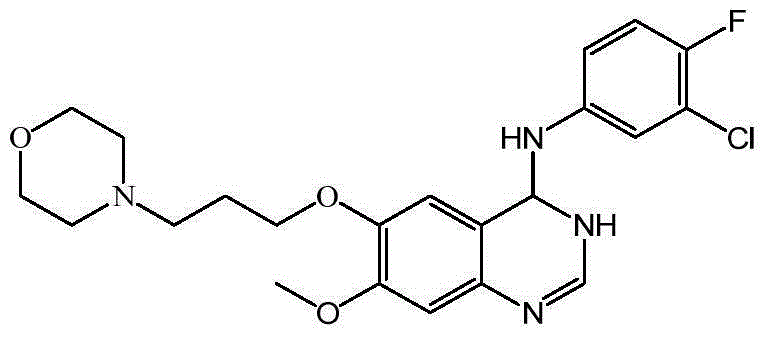
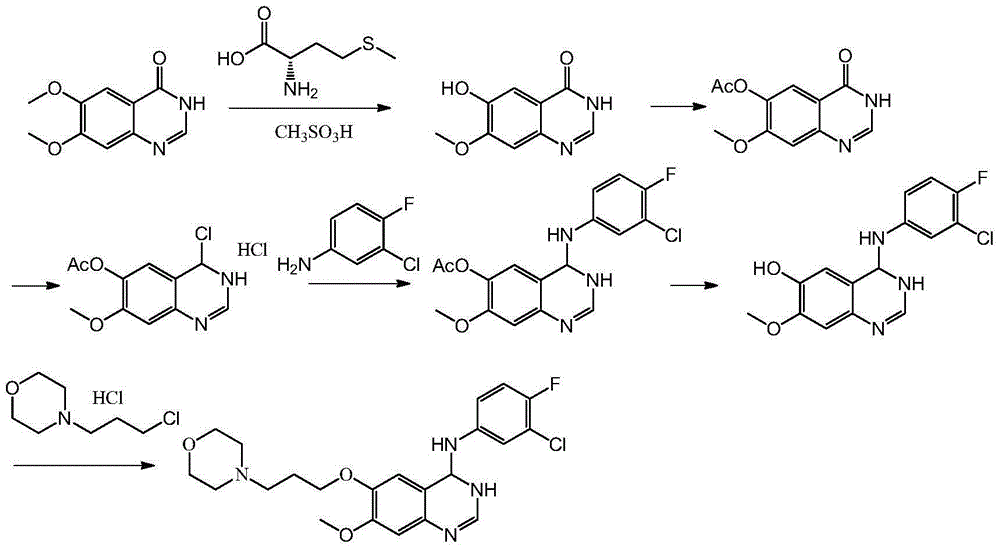
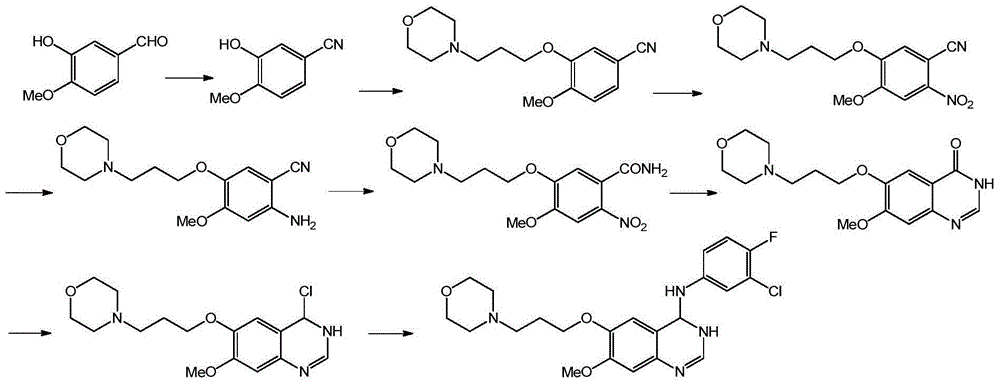
Preparation method of 4-(3-chloro-4-fluorophenyl amido)-7-methoxy-6-(3-morpholine propoxy) quinazoline
A technology of methoxybenzoic acid and morpholine, which is applied in the field of preparation of 4--7-methoxy-6-quinazoline, and can solve the problems of many side reactions, low yield, and decreased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] Preparation of Gefitinib
[0090] The present invention provides a kind of preparation method of gefitinib, specifically, described preparation method prepares a kind of compound shown in following formula IV as intermediate:
[0091]
[0092] Described formula IV compound can be prepared by the following method:
[0093]
[0094] (3) In an inert solvent, in the presence of a catalyst, the compound of formula III is reacted with ammonium formate oxime to obtain the compound of formula IV.
[0095] In another preference, in the step (3), the catalyst is selected from the group consisting of trimethylaluminum, tri-n-propylaluminum, trioctylaluminum, triisobutylaluminum, triethylaluminum , tetrabutyl titanate, aluminum sulfate, trimethylaluminum silane; preferably trimethylaluminum.
[0096] In the step (3), all the other conditions can be determined according to actual needs, such as the scale of the reaction, the selected specific catalyst and other factors. Pref...
Embodiment 1
[0158] (1) Synthesis of 3-hydroxyl-4-methoxybenzoic acid methyl ester (II)
[0159] 3-Hydroxy-4-methoxybenzaldehyde (10g, 65.7mmol) was dissolved in formic acid (9.1g, 197.1mmol), the solution was lowered to 0°C, and 35% hydrogen peroxide (22.3g, 230mmol) was slowly added dropwise as above The solution was kept at 4°C for overnight reaction. After the reaction, the solid matter was filtered, washed with ice water, and dried to obtain a solid matter. The solid was dissolved in methanol (50ml), and concentrated sulfuric acid (98%, 3.2ml, 59.1mmol) was slowly added dropwise, heated to reflux, and the solvent was removed to obtain a crude product, which was recrystallized from 10% methanol aqueous solution to obtain 3-hydroxy-4-methanol Methyl oxybenzoate (II) (9.6 g), 80% yield.
[0160](2) Synthesis of methyl 4-methoxy-5-[3-(4-morpholinyl)propoxy]benzoate (III)
[0161] 3-Hydroxy-4-methoxybenzoic acid methyl ester (II) (15g, 82.3mmol), anhydrous sodium carbonate (23.2g, 167.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com