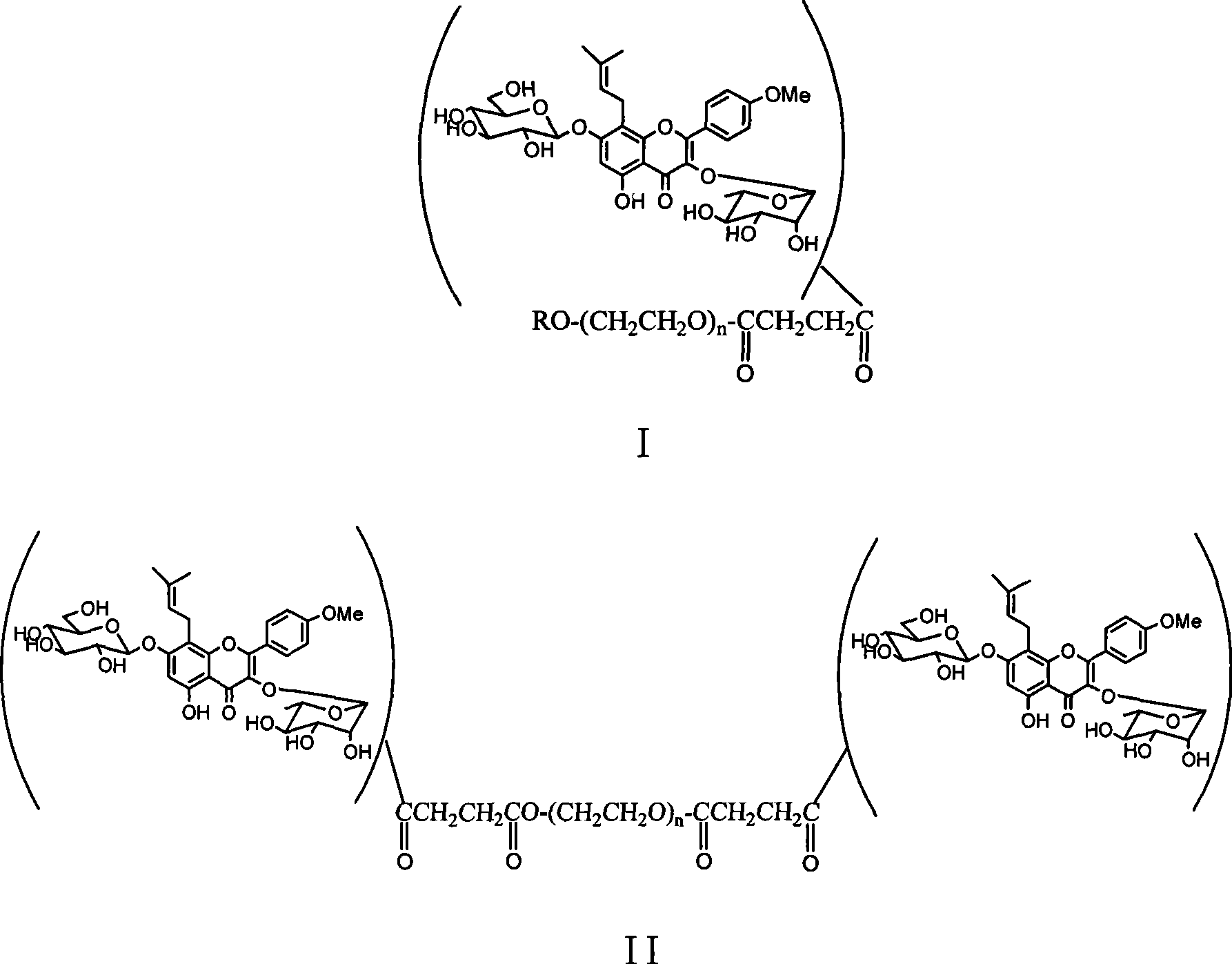
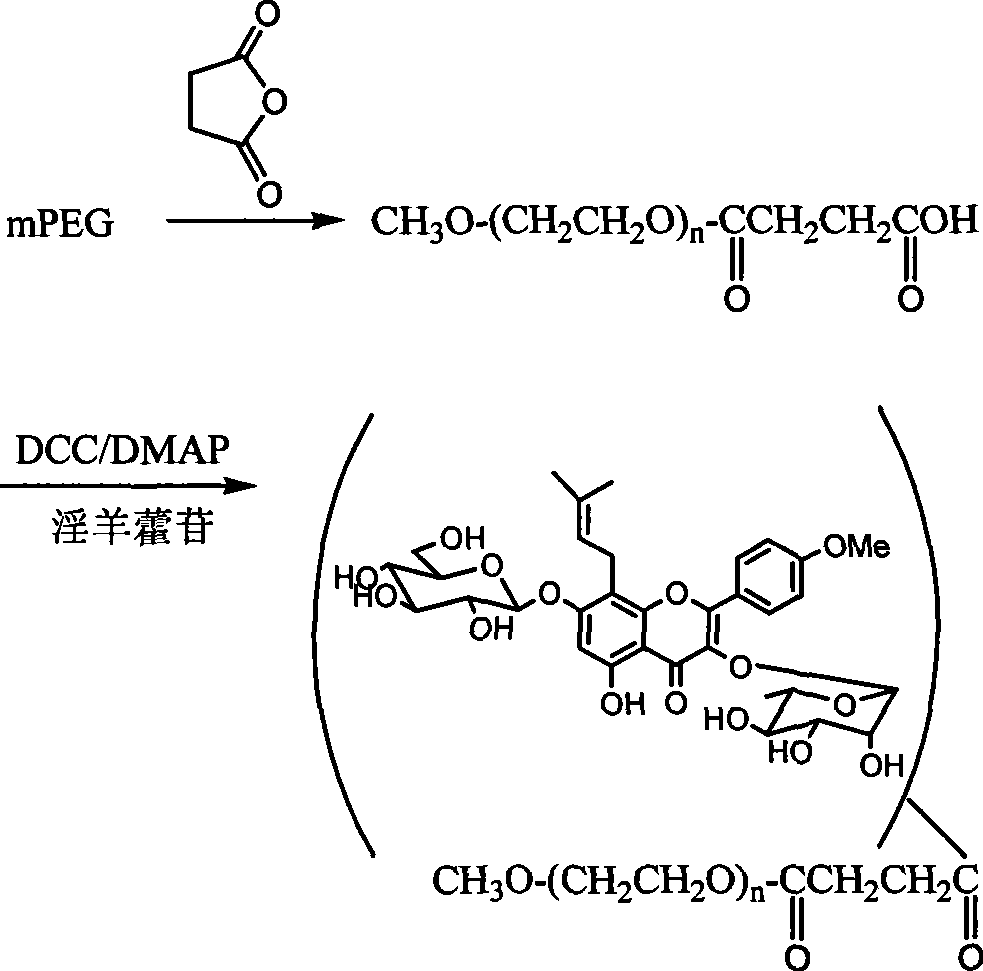
Pegylated glycosides of aerial parts of epimedium, and preparation and use thereof
A technology of PEGylation and icariin, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as poor water solubility, limited application, and reduced bioavailability question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 PEGylated icariin ICA-mPEG5000 was prepared by using polyethylene glycol monomethyl ether with a molecular weight of 5000.
[0018] Weigh 5.0g (1.0mmol) of polyethylene glycol monomethyl ether-5000 and dissolve it in 10mL of pyridine, then add 0.11g (1.1mmol) of succinic anhydride and 61mg (0.5mmol) of 4-dimethylaminopyridine (DMAP) ). The reaction was stirred at 50°C for 5h. After distilling off the solvent under reduced pressure, the residue was dried in vacuo and used directly in the next reaction. Take 336mg (~0.067mmol) of polyethylene glycol monomethyl ether-5000 derivative prepared above and 50mg (0.074mmol) of icariin and dissolve it in 2mL DMF, then add DCC28mg (0.14mmol) and DMAP9mg (0.074mmol) . After stirring at room temperature for 24 hours, the insoluble matter in the reaction solution was filtered off, and anhydrous ether was added to produce a white precipitate, which was filtered to obtain a light yellow solid. The product was further puri...
Embodiment 2
[0019] Example 2 PEGylated icariin ICA-mPEG2000 was prepared by using polyethylene glycol monomethyl ether with a molecular weight of 2000.
[0020] Repeat the preparation steps of Example 1, replace polyethylene glycol monomethyl ether-5000 with polyethylene glycol monomethyl ether-2000, and use a dialysis membrane with a cut-off molecular weight of 1000 to purify the product to obtain ICA-mPEG2000 with a yield of 78% . The solubility in water at room temperature is about 650mg / mL. The structure of this compound was confirmed by mass spectrum, nuclear magnetic resonance spectrum, infrared spectrum and so on. From 1 H-NMR spectrum calculates that the content of its icariin is 22%. Through gel exclusion chromatography (GPC) analysis, its Mn (number average molecular weight) is 3398, and Mw (weight average molecular weight) is 3708.
Embodiment 3
[0021] Example 3 PEGylated icariin ICA-mPEG750 was prepared by using polyethylene glycol monomethyl ether with a molecular weight of 750.
[0022] Repeat the preparation steps of Example 1, replace polyethylene glycol monomethyl ether-5000 with polyethylene glycol monomethyl ether-750, and use a dialysis membrane with a cut-off molecular weight of 500 to purify the product to obtain ICA-mPEG750 with a yield of 85% . The structure of this compound was confirmed by mass spectrum, nuclear magnetic resonance spectrum, infrared spectrum and so on. From 1 H-NMR spectrum calculated the content of icariin to be 47%. The solubility in water at room temperature is about 260mg / mL. The compound was analyzed by gel exclusion chromatography (GPC), and its Mn (number average molecular weight) was 2134, and its Mw (weight average molecular weight) was 2383.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com