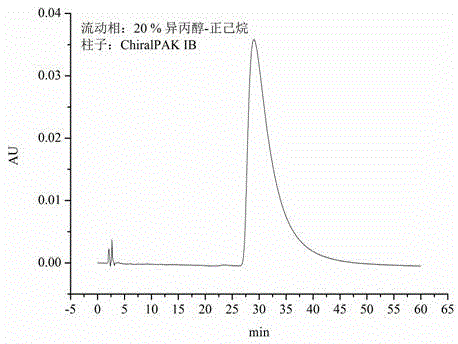
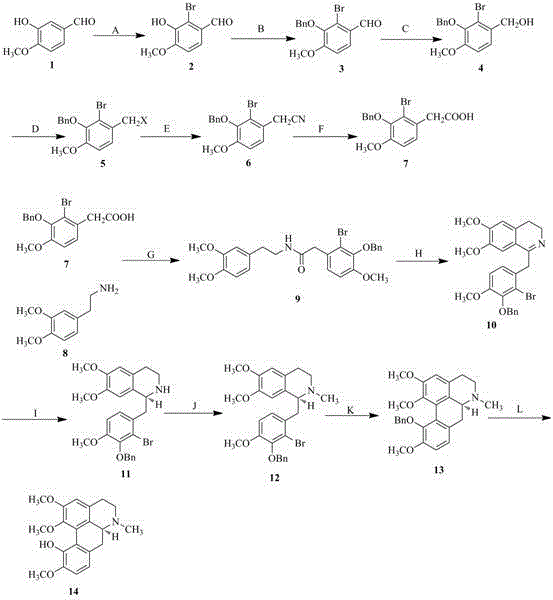
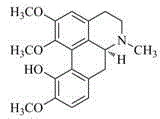
Preparation method of isocorydaline
A technology of isovioletine and benzyloxy, applied in the field of preparation of isovioletine, can solve the problems of large time consumption and labor, long time consumption, waste of solvent and the like, and achieve the effect of sufficient source of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 2-bromo-3-hydroxy-4-methoxybenzaldehyde (2) in Step A
[0048] Dissolve 10.0 g of isovanillin (1), 10.8 g of sodium acetate, and 0.34 g of iron powder in 60 mL of glacial acetic acid, stir at room temperature for 30 min, and dissolve 3.5 mL of Br 2 Dissolve in 15 mL of glacial acetic acid, slowly add dropwise to the above reaction solution, stir at room temperature for 3 h, TLC detection reaction (developing solvent, petroleum ether: acetone = 4:1, v / v), add 125 mL of ice water to the above In the reaction solution, the stirring was continued for 1 h, and the obtained white solid was recrystallized with absolute ethanol to obtain 9.9 g of light gray crystals with a yield of 65%.
[0049] HR-ESI-MS m / z 252.9457 [M+Na] + (calculated value C 8 h 7 BrNaO 3 Be 252.9471), the proton nuclear magnetic resonance spectrum of compound, the carbon spectrogram data are as follows: 1 H-NMR (400 Hz, Acetone- d 6 ): δ 10.23(s, 1H), 7.49(d, J = 8.0 Hz, 1H), 7.14 (...
Embodiment 2
[0084] Synthesis of 2-bromo-3-hydroxy-4-methoxybenzaldehyde (2) in Step A
[0085] Dissolve 10.0 g of isovanillin (1), 10.8 g of sodium acetate, and 0.36 g of iron powder in 60 mL of glacial acetic acid, stir at room temperature for 40 min, and dissolve 3.5 mL of Br 2 Dissolve in 15 mL glacial acetic acid, slowly add dropwise to the above reaction solution, stir at room temperature for 2 h, TLC detection reaction, (developing solvent, petroleum ether: acetone = 4:1, v / v), add 125 mL ice water In the above reaction solution, the stirring was continued for 1 h, and the filtered white solid was recrystallized with absolute ethanol to obtain 7.8 g of light gray crystals, with a yield of 52%.
[0086] Synthesis of 2-bromo-3-benzyloxy-4-methoxybenzaldehyde (3) in Step B
[0087] Dissolve 7.8 g of compound 2 in 85 mL of anhydrous DMF, and slowly add 4.3 mL of benzyl bromide dropwise to the above solution under mechanical stirring, then add 11.12 g of potassium carbonate solid powder...
Embodiment 3
[0110]Synthesis of 2-bromo-3-hydroxy-4-methoxybenzaldehyde (2) in Step A
[0111] Dissolve 10.0 g of isovanillin (1), 10.8 g of sodium acetate, and 0.34 g of iron powder in 60 mL of glacial acetic acid, stir at room temperature for 1 h, and dissolve 3.5 mL of Br 2 Dissolve in 15 mL glacial acetic acid, slowly add dropwise to the above reaction solution, stir at room temperature for 2 h, TLC detection reaction, (developing solvent, petroleum ether: acetone = 4:1, v / v), add 125 mL ice water In the above reaction solution, the stirring was continued for 1 h, and the filtered white solid was recrystallized with absolute ethanol to obtain 7.9 g of light gray crystals with a yield of 52%.
[0112] Synthesis of 2-bromo-3-benzyloxy-4-methoxybenzaldehyde (3) in Step B
[0113] Dissolve 7.9 g of compound 2 in 85 mL of anhydrous DMF, and slowly add 4.4 mL of benzyl bromide dropwise to the above solution under mechanical stirring, then add 11.3 g of potassium carbonate solid powder, reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com