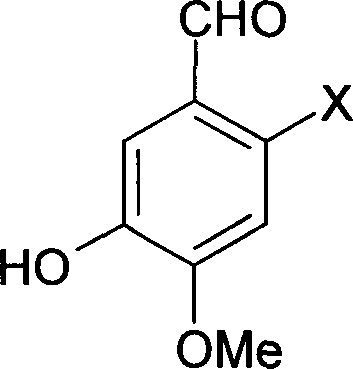
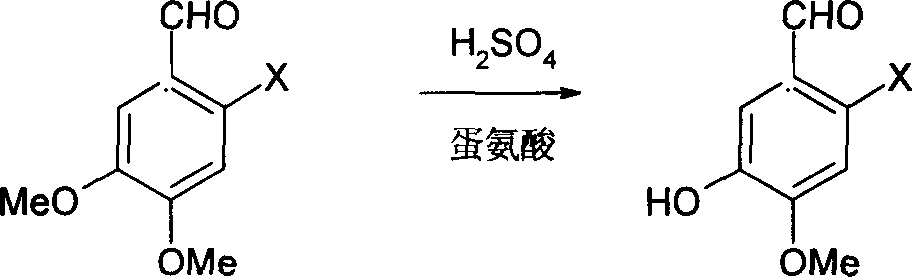
Method for synthesizing 6-substituent-3-hydroxy-4-methoxybenxaldchyde
A technology of methoxybenzaldehyde and dimethoxybenzaldehyde, which is applied in the field of synthesis of organic compounds, can solve problems such as poor product color, difficulty in meeting commercial production needs, and synthetic methods that have not been adopted. The effect of low cost, reduced production cost, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of 6-bromo-3-hydroxyl-4-methoxybenzaldehyde
[0019] (1) Demethylation reaction:
[0020] Dissolve 6-bromo-3,4-dimethoxybenzaldehyde (24.5g, 0.1mol) in 200mL of concentrated sulfuric acid with a concentration of 96%, and add DL-methionine in batches for 30min under stirring at 20-25°C (17.9g, 0.12mol), heated up to 90-95°C, and reacted for 3h. (At this time, the reaction liquid was taken for HPLC analysis, and the content of 6-bromo-3,4-dimethoxybenzaldehyde was <1%). Under stirring, the reaction solution was poured into 1500mL of ice water in a thin stream while it was hot, filtered (retain the filtrate for the third step experiment), and the filter cake was washed with 3×10mL saturated aqueous sodium chloride solution to obtain 6-bromo-3-hydroxyl-4 - crude product of methoxybenzaldehyde.
[0021] (2) Purification:
[0022] Dissolve the crude product obtained in the above operation with 100mL of 40% aqueous sodium hydroxide solution, fil...
Embodiment 2
[0025] Embodiment 2: the synthesis of 6-nitro-3-hydroxyl-4-methoxybenzaldehyde
[0026] (1) Demethylation reaction:
[0027] Dissolve 6-nitro-3,4-dimethoxybenzaldehyde (21.1g, 0.1mol) in 220mL of 97% concentrated sulfuric acid, add DL-methionine in batches for 30min under stirring at 20-25°C (20.9g, 0.14mol), heated up to 60-65°C, and reacted for 6h. (At this time, the reaction liquid was analyzed by HPLC, and the content of 6-nitro-3,4-dimethoxybenzaldehyde was <1%). Under stirring, the reaction solution was poured into 1650mL of ice water in a thin stream while it was hot, filtered (the filtrate was retained for the third step experiment), and the filter cake was washed with 3×10mL saturated aqueous sodium chloride solution to obtain 6-nitro-3-hydroxy- Crude 4-methoxybenzaldehyde.
[0028] (2) Purification:
[0029] Dissolve the crude product obtained in the above operations with 80 mL of 40% aqueous sodium hydroxide solution, filter, adjust the pH value of the filtrate ...
Embodiment 3
[0032] Embodiment 3: the synthesis of 6-amino 3-hydroxyl-4-methoxybenzaldehyde
[0033] (1) Demethylation reaction:
[0034] 6-Amino-3,4-dimethoxybenzaldehyde (18.1 g, 0.1 mol) was dissolved in 250 mL of 98% concentrated sulfuric acid, and DL-methionine ( 17.9g, 0.12mol), heated up to 70-75°C, and reacted for 4h. (At this time, the reaction liquid was analyzed by HPLC, and the content of 2-amino-3,4-dimethoxybenzaldehyde was <1%). Under stirring, pour the reaction solution into 2000mL ice water in a thin stream while it is hot, adjust the pH value to 7-8 with 40% sodium hydroxide solution, filter (retain the filtrate for the third step experiment), filter the cake with 3×10mL saturated chlorine Washing with aqueous sodium chloride solution gave crude 6-amino-3-hydroxy-4-methoxybenzaldehyde.
[0035] (2) Purification:
[0036] Dissolve the crude product obtained by the above operation with 90 mL of 40% aqueous sodium hydroxide solution, filter, adjust the pH value of the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com