Preparation method of combretastatin furan type analogues
A combretastatin and furan-type technology is applied in the field of preparation of combretastatin furan-type analogs, can solve problems such as unsatisfactory product yield, heavy metal residues, expensive raw materials, etc., and achieves easy industrialized production, mild reaction conditions, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The following examples can enable those skilled in the art to understand the present invention more comprehensively, but do not limit the present invention in any way. The raw materials used in the present invention are all known compounds, which can be purchased from the market or synthesized by methods known in the art.
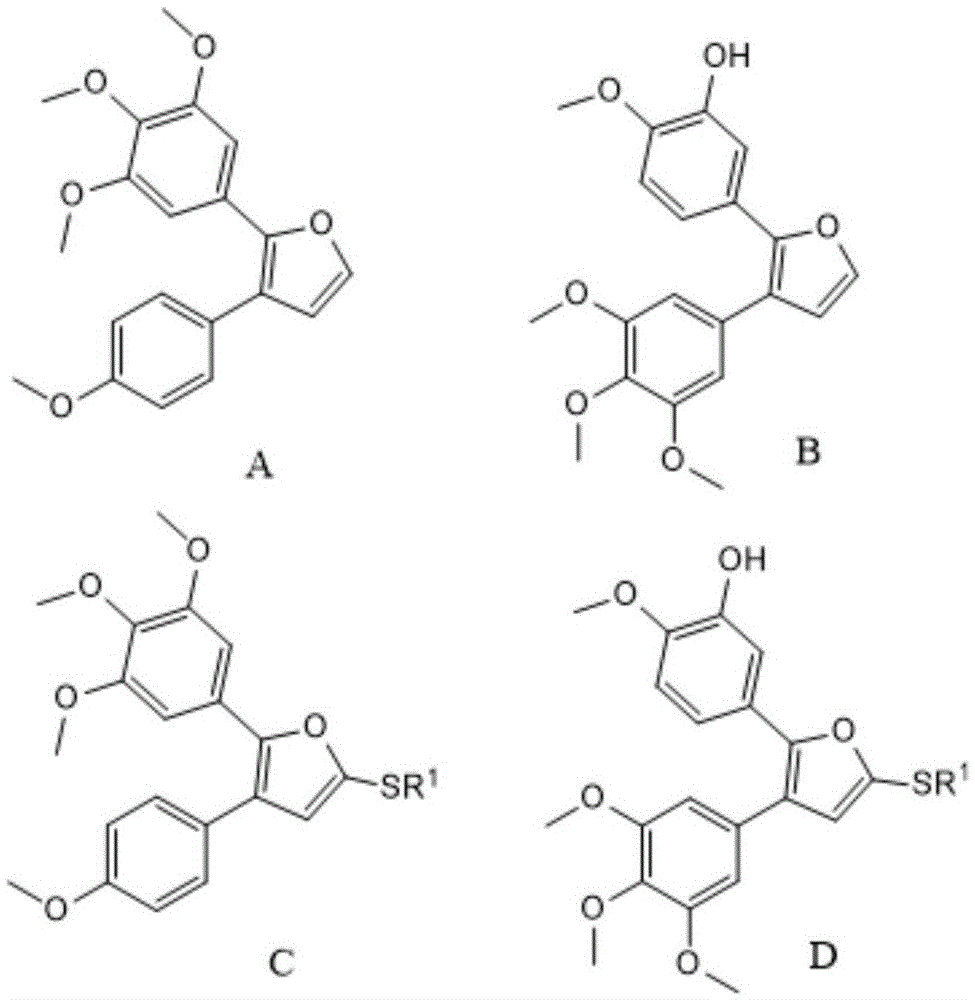
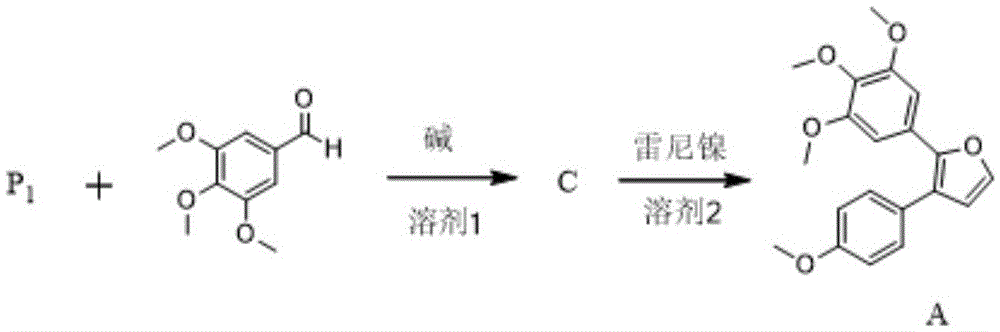
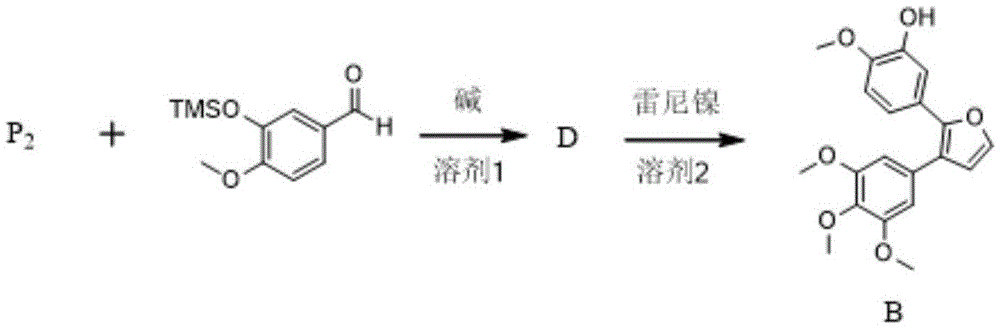
[0028] according to figure 2 The reaction steps of the method for preparing combretastatin furan type analogue A shown:
[0029] Weigh 2.90g compound P 1 , 2.00g of 3,4,5-trimethoxybenzaldehyde and 100mL of THF were added to the reaction flask, and 2.20g of potassium tert-butoxide was added under stirring. After reacting for 10 minutes, TLC detected that after the reaction was complete, the solvent was evaporated, and the residue was separated and purified by silica gel column chromatography with a mobile phase of petroleum ether / ethyl acetate=50 / 2 to obtain compound C-1, 4.12 g, with a yield of 92%. Weigh 2.00 g of compound C-1 into a reaction f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com