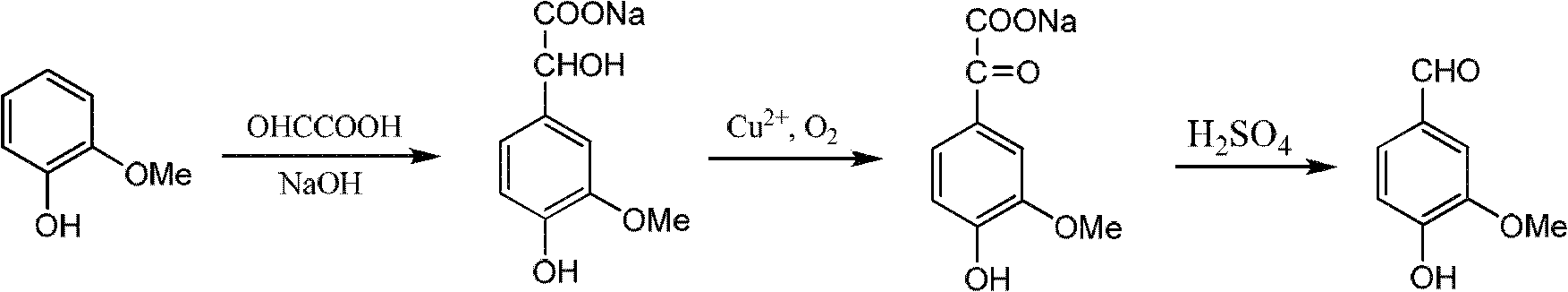Patents
Literature
51 results about "4-methoxybenzaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
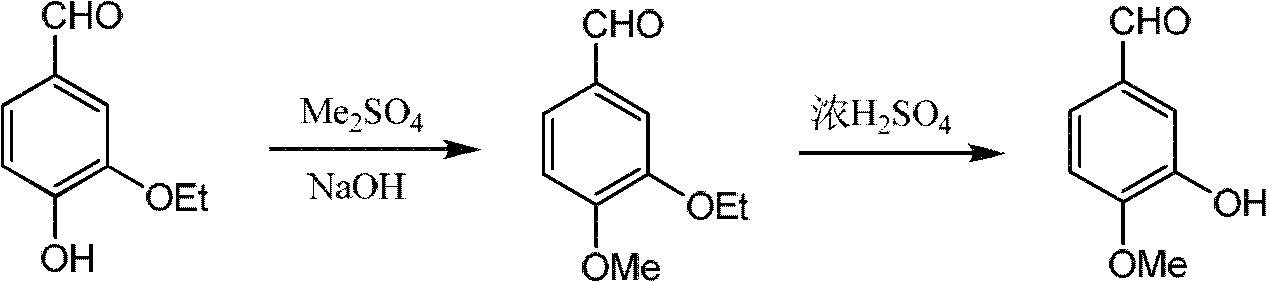
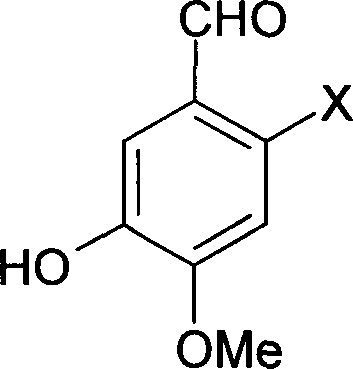
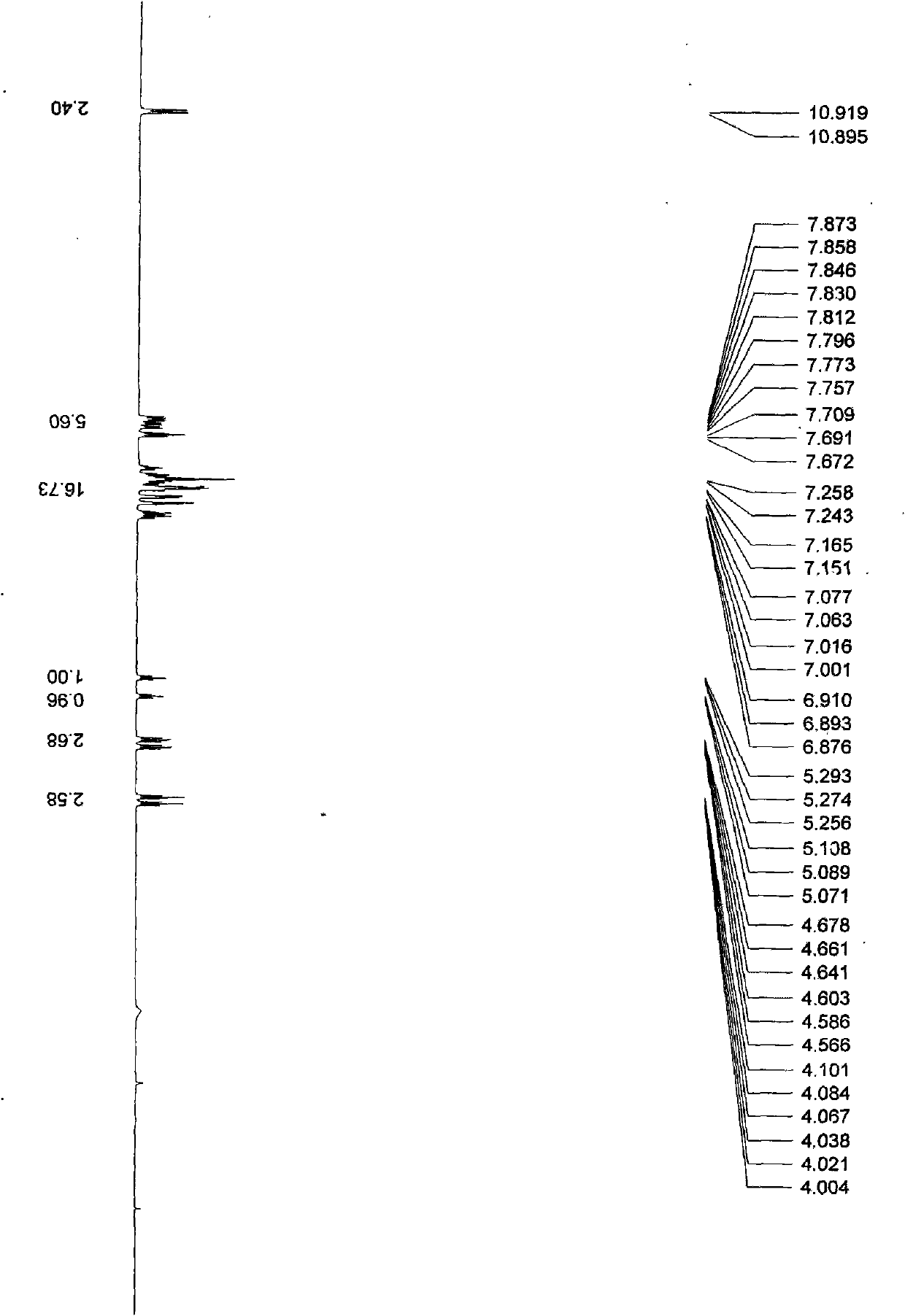
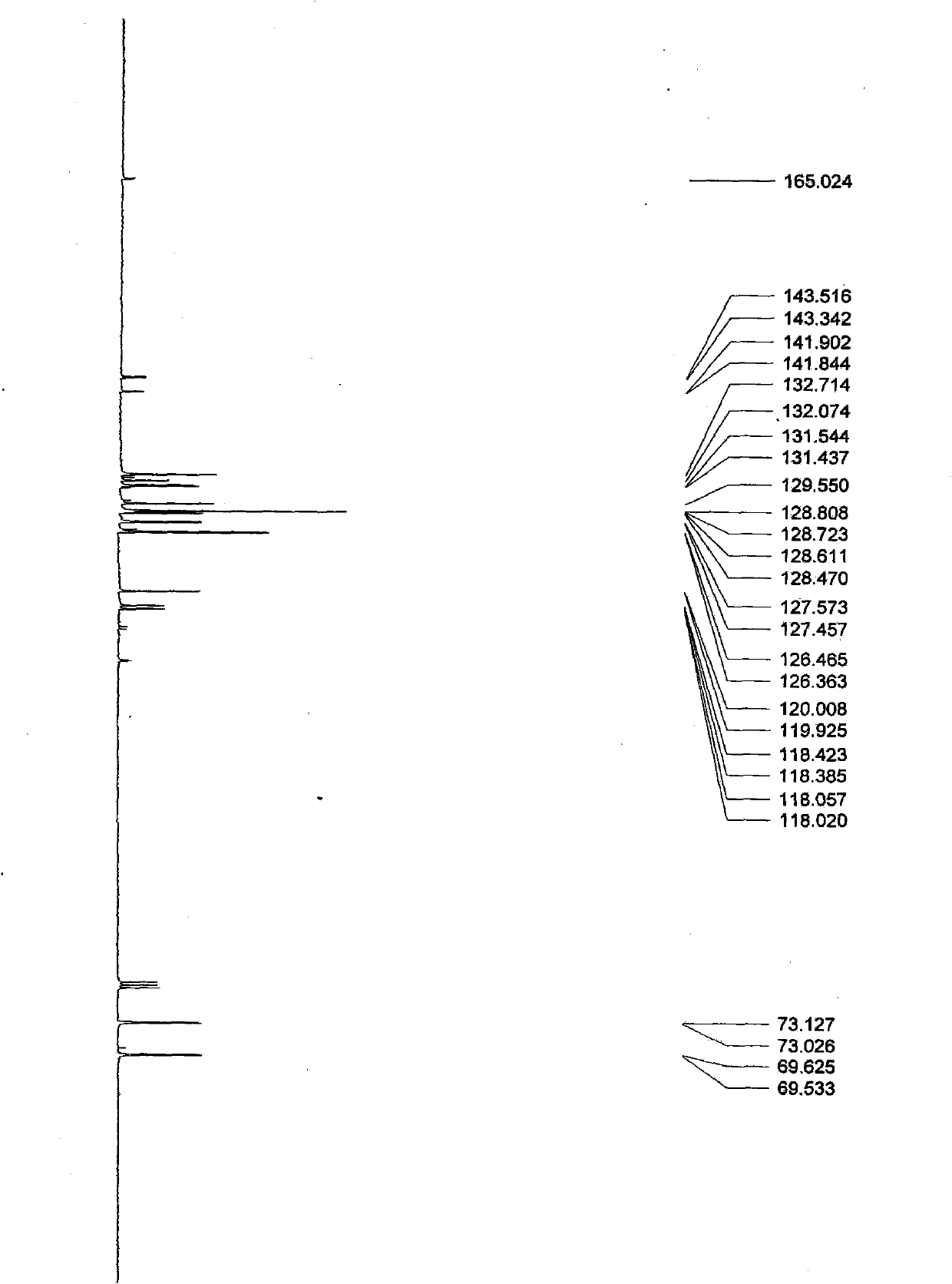
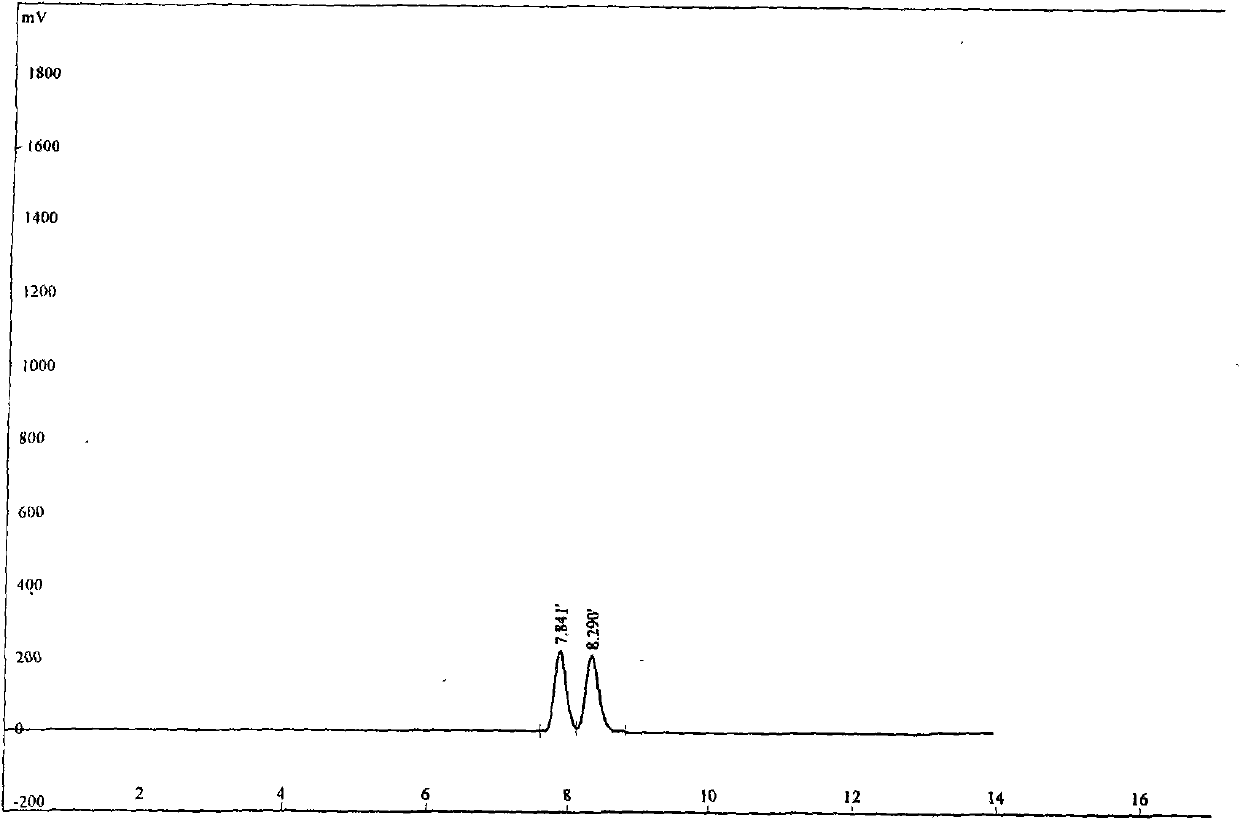
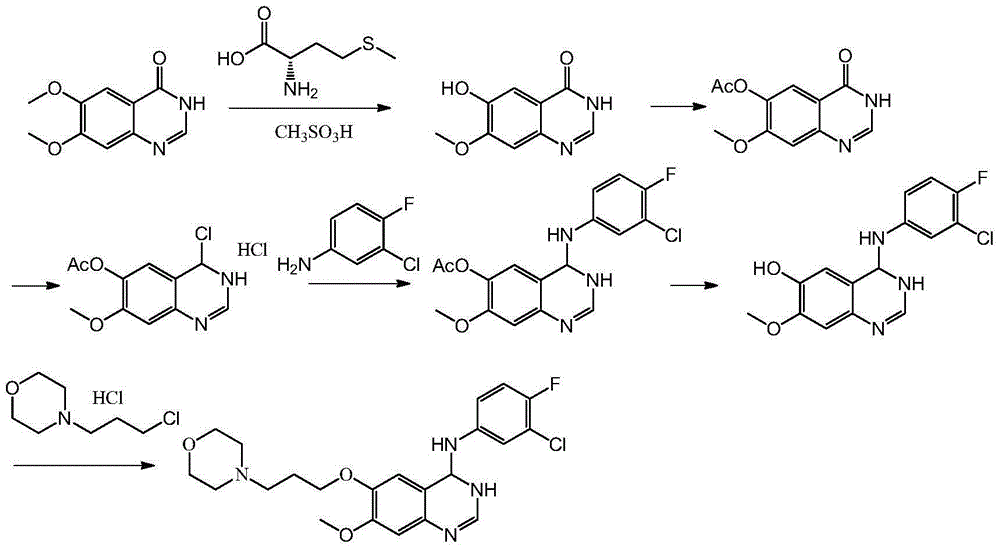
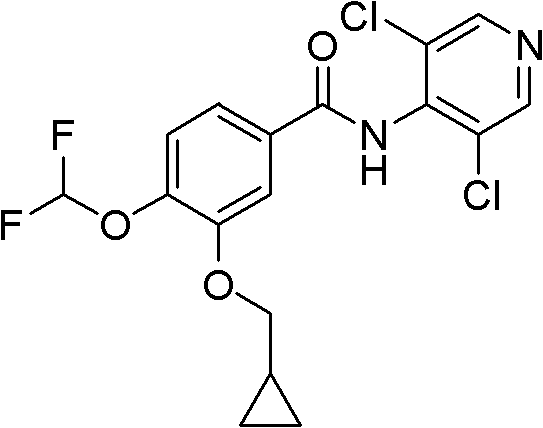
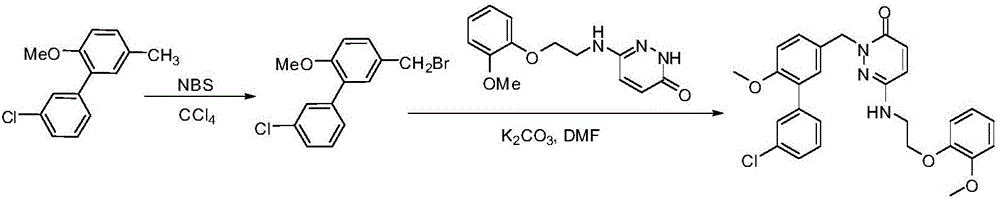
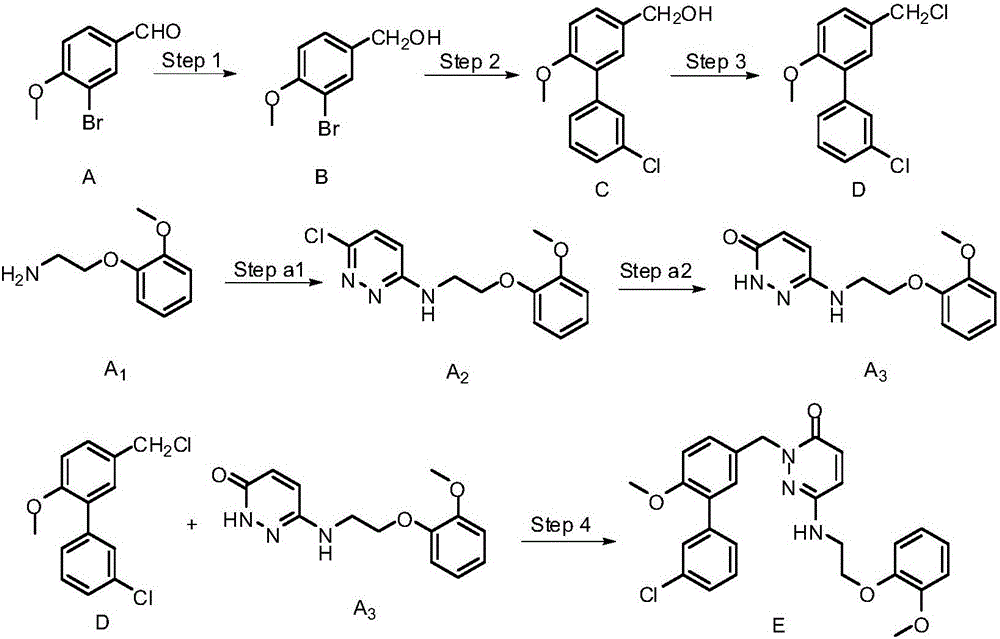
Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline
The invention discloses a method for synthesizing 4-(3-chloro-4-fluorophenylamino)-7-methoxy -6-[3-(4-morpholinyl)-propoxy] quinoline. The method comprises the following steps: 1) 3-hydroxide radical-4-methoxybenzaldehyde is used as a raw material to prepare 3-hydroxide radical-4-methoxy-benzonitrile; 2) the 3-hydroxide radical-4-methoxy-benzonitrile and 3- chloropropy morpholinehydrochloride are heated to have reflux reaction to obtain 4- methoxy-3-[3-(4- morpholinyl) propoxy] benzonitrile; 3) the 4- methoxy-3-[3-(4-morpholinyl) propoxy] benzonitrile is subjected to nitration to obtain 2- nitryl-4- methoxy -5-[3-(4-morpholinyl) propoxy] benzonitrile; 4) the 2- nitryl-4- methoxy -5-[3-(4-morpholinyl) propoxy] benzonitrile is subjected to reduction to obtain 2-amido-4- methoxy-5-[3-(4-morpholinyl) propoxy] benzonitrile; and 5) the 2-amido-4- methoxy-5-[3-(4-morpholinyl) propoxy] benzonitrile and an azomethine intermediate of 3-chloro-4-fluoroaniline have rearrangement reaction to obtain 4-(3-chloro-4-fluorophenylamino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline. The method has characteristics of environmental protection and high production rate.
Owner:浙江精进药业有限公司
Flavors for oral compositions
The present invention relates to oral care compositions comprising stannous ions, a mint-type flavor oil, a protectant component that prevents generation of off odor and off taste in the composition, and orally acceptable carriers. The mint-type oils include peppermint, spearmint and corn mint. Suitable protectant components include copper salts and carbonyl compounds such as ascorbic acid; cis-jasmone; 2,5-dimethyl-4-hydroxy-3(2H)-furanone; 5-ethyl-3-hydroxy-4-methyl-2(5H)-furanone; vanillin; ethyl vanillin; anisaldehyde; 3,4-methylenedioxybenzaldehyde; 3,4-dimethoxybenzaldehyde; 4-hydroxybenzaldehyde; 2-methyoxybenzaldehyde; 4-methoxybenzaldehyde; benzaldehyde; cinnamaldehyde (3-phenyl-2-propenal); hexyl cinnamaldehyde; α-methyl cinnamaldehyde; ortho-methoxy cinnamaldehyde; α-amyl cinnamaldehyde; and combinations thereof. The oral care composition may be a dentifrice.
Owner:PROCTER & GAMBLE CO
Chiral zinc complex and copper complexes of alpha-phenylethylamine
InactiveCN102069014AGroup 4/14 element organic compoundsCarboxylic acid nitrile preparationBenzaldehyde4-chlorobenzaldehyde
The invention relates to a chiral zinc acetate complex of alpha-phenylethylamine, a chiral copper acetate complex of alpha-phenylethylamine and a chiral copper chloride complex of alpha-phenylethylamine which are used as catalyst. When the complexes are used in the nitrile silicification reactions of aromatic aldehydes such as benzaldehyde, 2-fluorobenzaldehyde, 2-methoxybenzaldehyde, 2-methylbenzaldehyde, 4-methylbenzaldehyde, 4-methoxybenzaldehyde, 4-fluorobenzaldehyde, 4-chlorobenzaldehyde and 4-bromobenzaldehyde to prepare chiral target products, the chiral catalysts have good catalytic activities and high enantioselectivity in the nitrile silicification reactions.
Owner:罗梅
Method for preparing Roflumilast
InactiveCN102336704AShort stepsRaw materials are cheap and easy to getOrganic chemistryBenzaldehydeEthyl acetate
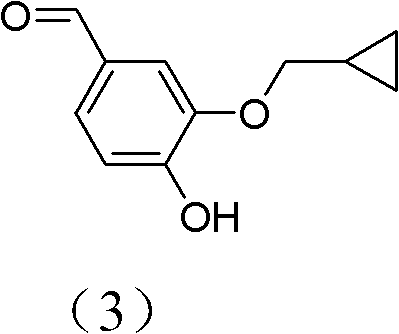
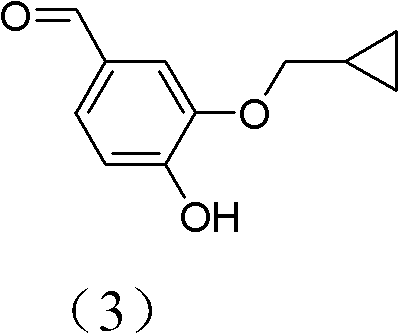
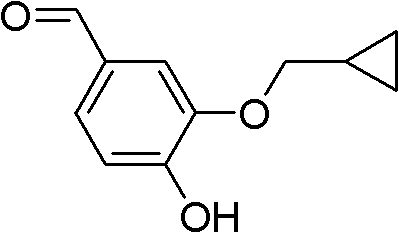
The invention discloses a method for preparing Roflumilast. The method comprises the following steps of: performing cyclopropyl methylation on isovanillin to obtain 3-cyclopropylmethoxy-4-methoxybenzaldehyde; performing demethylation to synthesize an important intermediate of the Roflumilast, namely 3-cyclopropylmethoxy-4hydroxyl-benzaldehyde; and further synthesizing a key intermediate in a formula (5) according to American patent US5712298 and finally synthesizing the Roflumilast in a formula (7). A crude product of the Roflumilast is treated by isopropanol and water, and is recrystallized by ethyl acetate and petroleum ether. The preparation method has a few steps, raw materials are readily available and cheap, the reaction selectivity is high, the yield is high and the post treatment is simple.
Owner:SHANDONG RUIHE PHARMA R&D CO LTD
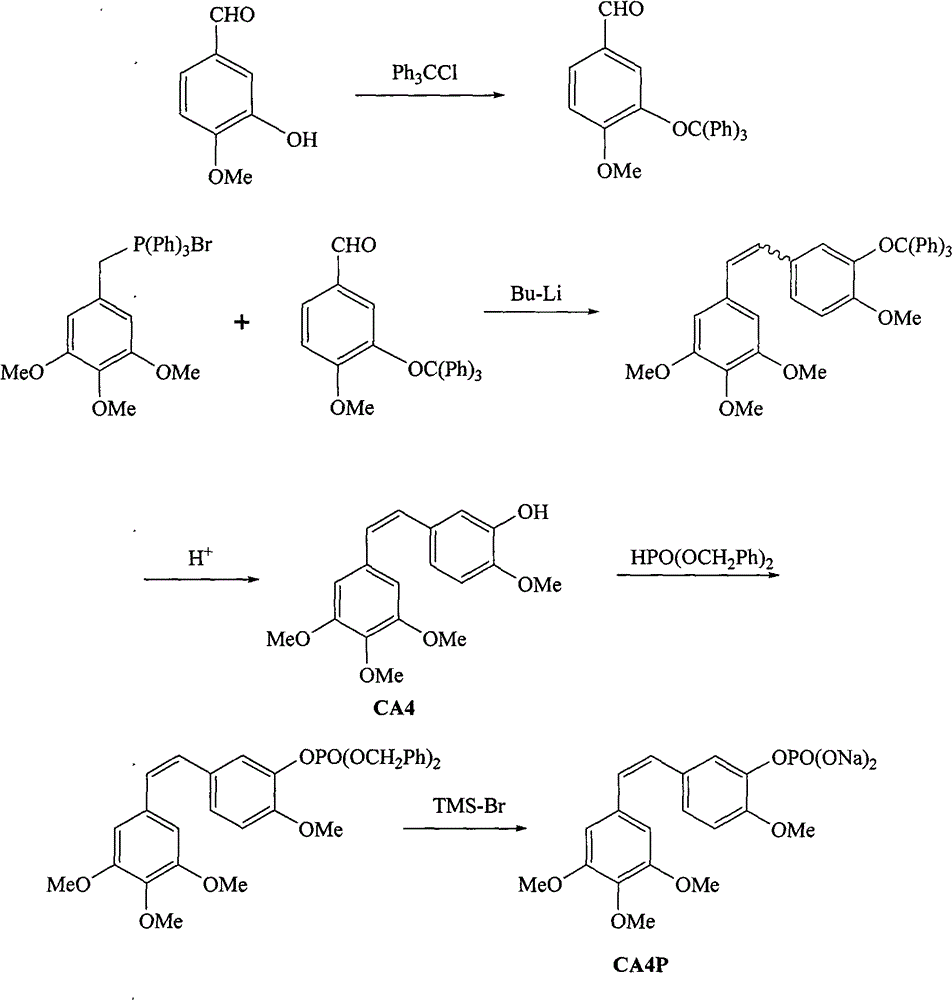
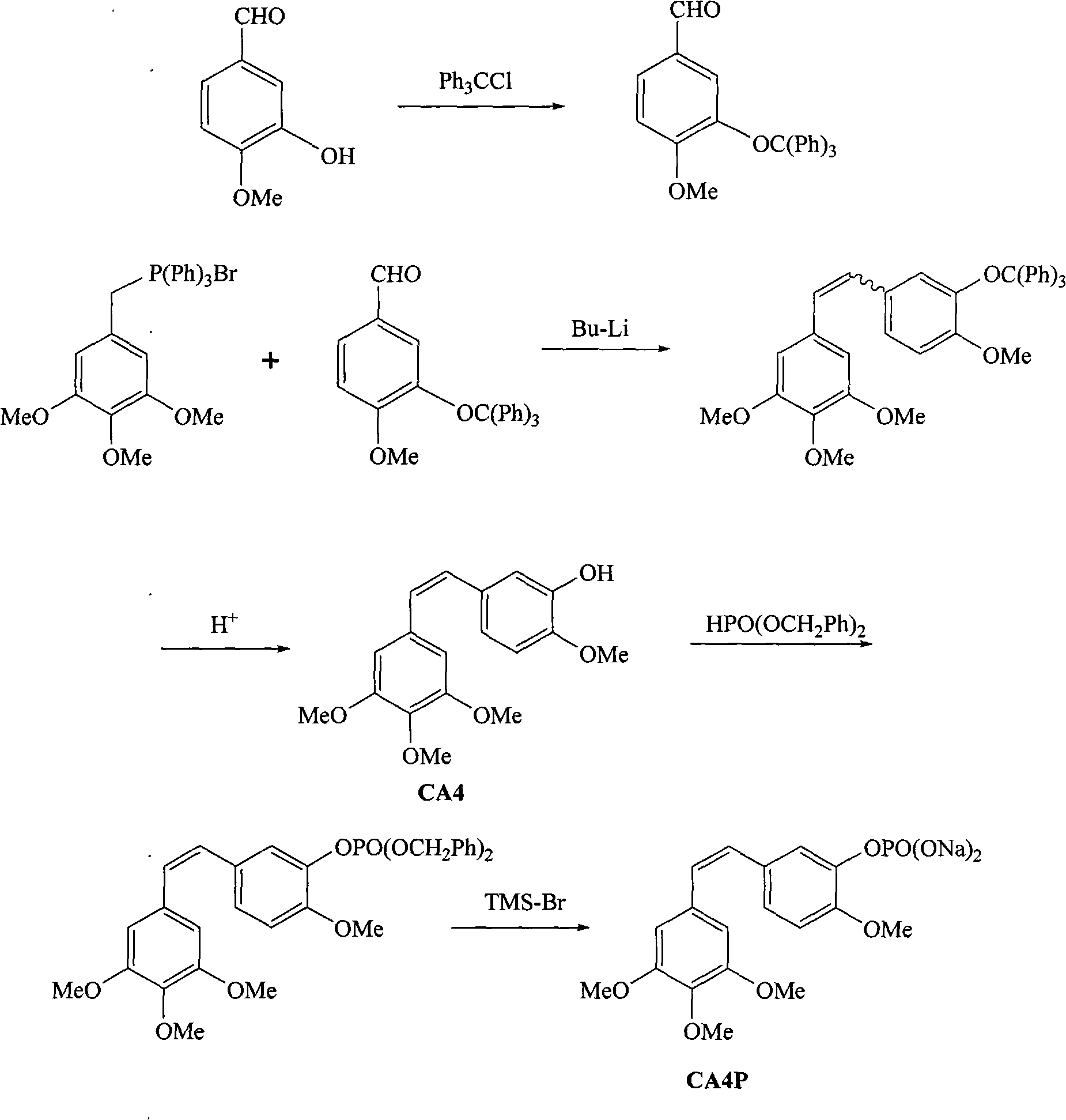
Method for synthesizing CA4P
ActiveCN101885738ARaw materials are cheap and easy to getMild reaction conditionsMethine/polymethine dyesGroup 5/15 element organic compoundsChemical synthesisPhosphate
The invention belongs to the field of chemical synthesis and relates to a method for preparing CA4P, in particular to a method for synthesizing CA4P by the following steps that: isovanillin and trityl chloride, which serve as raw materials, are used to form 3- triphenylmethoxy-4-methoxybenzaldehyde which is an intermediate isovanillin protector; the 3- triphenylmethoxy-4-methoxybenzaldehyde and 3,4,5-trimethoxy-triphenyl benzylidene bromide phosphine salt undergo a Wittig reaction , and the protective group is removed by hydrolysis to obtain CA4; and the CA4 and phosphonic acid bis(phenylmethyl)ester react to form benzyl phosphate, and the benzyl group is removed to form a sodium salt to obtain the target compound, namely CA4P.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
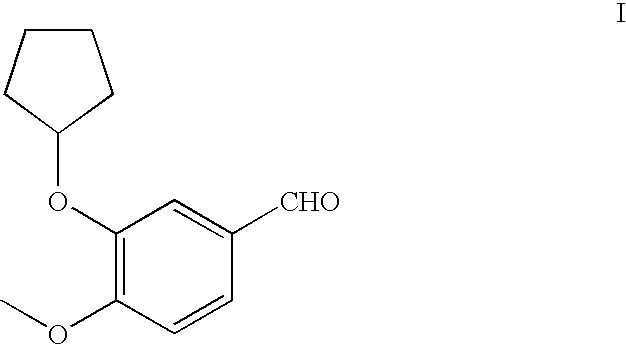
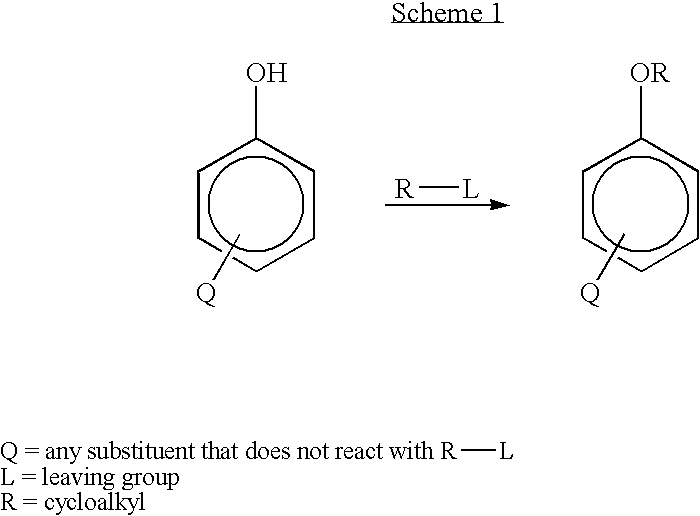
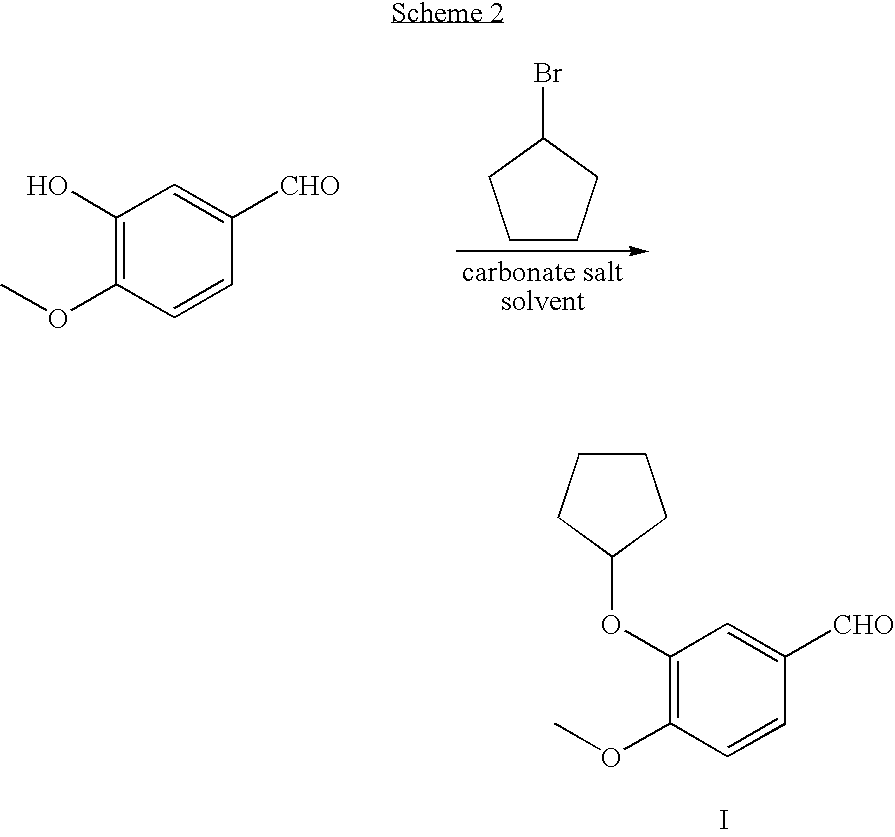
Method for preparing 3-cyclopentyloxy-4-methoxybenzaldehyde
Owner:WYETH LLC
Preparation method for chlorbipram PDE4-inhibitor
ActiveCN106632070AHigh yieldRaw materials are cheap and easy to getOrganic chemistryPDE4 InhibitorsBromine
The invention discloses a preparation method for a chlorbipram PDE4-inhibitor. The method is capable of using 3-bromine-4-methoxybenzaldehyde as a starting raw material, and synthesizing a target product of E chlorbipram by steps, such as reduction reaction, Suzuki reaction and chlorination. Compared with the prior art, the yield is greatly improved, and the yield is up to 71% proved by experiment. The raw material is easily obtained and cheap, the reaction condition is moderate, the equipment request is low, and the industrial production can be realized. The preparation method is a new efficient and easy way for the synthetic method of the chlorbipram.
Owner:苏州兰晟医药有限公司
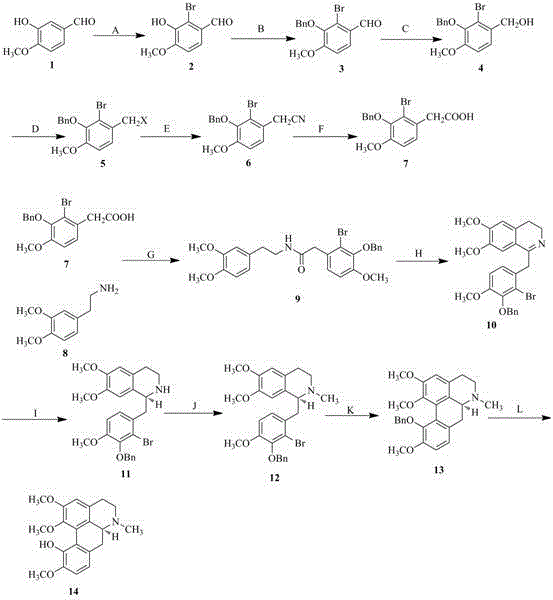
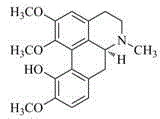
Preparation method of isocorydaline
InactiveCN104892510AWell sourcedDoes not damage the ecological environmentOrganic chemistryChemical reactionAsymmetric hydrogenation
The invention discloses a preparation method of isocorydaline. In the method, the isocorydaline is synthesized by adopting 3-hydroxyl-4-methoxybenzaldehyde (isovanillin) and 3,4-dimethoxyphenylethylamine as start raw materials through 12 steps of chemical reactions, wherein through the key Bischer-Napieralski reaction as well as the key chemical reactions such as an asymmetric hydrogenation reduction in the presence of a chiral catalyst and a coupling reaction of double benzene rings in the presence of transition metal, an organic total synthesis path of the target compound isocorydaline is established finally, and a raw material source is provided for the development of isocorydaline-based anti-cancer drugs.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Fly attractant and preparing method thereof
InactiveCN105918353AStrong ability to seduceEasy to useBiocidePest attractantsCis-3-HexenalHouttuynin
The invention relates to the technical field of attractants, in particular to fly attractant. The fly attractant is prepared from, by weight, 2-3 parts of fish oil, 1-2 parts of houttuynin, 1.5-3 parts of fly maggot powder, 2-2.4 parts of shrimp meal, 3-4 parts of rose oxide, 30-35 parts of composite yeast, 20-25 parts of white sugar, 10-15 parts of brown sugar, 3-4 parts of malt sugar, 3-4 parts of fish bone meal, 1-3 parts of ionone, 0.8-1 part of 4-methoxybenzaldehyde, 0.1-0.5 part of cis-3-hexenal, 1-3 parts of 1-ethylhexenol, 5-7 parts of lard oil, 0.3-0.4 part of sunset yellow, 0.1-0.3 part of 2,6-di-tert-butyl-4-methyl phenol and 0.5-1 part of calcium carbonate. The fly attractant has a strong attracting effect on flies, and is free of toxicity, easy to use and is non-toxic to people and livestock, the preparation method is simple, the production cost is low, and the effect is durable.
Owner:张芳
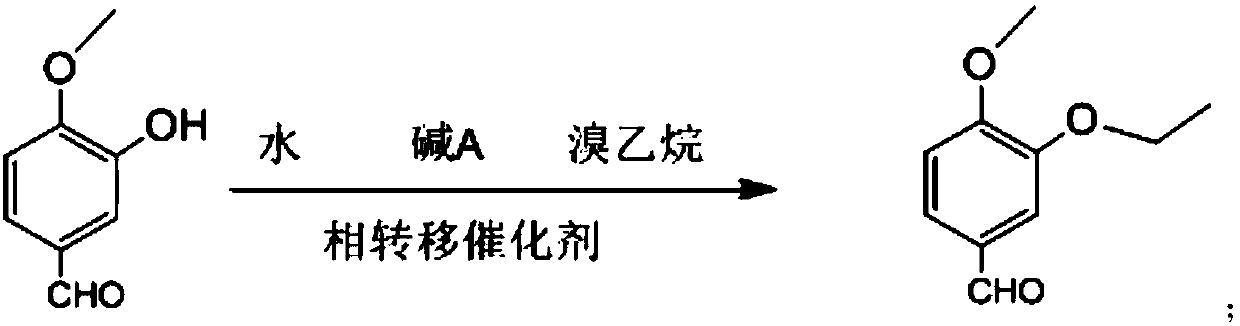
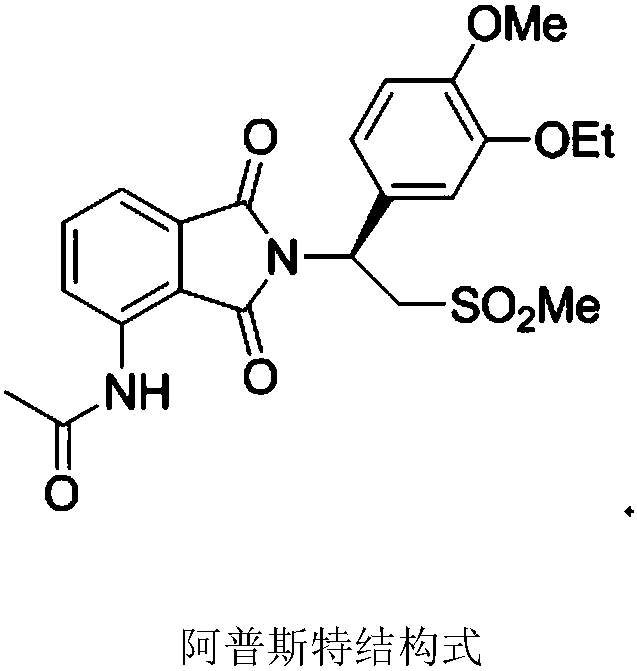
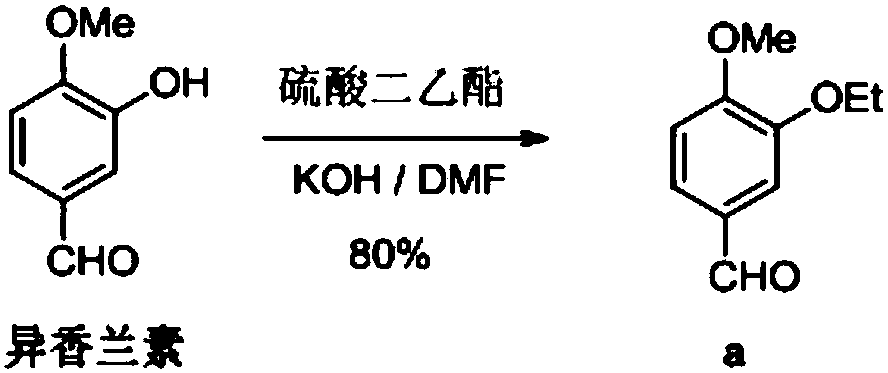
Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol
ActiveCN105461602AShort reaction timeReduce waste disposalCarboxylic acid nitrile preparationOrganic compound preparationBENZYL ALCOHOL/WATERBromoethane
The invention relates to a preparation method of chiral S / R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol. The preparation method comprises the following steps: 3-hydroxy-4-methoxybenzaldehyde is taken as a starting material and reacts with hydroxylammonium hydrochloride to produce 3-hydroxy-4-methoxybenzonitrile; 3-hydroxy-4-methoxybenzonitrile reacts with bromoethane to produce 3-ethoxy-4-methoxybenzonitrile; 3-ethoxy-4-methoxybenzonitrile reacts with dimethyl sulfone under the action of n-butyllithium, a product is hydrolyzed in an aqueous hydrochloric acid solution, and 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanone is obtained; finally, S-(-)-alpha,alpha-diphenyl-2-pyrrolidinemethanol or R-(+)-alpha,alpha-diphenyl-2-pyrrolidinemethanol is taken as a chiral catalyst, a borane dimethyl sulfide solution is taken as a reducing agent, carbonyl is reduced, and a product is obtained. The reaction conditions are mild, the product yield is higher, the technology level is increased, the operability is improved, and large-scale industrial production is facilitated.
Owner:DONGHUA UNIV
Compositions
ActiveUS20100284942A1Reduction of spore countExtended shelf lifeCosmetic preparationsBiocide3-HydroxybenzaldehydeBenzaldehyde
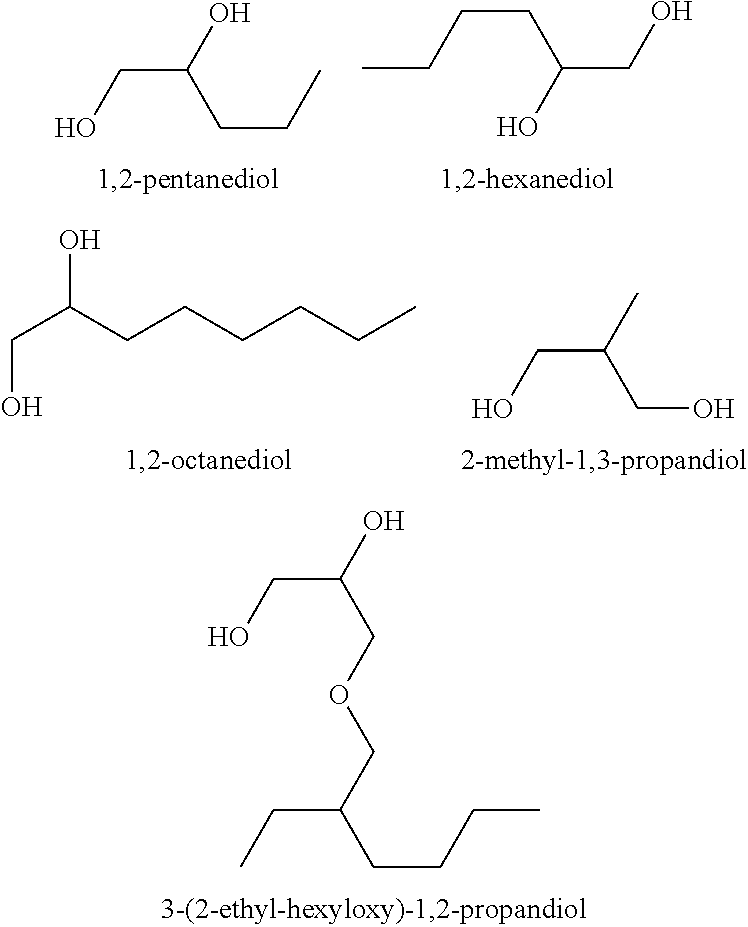
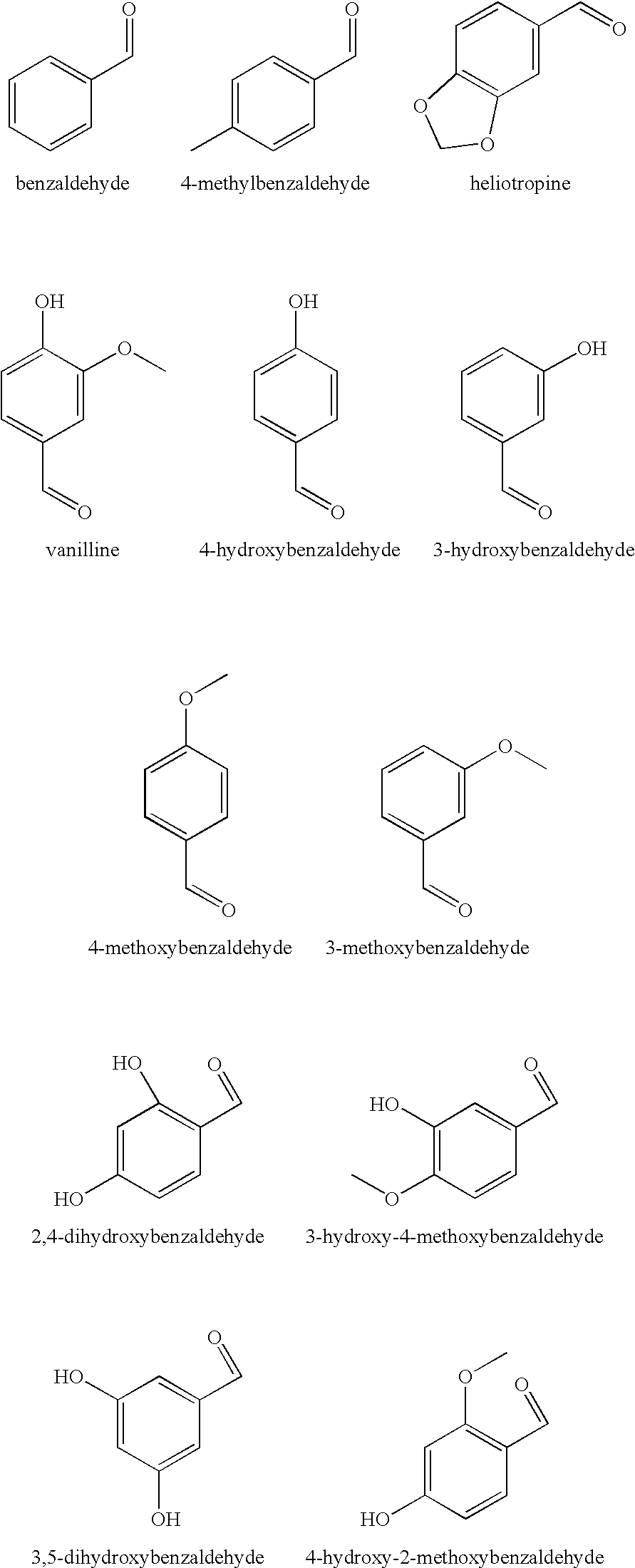
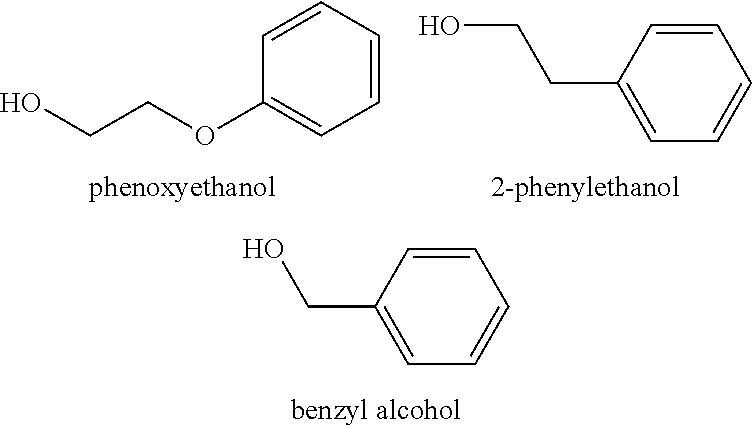
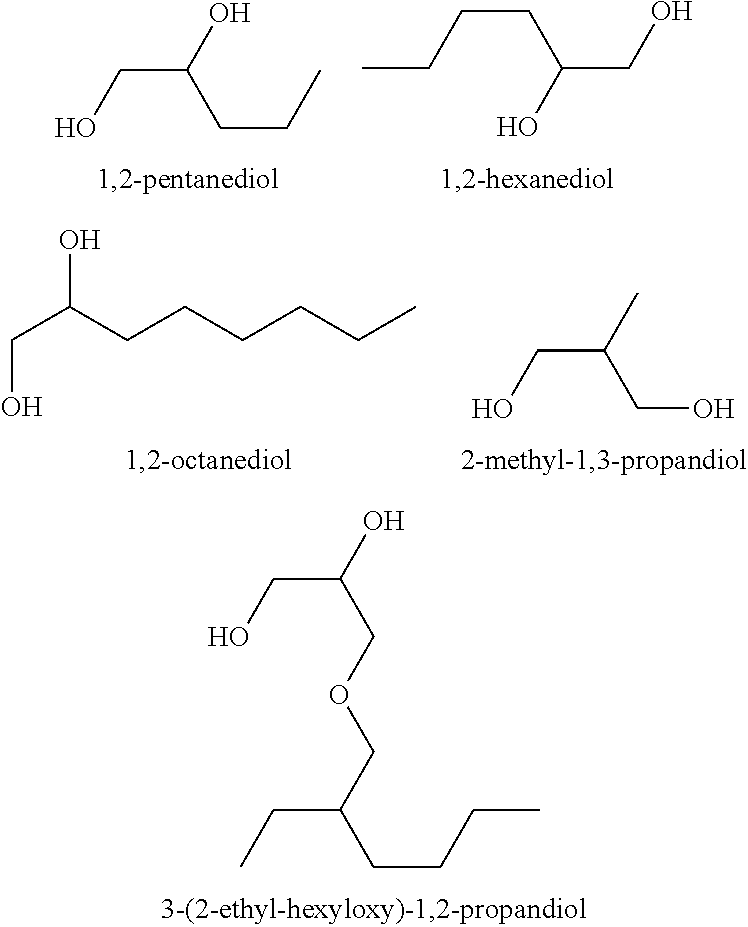
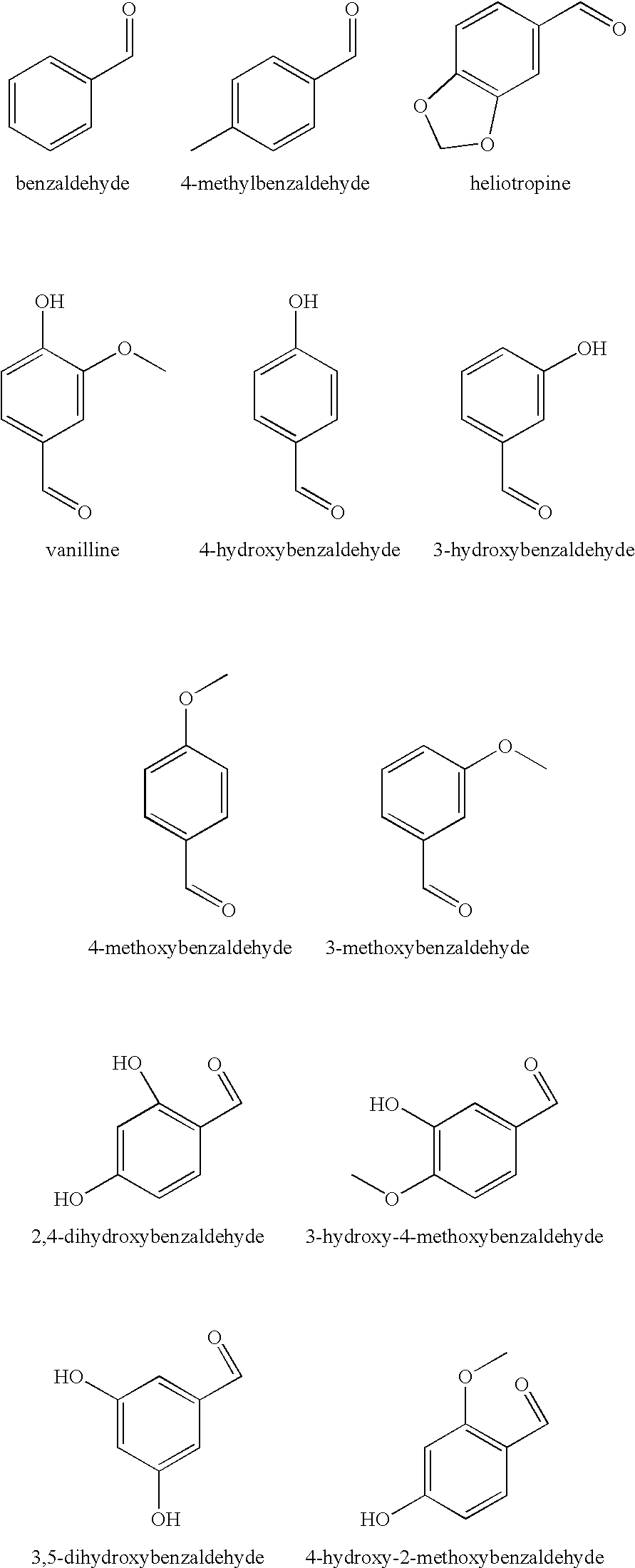
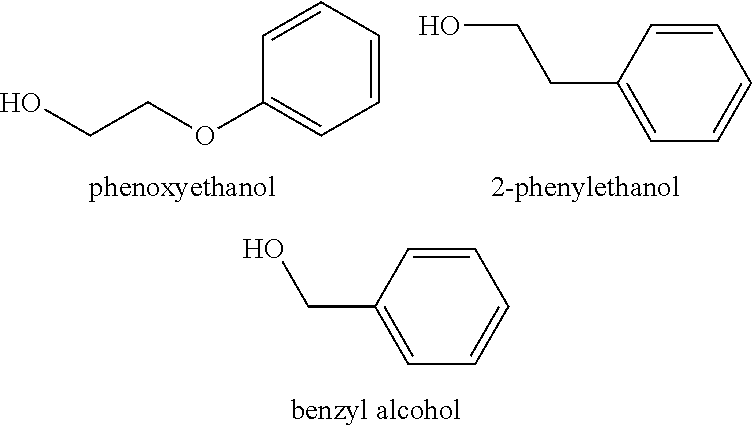
There are provided personal care products and compositions that comprise at least one diol compound selected from the group consisting of 1,2-pentanediol, 1,2-hexanediol, 1,2-octanediol, 2-methyl-1,3-propandiol, and 3-(2-ethyl-hexyloxy)-1,2-propandiol in a total concentration of 0.1% to 0.5% (w / w); and at least one preservative enhancer compound selected from the group consisting of benzaldehyde, 4-methylbenzaldehyde, heliotropine, vanilline, 4-hydroxybenzaldehyde, 3-hydroxybenzaldehyde, 4-methoxybenzaldehyde and 3-methoxybenzaldehyde, 2,4-dihydroxybenzaldehyde, 3-hydroxy-4-methoxybenzaldehyde, 3,5-dihydroxybenzaldehyde, and 4-hydroxy-2-methoxybenzaldehyde, in a total concentration of 0.05 to 0.5% (w / w), in a cosmetically acceptable base. The composition optionally contains at least one compound selected from the group consisting of phenoxyethanol, 2-phenylethanol, and benzylalcohol, in a total concentration of 0.05 to 0.3% (w / w), but does not contain other classic bactericidal, fungicidal, sporicidal or preservative compounds. The invention is further directed to methods of forming such compositions and products and the use of preservatives and preservative enhancers in such compositions and products.
Owner:GIVAUDAN SA
Fly attractant and preparing method thereof
InactiveCN105994263AStrong ability to seduceEasy to useBiocidePest attractantsHouttuyninCis-3-Hexenal
The present invention relates to the technical field of attractants, in particular to fly attractants, which are composed of the following components in parts by weight: 2-3 parts of fish oil, 1-2 parts of fishantin, 1.5-3 parts of fly maggot powder, and 2-3 parts of shrimp powder. 2.4 parts, 3-4 parts of baitene, 30-35 parts of compound fermented product, 20-25 parts of white sugar, 10-15 parts of brown sugar, 3-4 parts of maltose, 3-4 parts of fish bone meal, dodecylbenzenesulfonate 1-3 parts of sodium bicarbonate, 0.8-1 part of 4-methoxybenzaldehyde, 0.1-0.5 parts of cis-3-hexenal, 1-3 parts of 1-octenol, 5-7 parts of lard, sunset yellow 0.3-0.4 parts, 0.1-0.3 parts of 2,6-di-tert-butyl-4-methylphenol, 0.5-1 part of calcium carbonate. The fly attractant provided by the invention has strong luring effect on flies, is non-toxic, easy to use, non-toxic to humans and animals, has simple preparation method, low production cost and long-lasting effect.
Owner:周莉莉
Chiral platinum complex and preparation method thereof
InactiveCN104530136AStable structureThe synthesis method is simplePlatinum organic compoundsAntineoplastic agentsPotassium tetrachloroplatinatePhenanthroline
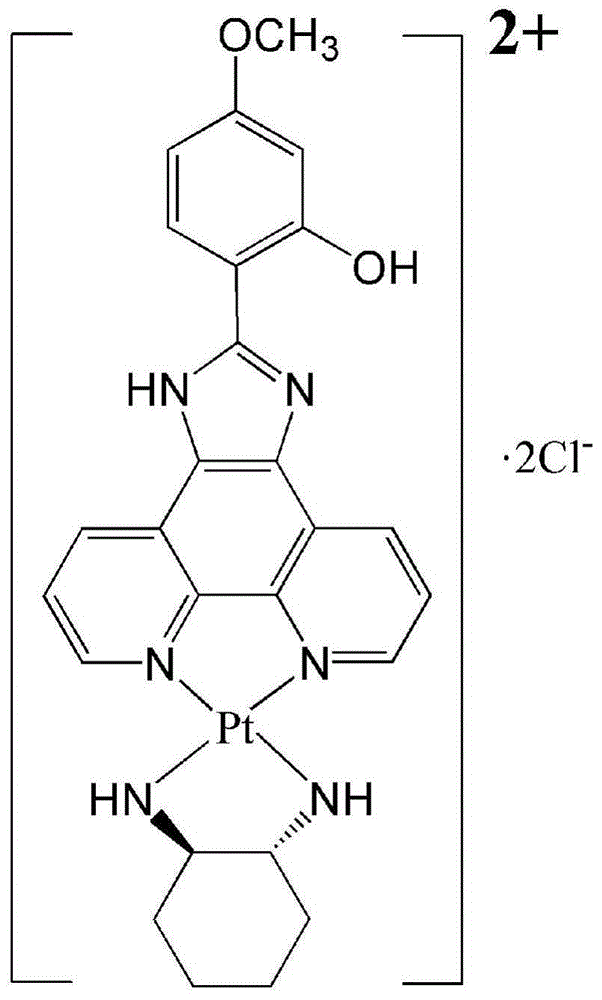
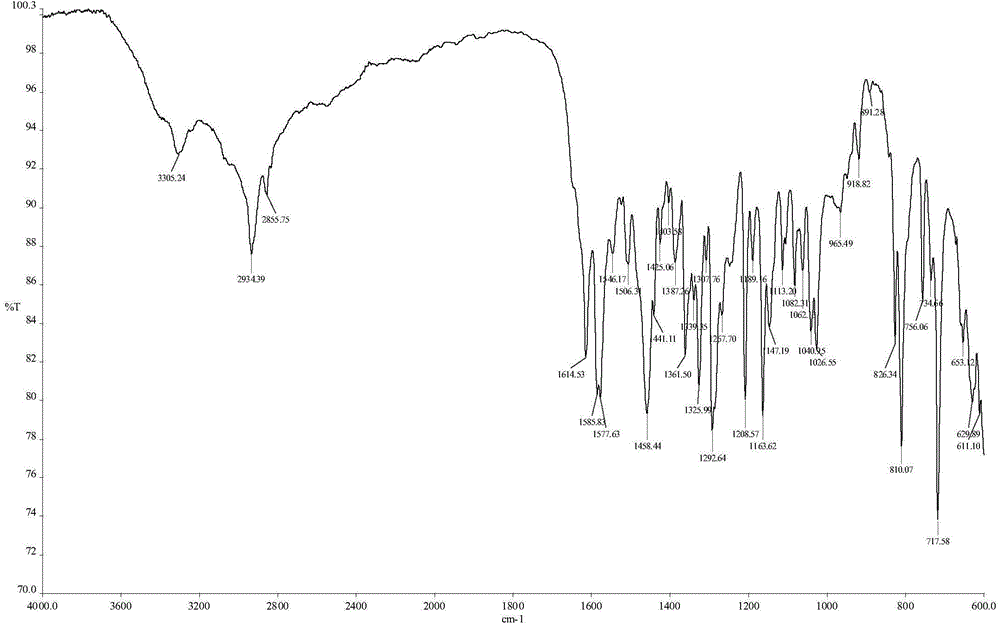
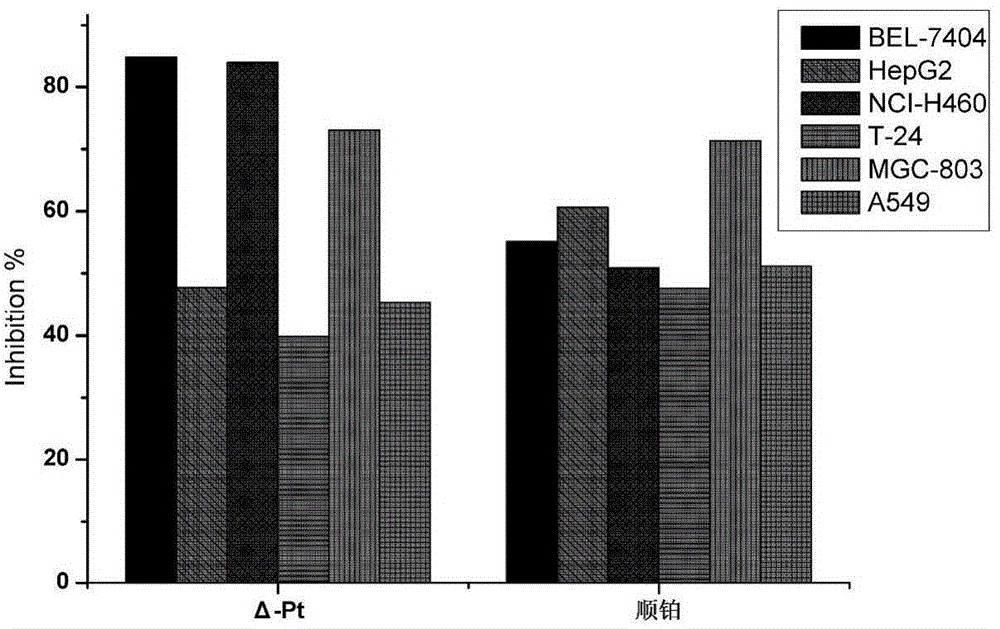
The invention discloses a chiral platinum complex and a preparation method thereof. The complex, namely dichloride-2-[2-(hydroxyl)-4-(methoxyl)-phenyl] imidazo[4,5-f][1,10]-phenanthroline.S, S-cyclohexanediamine platinum (II) has chirality. The preparation method comprises the following steps: preparing 2-[2-(hydroxyl)-4-(methoxyl)-phenyl] imidazo[4,5-f][1,10]-phenanthroline by taking 1,10-phenanthroline-5,6-dione and 2-hydroxyl-4-methoxybenzaldehyde as raw materials; adding potassium chloroplatinite to prepare 2-[2-(hydroxyl)-4-(methoxyl)-phenyl] imidazo[4,5-f][1,10]-phenanthroline.platinum (II) dichloride; finally adding cyclohexanediamine, thereby obtaining the chiral platinum complex disclosed by the invention. The complex has the characteristic of excellent anti-tumor activity, and can be applied to drugs resistant to the liver cancer, the lung cancer and the gastric cancer.
Owner:YULIN NORMAL UNIVERSITY
Sharing synthesis method for vanillin and isovanillin
InactiveCN102617313AReasonable designHigh yieldOrganic compound preparationCarbonyl compound preparationAlkyl transferSynthesis methods
The invention relates to a sharing synthesis method for vanillin and isovanillin. The method comprises the following steps of: performing alkylation reaction on guaiacol and halogenated hydrocarbon to prepare a 1-methoxy-2-alkoxylbenzene compound 1 in a high-yield way; performing Vilsmeier-Haack reaction on the 1-methoxy-2-alkoxylbenzene compound 1 and N,N-methylformanilide under the action of phosphorus oxychloride to generate a mixture of 3-methoxy-4-alkyloxybenzaldehyde 2 and 3-methoxy-4-methoxybenzaldehyde 3, separating the mixture out, selectively removing alkyl groups by directly using lewis acid to obtain a mixture of vanillin and isovanillin, and separating to prepare two compounds, namely vanillin and isovanillin. The method has the advantages of simplicity and high yield.
Owner:EAST CHINA UNIV OF SCI & TECH
Compositions
ActiveUS9192559B2Extended shelf lifeGood broad band preservative activityCosmetic preparationsBiocide3-HydroxybenzaldehydeBenzaldehyde
There are provided personal care products and compositions that comprise at least one diol compound selected from the group consisting of 1,2-pentanediol, 1,2-hexanediol, 1,2-octanediol, 2-methyl-1,3-propandiol, and 3-(2-ethyl-hexyloxy)-1,2-propandiol in a total concentration of 0.1% to 0.5% (w / w); and at least one preservative enhancer compound selected from the group consisting of benzaldehyde, 4-methylbenzaldehyde, heliotropine, vanilline, 4-hydroxybenzaldehyde, 3-hydroxybenzaldehyde, 4-methoxybenzaldehyde and 3-methoxybenzaldehyde, 2,4-dihydroxybenzaldehyde, 3-hydroxy-4-methoxybenzaldehyde, 3,5-dihydroxybenzaldehyde, and 4-hydroxy-2-methoxybenzaldehyde, in a total concentration of 0.05 to 0.5% (w / w), in a cosmetically acceptable base. The composition optionally contains at least one compound selected from the group consisting of phenoxyethanol, 2-phenylethanol, and benzylalcohol, in a total concentration of 0.05 to 0.3% (w / w), but does not contain other classic bactericidal, fungicidal, sporicidal or preservative compounds. The invention is further directed to methods of forming such compositions and products and the use of preservatives and preservative enhancers in such compositions and products.
Owner:GIVAUDAN SA
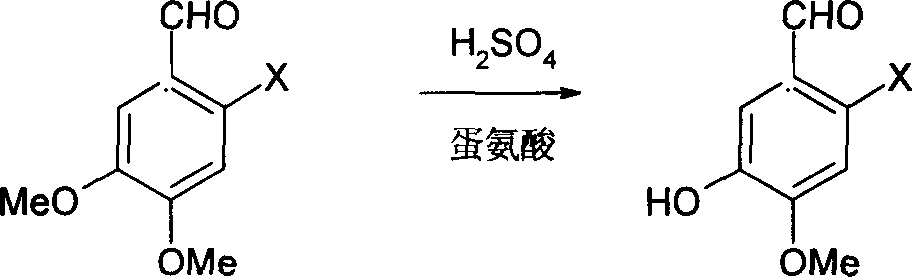
Method for synthesizing 6-substituent-3-hydroxy-4-methoxybenxaldchyde
InactiveCN1686991AThe synthesis process is simpleReduce manufacturing costOrganic compound preparationCarbonyl compound preparationSynthesis methodsDL-methionine
The present invention discloses a synthesis method of 6-substituent-3-hydroxy-4-methoxybenzaldehyde. Said method includes the following steps: (1). demethylation; under the action of concentrated sulfuric acid making 6-substituent-3,4-dimethoxybenzaldehyde and DL-methionine be reacted, after the reaction is completed, diluting and filtering to obtain filtrate and filter cake, washing filter cake to obtain 6-substituent-3-hydroxy-4-methoxybenzaldehyde crude product; (2). purification: using alkaline solution to dissolve the above-mentioned crude product, filtering, using acidic solution to regulate pH value of obtained filtrate to acidity, filtering, washing, drying so as to obtain 6-substituent-3-hydroxy 4-methoxybenzaldehyde; and (3) recovering DL-methionine.
Owner:ZHEJIANG UNIV
Two-photon fluorescent dye based on 4-methoxyphenyl-substituted BODIPY and diphenylaminofluorene and synthesis method of two-photon fluorescent dye
ActiveCN106432314AHigh synthetic yieldGood single photonAzo dyesGroup 3/13 element organic compoundsQuantum yieldSynthesis methods
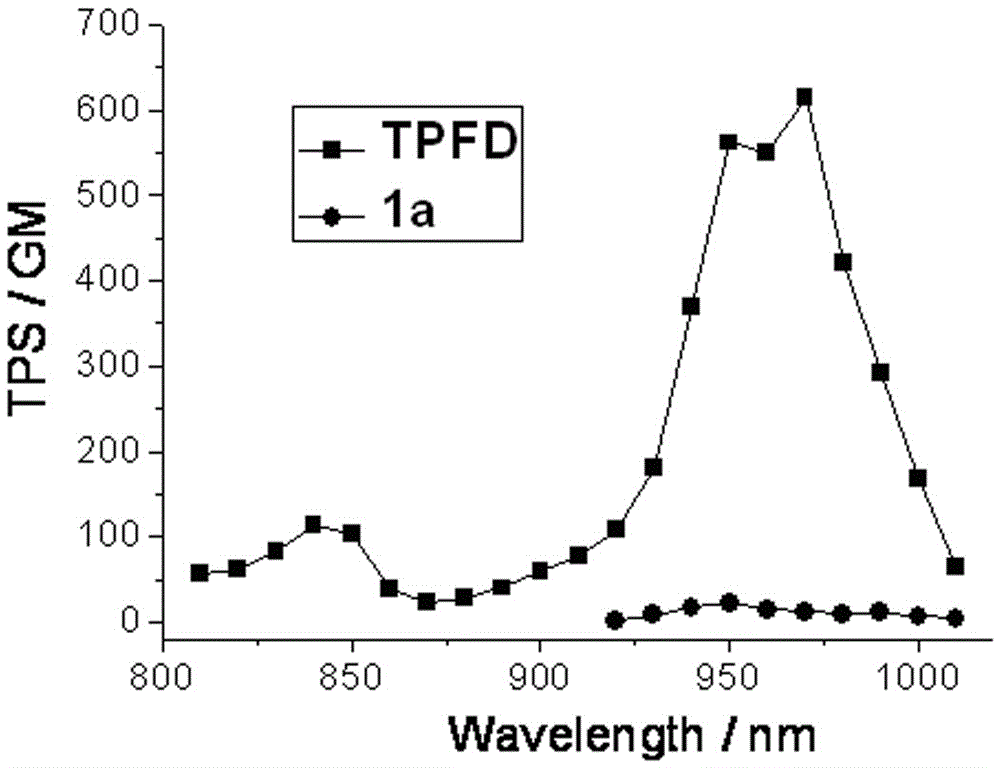
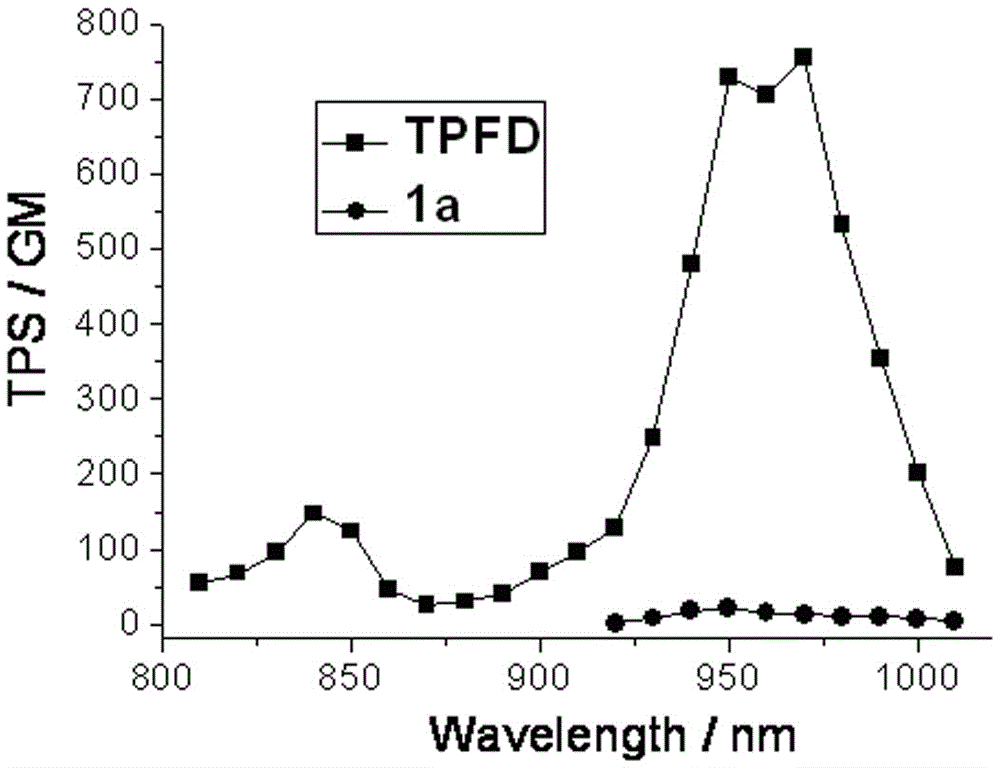
The invention provides a two-photon fluorescent dye based on 4-methoxyphenyl-substituted BODIPY and diphenylaminofluorene and a synthesis method of the two-photon fluorescent dye. The structural formula of the two-photon fluorescent dye is shown in the specification, wherein R is C1-C18 alkyl. The synthesis method of the synthesized two-photon fluorescent dye comprises steps as follows: (1) 2,6-bit iodinated BODIPY is prepared from 2,4-dimethyl pyrrole and 4-methoxybenzaldehyde as raw materials; (2) fluorene as a raw material is subjected to bromination, alkylation, Pd (0) catalytic amination, Pd (0) and CuI catalysis and the like, and a diphenylamine-fluorene-acetylene compound is produced; (3) the two products react, and the two-photon fluorescent dye is obtained. The synthesized target compound has higher two-photon fluorescent performance; in the toluene, the maximum two-photon absorption cross section of the compound reaches 615 GM and the fluorescence quantum yield is 0.39; a new design idea is provided for synthesis and application of the two-photon fluorescent dye based on BODIPY.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Two-photon fluorescent dye based on 4-methoxyphenyl-substituted boron-dipyrromethene and diphenylaminoindenofluorene and its synthesis method
ActiveCN106478705AIncreased absorption cross sectionHigh fluorescence quantum yieldAzo dyesGroup 3/13 element organic compoundsQuantum yieldSynthesis methods
The invention discloses a two-photon fluorescent dye based on 4-methoxyphenyl-substituted boron-dipyrromethene and diphenylaminoindenofluorene and its synthesis method. The two-photon fluorescent dye has a general structural formula shown in the description. In the formula, R represents a C1-C18 alkyl group. The synthesis method comprises that 1) 2, 4-dimethylpyrrole and 4-methoxybenzaldehyde as raw materials undergo a reaction to produce 2, 6-bit iodinated boron-dipyrromethene, 2) indenofluorene dione as a raw material is reduced, the reduced product undergoes an alkylation reaction, the product undergoes a bromination reaction, the product undergoes a Pd(0) catalytic amination reaction, and the product undergoes a Pd(0) and CuI catalytic Sonogashira cross-coupling reaction to produce a diphenylamino-indenofluorene-acetylene compound, 3) the two products obtained by the steps 1 and 2 undergo a reaction to produce the two-photon fluorescent dye. The dye has strong two-photon fluorescence performances. In toluene, the two-photon fluorescent dye has the maximum two-photon absorption cross section of 757 GM and a fluorescence quantum yield of 0.49. The two-photon fluorescent dye provides a novel design idea for synthesis and application of the boron-dipyrromethene-based two-photon fluorescent dye.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
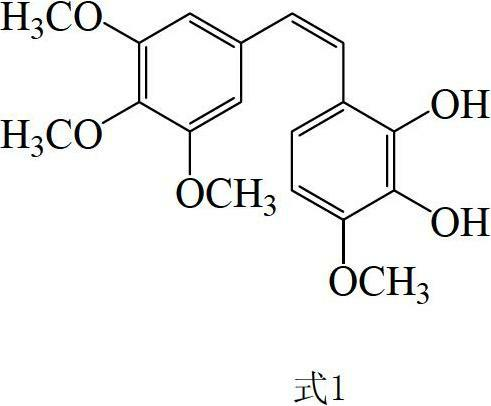
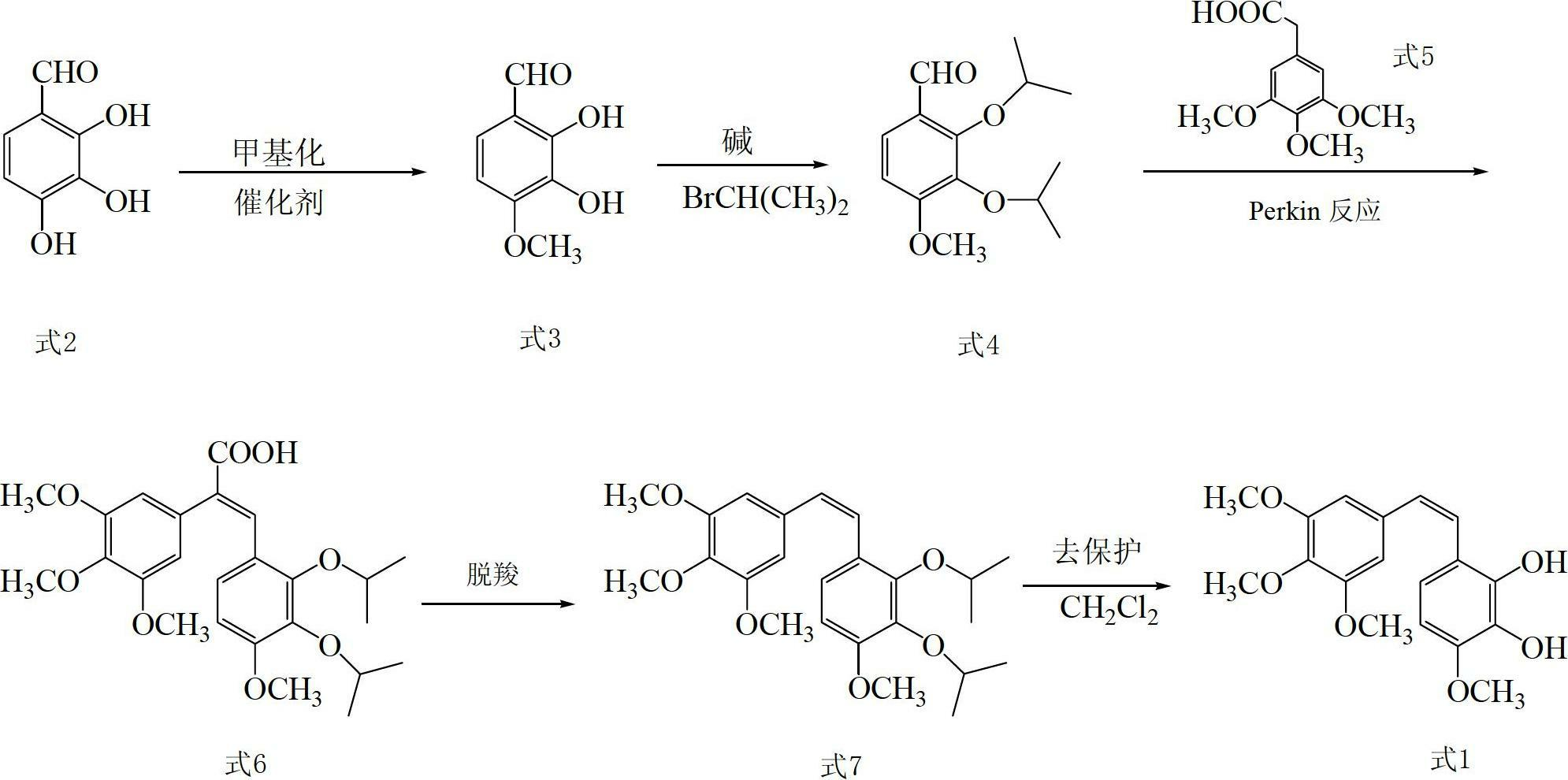
Preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene
The invention discloses a preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene. The preparation method comprises the following steps of: taking 2,3,4-trihydroxy phenylfluorone as a raw material; carrying out single methylation on the 2,3,4-trihydroxy phenylfluorone; carrying out substitution reaction on the methylated 2,3,4-trihydroxy phenylfluorone with bromopropane so as to obtain 2,3-diisopropoxy-4-methoxybenzaldehyde; carrying out Perkin reaction on 2,3-diisopropoxy-4-methoxybenzaldehyde with 3,4,5-trimethoxyphenylacetic acid so as to obtain E-2-(3,4,5-trimethoxyphenyl)-3-(2',3'-diisopropoxy-4'-methoxyphenyl)acrylic acid; carrying out decarboxylation to obtain Z-3,4,4',5-tetramethoxy-2',3'-diisopropoxy diphenylethylene; and finally obtaining Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene after carrying out deprotection. The synthetic route provided by the invention is simple and direct, cis-selectivity is higher, and the yield of the Perkin reaction as well as the total yield of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene are both higher. The preparation method disclosed by the invention has the characteristics of simpleness and feasibility for operation, low price and easy obtainment of used raw materials and reagents, small pollution on environment, good atom economy, low cost and capability of being applied to industrialized production.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Simple and effective preparation method for resveratrol
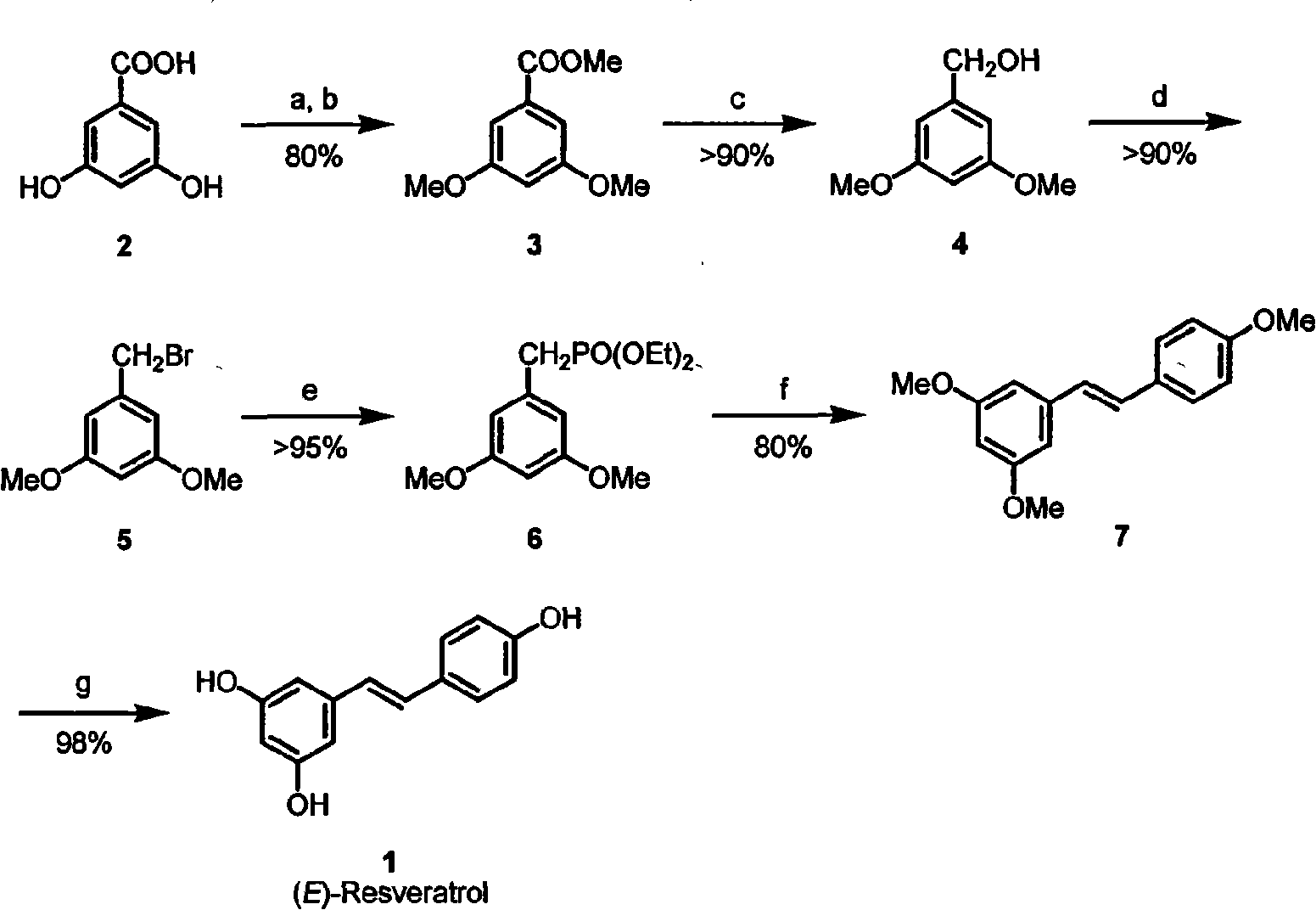
InactiveCN101585751ALow corrosive explosiveHigh purityOrganic chemistryOrganic compound preparationBenzoic acidMethyl group
The invention provides a simple and effective preparation method for a natural plant component resveratrol. The characteristic is following: 3,5-dihydric benzoic acid is used as initial material; steps of phenolic hydroxyl group protection, esterification, reduction, bromination, arbuzov reaction are adopted to prepare the key Intermediate 3,5 - dimethoxy-benzyl diethyl, processing the Widischy - Horner reaction with 4-methoxybenzaldehyde (anisaldehyde right) producing 3,4',5-trimethoxy-stilbene, finally removing the methyl protection to obtain the reaction formula resveratrol.
Owner:米启兮 +1
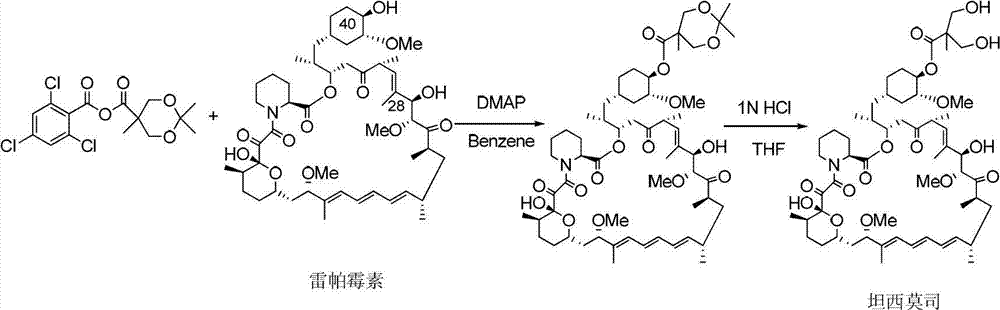
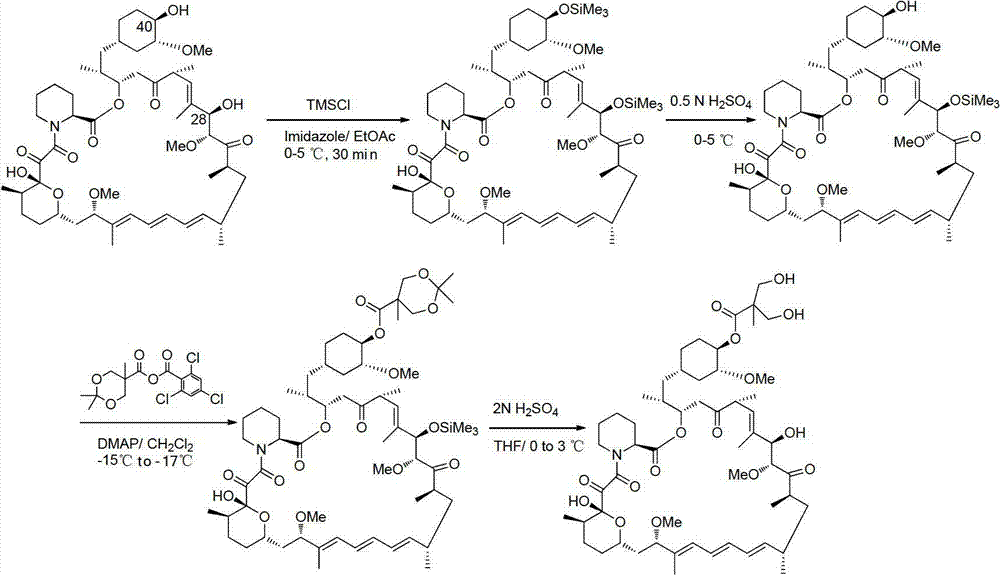
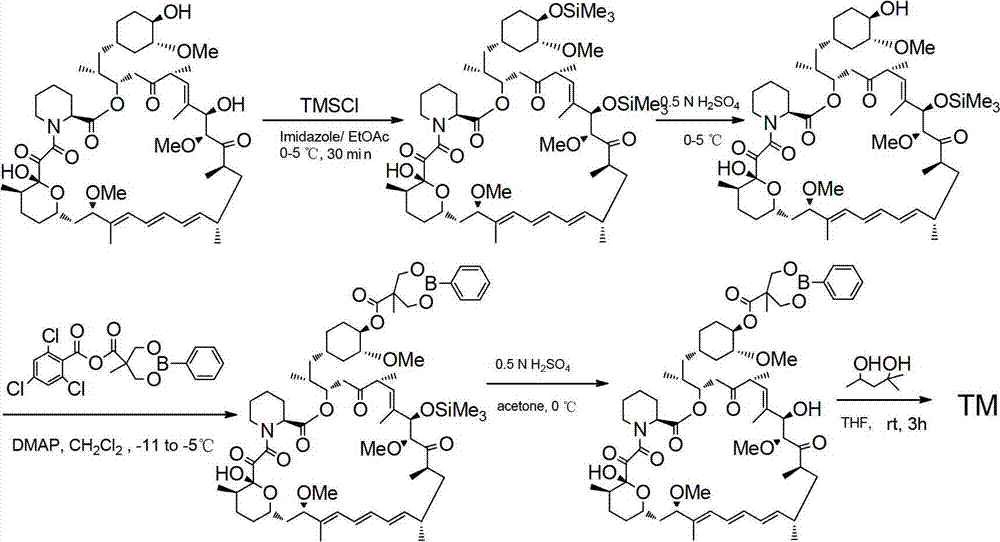
Method used for preparing temsirolimus and suitable for industrial production
InactiveCN102807571AHigh yieldEasy to operateOrganic chemistryBulk chemical productionCompound aOrganic solvent
The invention belongs to the technical field of methods for preparing temsirolimus. The method for preparing the temsirolimus comprises the following steps of: 1) performing catalytic reaction on 2,2-bis(hydroxymethyl)propionic acid and 4-methoxybenzaldehyde dimethylacetal in the presence of an organic solvent; 2) reacting a compound II with 2,4,6-trichlorobenzoyl chloride at normal temperature in the presence of an organic solvent under the alkaline condition, adding an organic solvent containing a compound A and 4-(N,N-dimethylamino)pyridine into the reaction solution, and reacting to obtain a compound B, wherein P is a protecting group; and 3) reacting the compound B with acid in the presence of a solvent to obtain the temsirolimus. The method is easy to operate, low in cost and suitable for industrial production, the synthetic route is short, and yield is high.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
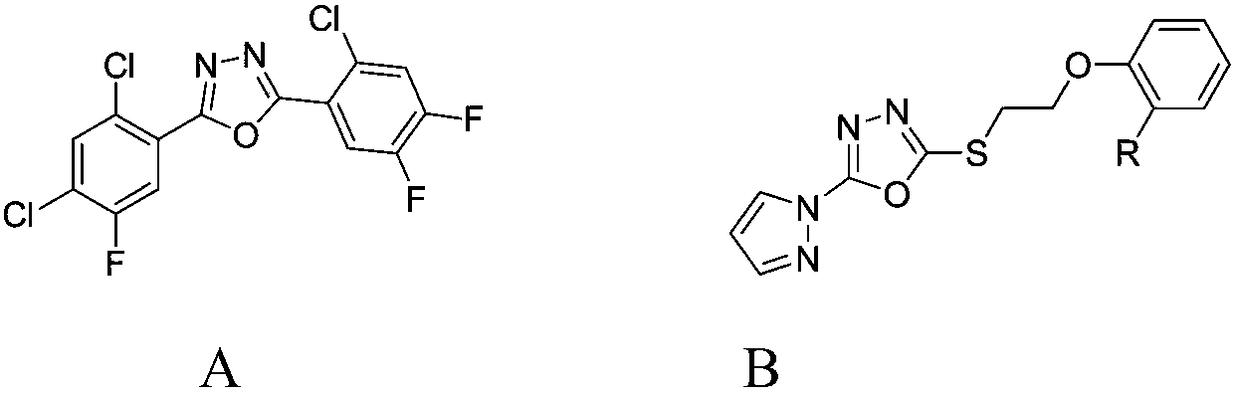
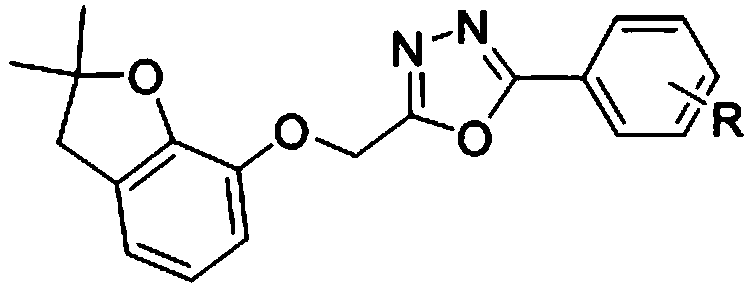
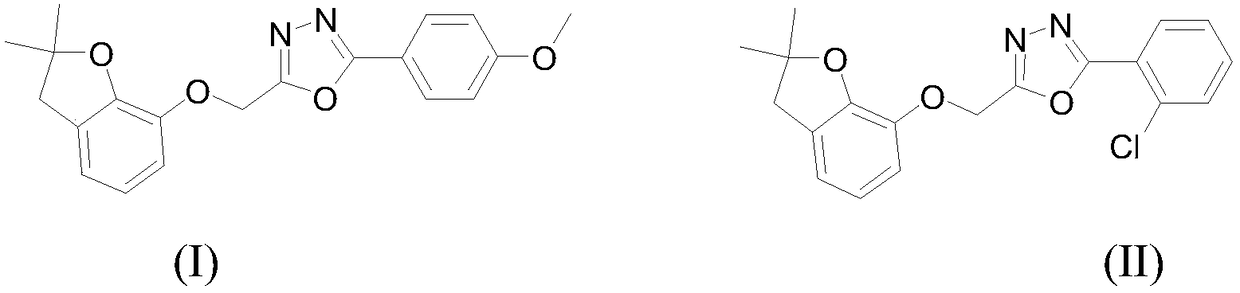
Benzofuryl-containing 1, 3, 4-oxadiazole compound
The invention discloses a benzofuryl-containing 1, 3, 4-oxadiazole compound. A preparation method of the benzofuryl-containing 1, 3, 4-oxadiazole compound comprises that 1) 2-((2, 2-dimethyl-2, 3-dihydrobenzofuran-7-yl)oxo)acethydrazide respectively reacts with 4-methoxybenzaldehyde and 2-chlorobenzaldehyde in an organic solvent I under catalyst conditions to produce an intermediate 2-((2, 2-dimethyl-2, 3-dihydrobenzofuran-7-yl)oxo)acetyl hydrazone, and 2) the intermediate 2-((2, 2-dimethyl-2, 3-dihydrobenzofuran-7-yl)oxo)acetyl hydrazone reacts in an organic solvent II under iodobenzene diacetate conditions to produce a desired compound (I) or (II). The compound has good insecticidal activity and good inhibitory activity to Tetranychus cinnabarinus at a test concentration. The preparationmethod is easy to operate, has a high yield and provides the candidate drug for the creation of the acaricide compound.
Owner:湖南博隽生物医药有限公司
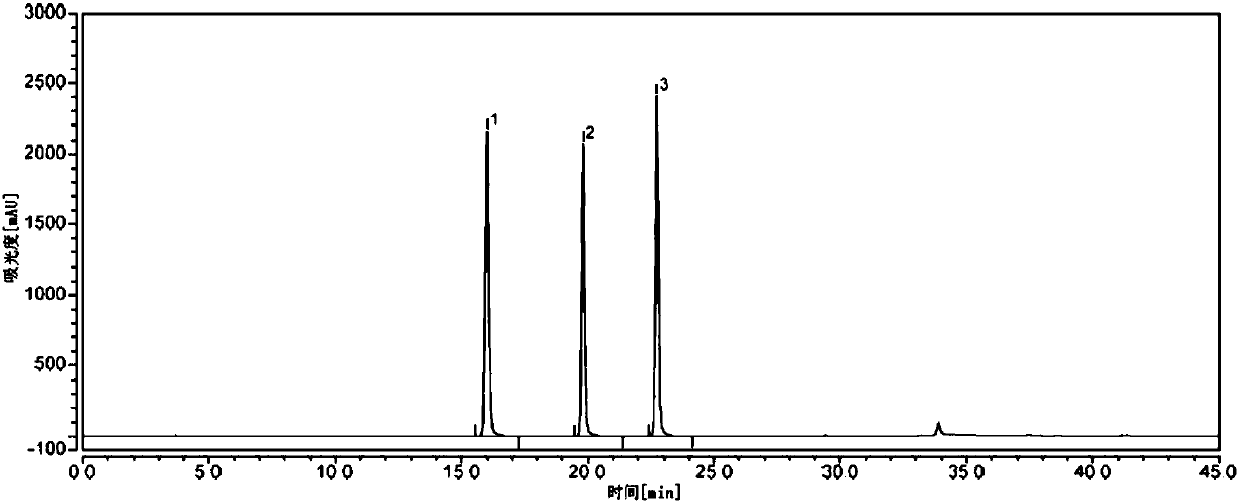
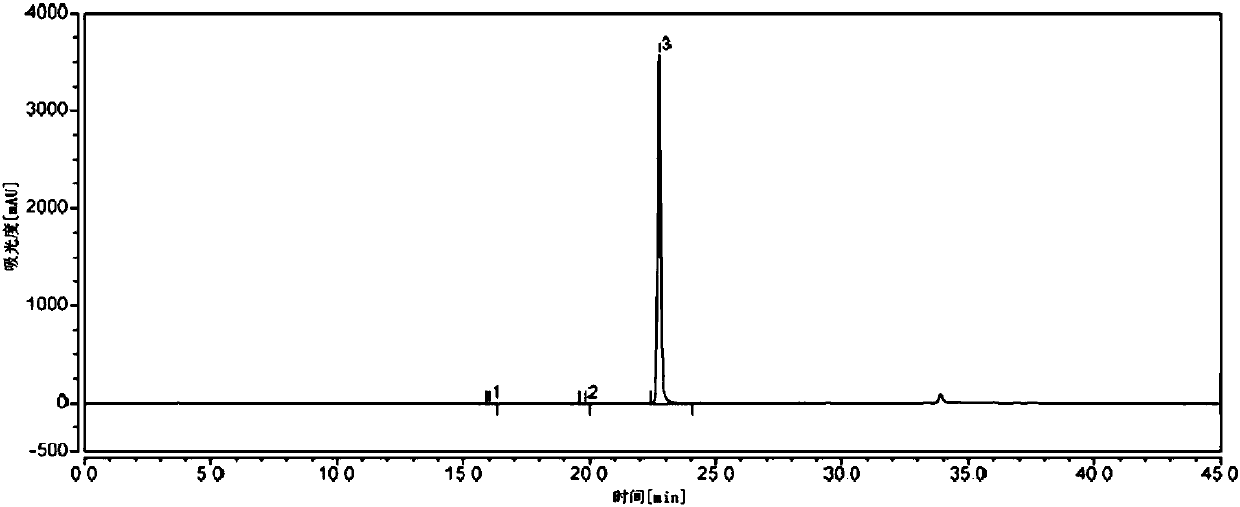
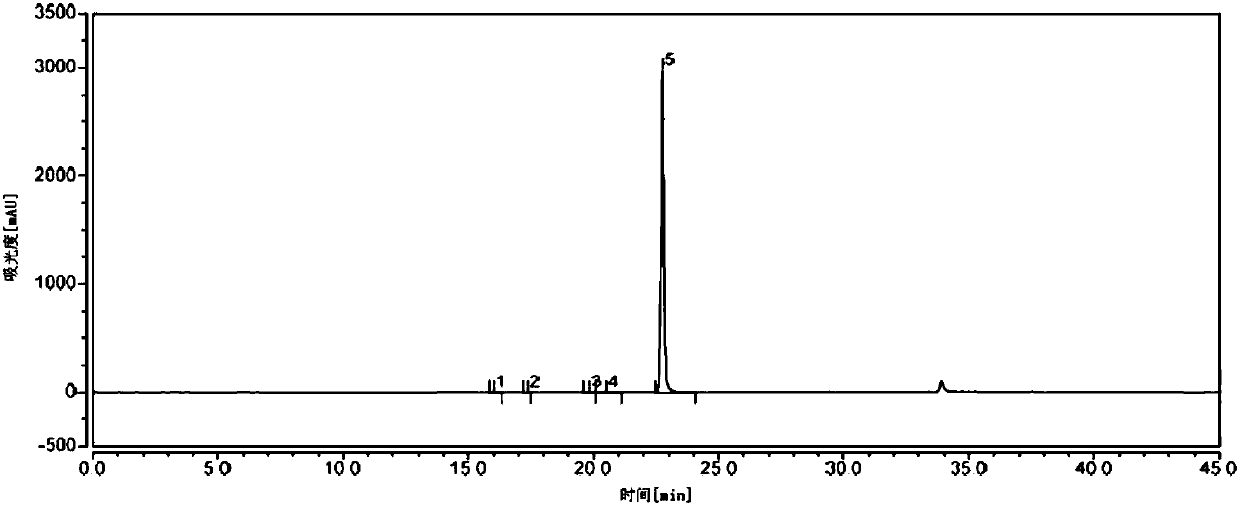
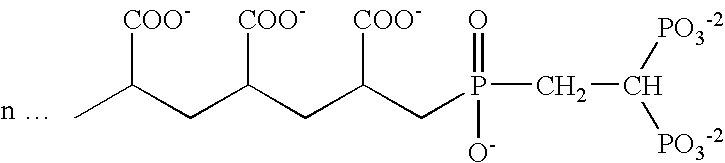
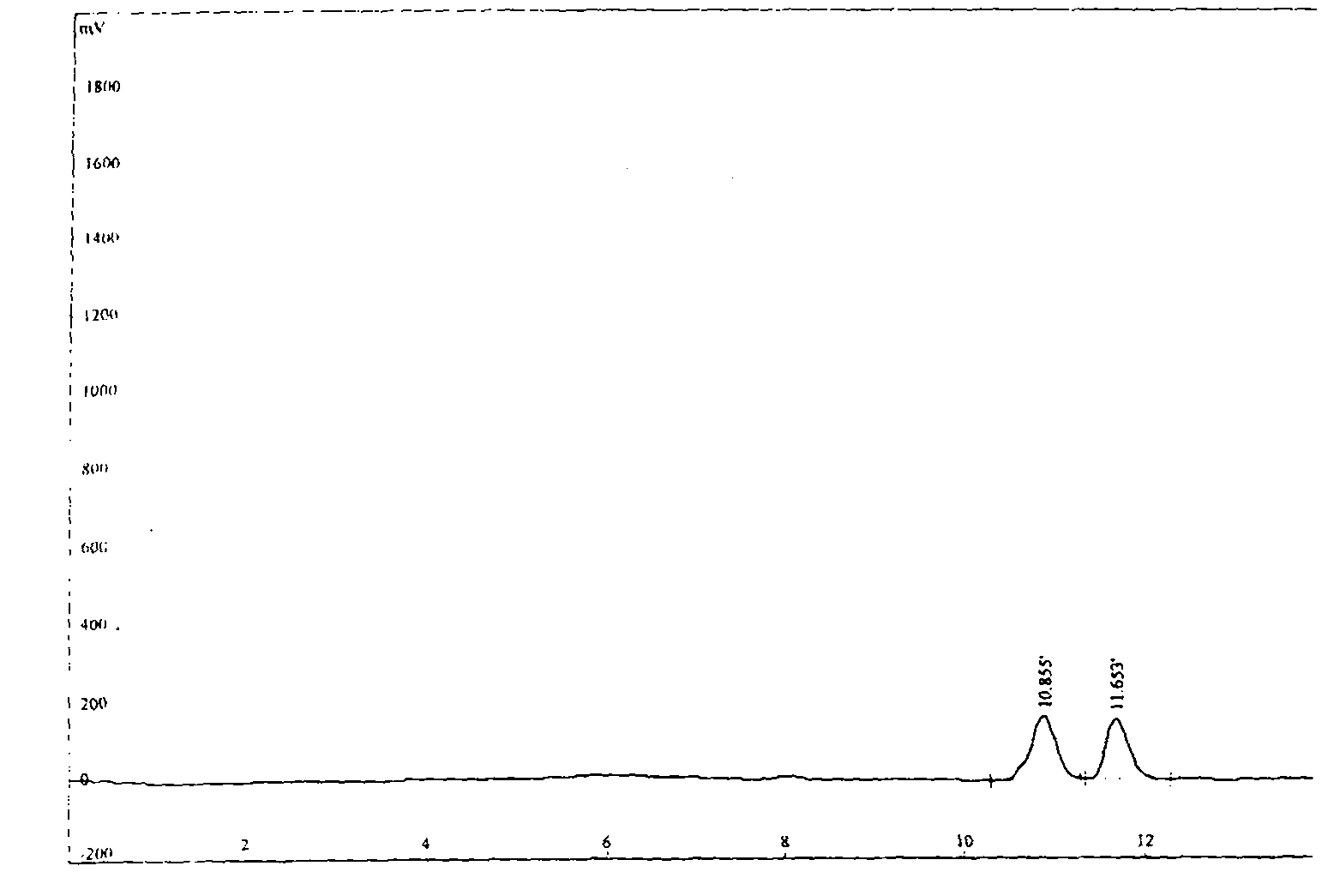
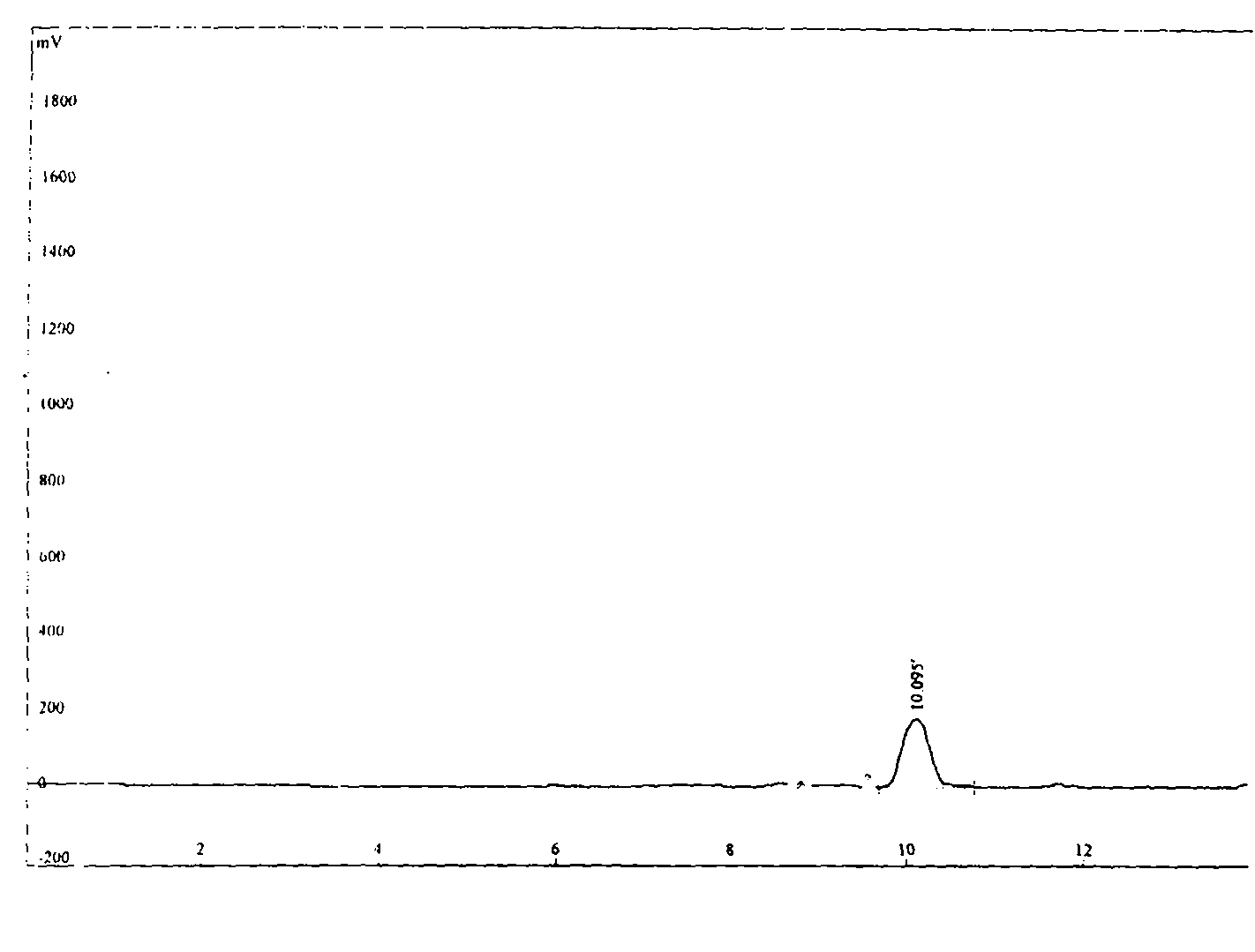
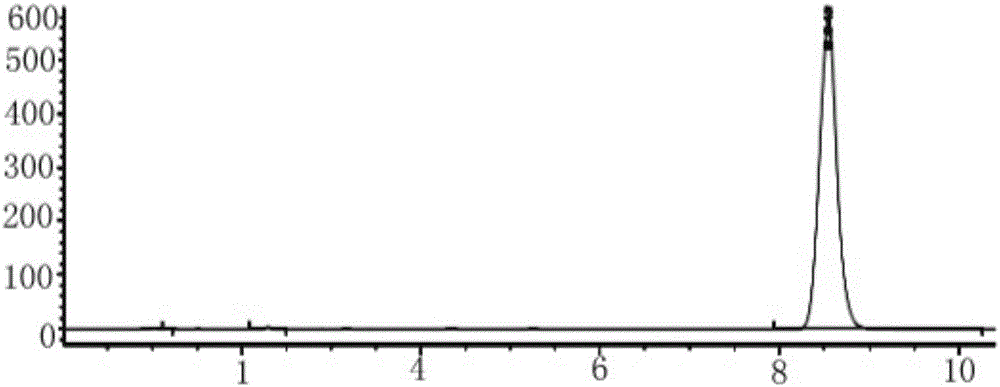
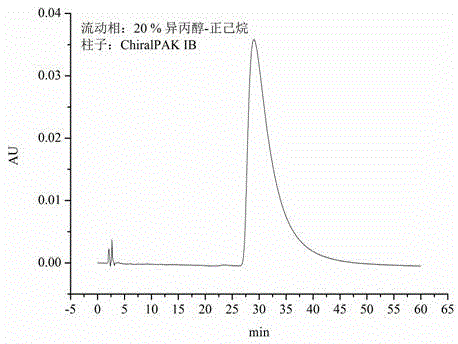
Detection method of purity of 3-ethoxy-4-methoxybenzaldehyde
ActiveCN108008035AEfficient separationGood peak shapeComponent separationUltraviolet detectorsAcetonitrile
The invention discloses a detection method of purity of 3-ethoxy-4-methoxybenzaldehyde. In the detection method, a phenomenon chromatographic column is adopted, two mobile phases are adopted for gradient elution, a mobile phase A is KH2PO4 buffer solution, a mobile phase B is acetonitrile, and the flow velocity of the each mobile phase is 0.7-1.2mL / min; a detector is an ultraviolet detector. The detection method is simple, convenient, accurate, fast, reliable, highly repeatable and stable, the 3-ethoxy-4-methoxybenzaldehyde and known impurities thereof can be effectively separated, and the simple, convenient, accurate, fast and reliable detection method is provided for industrial production of the 3-ethoxy-4-methoxybenzaldehyde.
Owner:GUANGXI ENANTIOTECH PHARM CO LTD
Synthesis method of 3-ethyoxyl-4-methoxybenzaldehyde
ActiveCN107827722AReduce usageReduce harmOrganic compound preparationCarbonyl compound preparationSynthesis methodsWastewater
The invention discloses a synthesis method of 3-ethyoxyl-4-methoxybenzaldehyde. The synthesis method of the 3-ethyoxyl-4-methoxybenzaldehyde is characterized by comprising the following steps: by taking isovanillin and halogenated ethane as raw materials, stirring the isovanillin and the halogenated ethane for reaction in a solvent under the action of alkali and a catalyst, and carrying out filtering, so as to obtain the 3-ethyoxyl-4-methoxybenzaldehyde. The method disclosed by the invention is simple, convenient and safe in reaction operation, and an obtained product is easy to separate; treatments on waste gas, wastewater and solid wastes are easy and feasible; in the reaction process, used equipment is simple, and operation can be carried out without special equipment and conditions ofhigh pressure and high vacuum; the synthesis method is an environmentally-friendly green synthesis process.
Owner:ENANTIOTECH CORP
Method for synthesizing CA4P
ActiveCN101885738BRaw materials are cheap and easy to getMild reaction conditionsMethine/polymethine dyesGroup 5/15 element organic compoundsChemical synthesisPhosphate
The invention belongs to the field of chemical synthesis and relates to a method for preparing CA4P, in particular to a method for synthesizing CA4P by the following steps that: isovanillin and trityl chloride, which serve as raw materials, are used to form 3- triphenylmethoxy-4-methoxybenzaldehyde which is an intermediate isovanillin protector; the 3- triphenylmethoxy-4-methoxybenzaldehyde and 3,4,5-trimethoxy-triphenyl benzylidene bromide phosphine salt undergo a Wittig reaction , and the protective group is removed by hydrolysis to obtain CA4; and the CA4 and phosphonic acid bis(phenylmethyl)ester react to form benzyl phosphate, and the benzyl group is removed to form a sodium salt to obtain the target compound, namely CA4P.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
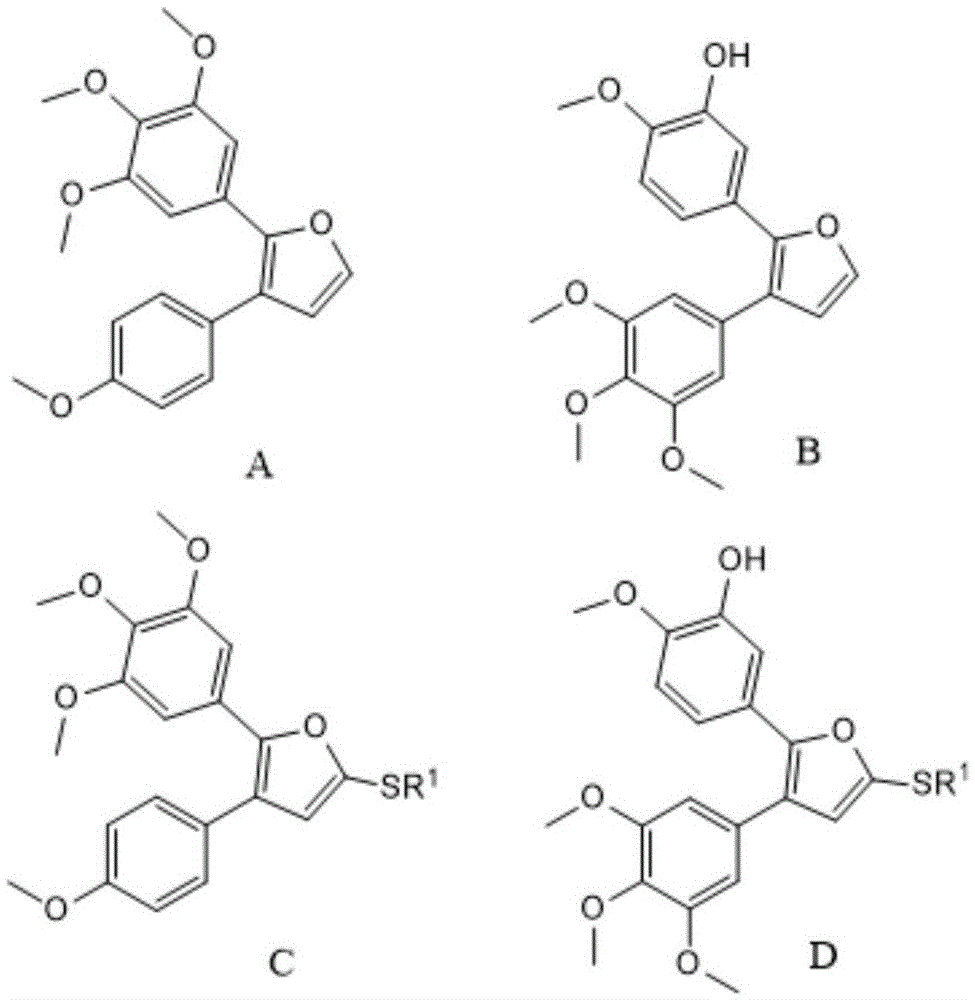
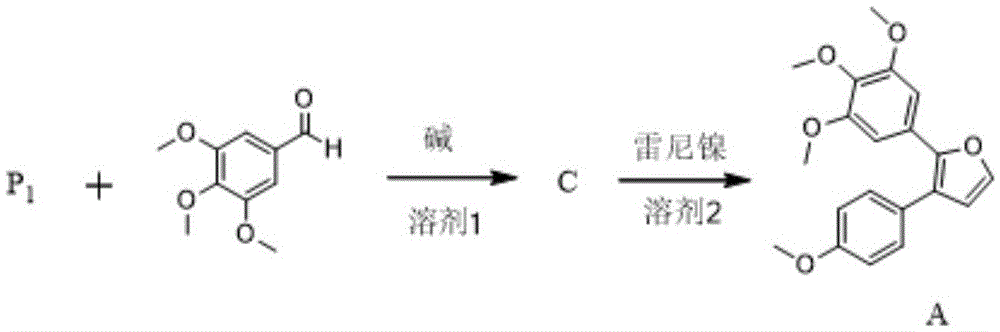
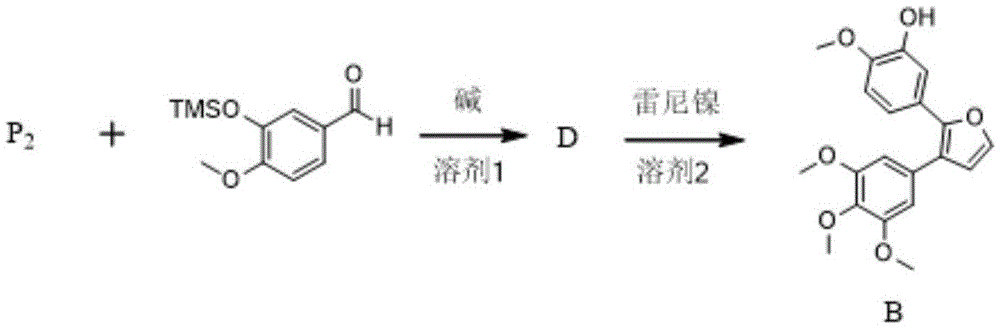
Preparation method of combretastatin furan type analogues
The invention provides a preparation method of combretastatin furan type analogues. The preparation method of the combretastatin furan type analogues (A and C) comprises the following steps of adding a solvent 1, a compound P1 and 3,4,5-triethoxy benzaldehyde into a reaction flask; adding alkali while stirring; reacting for 10 minutes at room temperature; adding diluted acid to regulate pH (Potential of Hydrogen) to be neutral; obtaining the combretastatin furan type analogue C through separation and purification; obtaining a compound A by reducing the C in a solvent 2 by using Raney Ni. The preparation method of combretastatin furan type analogues (B and D) comprises the following steps of adding the solvent 1, a compound P2 and 3-trimethylsilyl ether-4-methoxybenzaldehyde into the reaction flask; adding alkali while stirring; reacting for 10 minutes at the room temperature; adding the diluted acid to regulate pH (Potential of Hydrogen) to be neutral; obtaining the combretastatin furan type analogue D through separation and purification; obtaining a compound B by reducing the D in the solvent 2 by using the Raney Ni. The preparation method of the combretastatin furan type analogues, provided by the invention, has the advantages that the yield is high, the operation is simple, the reaction conditions are moderate, the used solvents are cheap and easy to get, industrial production is easy to realize, and the like.
Owner:LANZHOU UNIVERSITY
Method for preparing Roflumilast
InactiveCN102336704BShort stepsRaw materials are cheap and easy to getOrganic chemistryBenzaldehydeEthyl acetate
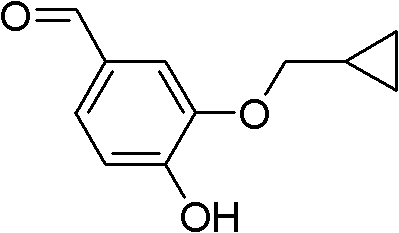
The invention discloses a method for preparing Roflumilast. The method comprises the following steps of: performing cyclopropyl methylation on isovanillin to obtain 3-cyclopropylmethoxy-4-methoxybenzaldehyde; performing demethylation to synthesize an important intermediate of the Roflumilast, namely 3-cyclopropylmethoxy-4hydroxyl-benzaldehyde; and further synthesizing a key intermediate in a formula (5) according to American patent US5712298 and finally synthesizing the Roflumilast in a formula (7). A crude product of the Roflumilast is treated by isopropanol and water, and is recrystallized by ethyl acetate and petroleum ether. The preparation method has a few steps, raw materials are readily available and cheap, the reaction selectivity is high, the yield is high and the post treatment is simple.
Owner:SHANDONG RUIHE PHARMA R&D CO LTD
Preparation method and application of chiral pimobendan
InactiveCN107344933AImprove securityOrganic chemistry methodsCardiovascular disorderN dimethylformamideHypertrophic cardiomyopathy
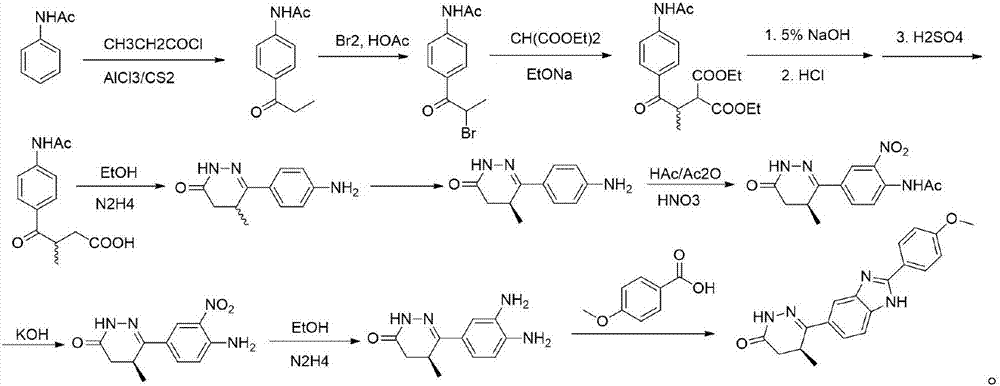
The present invention relates to the preparation method and application of chiral pimobendan, comprising the following steps: preparing chiral intermediate 1 with chiral reagent, diammonium phthalate and aluminum trichloride; using chiral intermediate 1, Prepare intermediate 2 with base, N,N-dimethylformamide and malonic acid substituted ester; prepare intermediate 3 with intermediate 2 and a catalytic hydrogenation catalyst; prepare intermediate 4 with intermediate 3, alcohol and hydrazine hydrate; Intermediate 4, N,N-dimethylformamide and 4-methoxybenzaldehyde are prepared to obtain chiral pimobendan, and the method of the present invention only needs 5 steps to obtain chiral pimobendan, and the current The steps are shortened by more than half, and the starting materials are simple and easy to obtain; and the chiral pimobendan can be used to treat mild, moderate or severe congestive heart failure in dogs caused by atrioventricular valve insufficiency or myocardial hypertrophy , hypertrophic cardiomyopathy in pets, etc.
Owner:蒿莱医药技术(上海)有限公司
Novel use of chiral diphosphonic diamide oxazoline
InactiveCN101972668AGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methods4-Methylbenzaldehyde
A chiral phosphonic diamide compound is N,N-2-[(4S)-4,5-dihydro-4-R-2-oxazoline]diphenyl-phenyl phosphonic diamide, and has a chemical formula. A synthesis method for the compound comprises the following steps of: refluxing and reacting 2-cyanoaniline and L-phenylglycinol for 24 hours in a chlorobenzene solvent under the conditions of no water and no oxygen and in the presence of a catalyst ZnCl2 to prepare an intermediate, and refluxing and reacting the intermediate and diphenyl phosphonic chloride for 24 hours in a methylbenzene-triethylamine mixed solvent under the conditions of no water and no oxygen to synthesize the target product. When the chiral target product is prepared by the cyanation-silication reaction of 4-methylbenzaldehyde, 4-methoxybenzaldehyde and 4-bromobenzaldehyde, the compound serves as a chiral catalyst.
Owner:罗梅
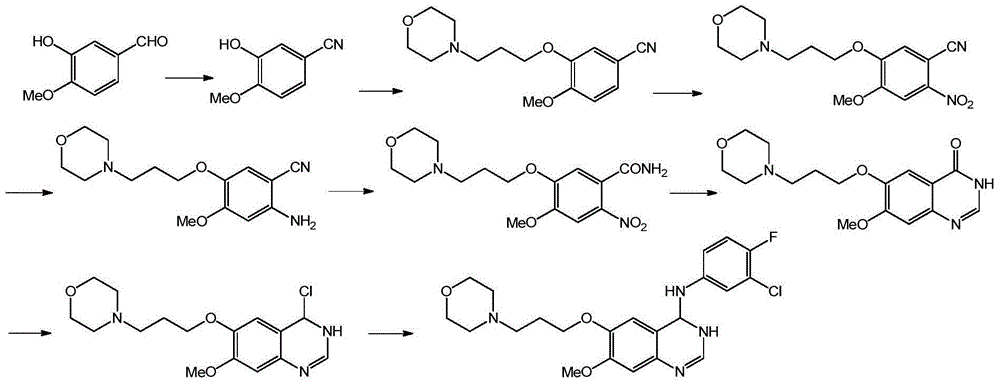
Preparation method of 4-(3-chloro-4-fluorophenyl amido)-7-methoxy-6-(3-morpholine propoxy) quinazoline
The invention provides a preparation method of 4-(3-chloro-4-fluorophenyl amido)-7-methoxy-6-(3-morpholine propoxy) quinazoline. Specifically, according to the method, 3-hydroxy-4-methoxybenzaldehyde (isovanillin) serves as raw materials, and the target product (I) is synthesized through the steps of oxidation, esterification, amidation, cyclization and the like. The method has the advantages of being short in synthetic route, low in cost, environmentally friendly, short in reaction step, easy to operate, high in yield and product purity and the like, and therefore the preparation method is suitable for industrial production.
Owner:上海天慈中商药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200041.PNG)
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200042.PNG)
![Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline Synthesis method of 4-(3-chlorine-4-fluorobenzene amino)-7-methoxy-6-[3-(4-morpholinyl)-propoxy] quinoline](https://images-eureka.patsnap.com/patent_img/37680de7-1f05-4d21-961b-fff7e39228d0/a20081012224200051.PNG)
![Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/97e6e5f8-76a4-40f2-bf53-c0138f8549ad/HDA0000860577330000011.PNG)
![Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/97e6e5f8-76a4-40f2-bf53-c0138f8549ad/HDA0000860577330000012.PNG)
![Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol Preparation method of chiral S/R-3-ethoxy-4-methoxy-alpha[(methylsulfonyl)methyl] benzyl alcohol](https://images-eureka.patsnap.com/patent_img/97e6e5f8-76a4-40f2-bf53-c0138f8549ad/HDA0000860577330000021.PNG)