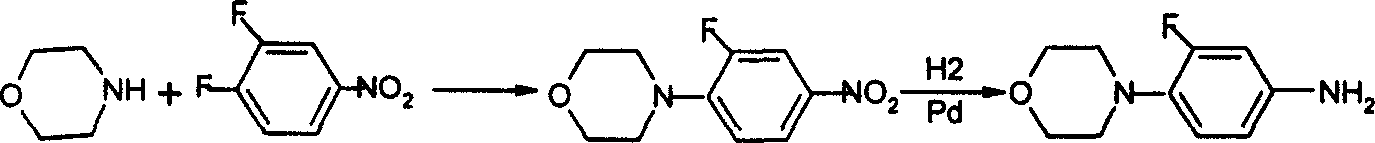
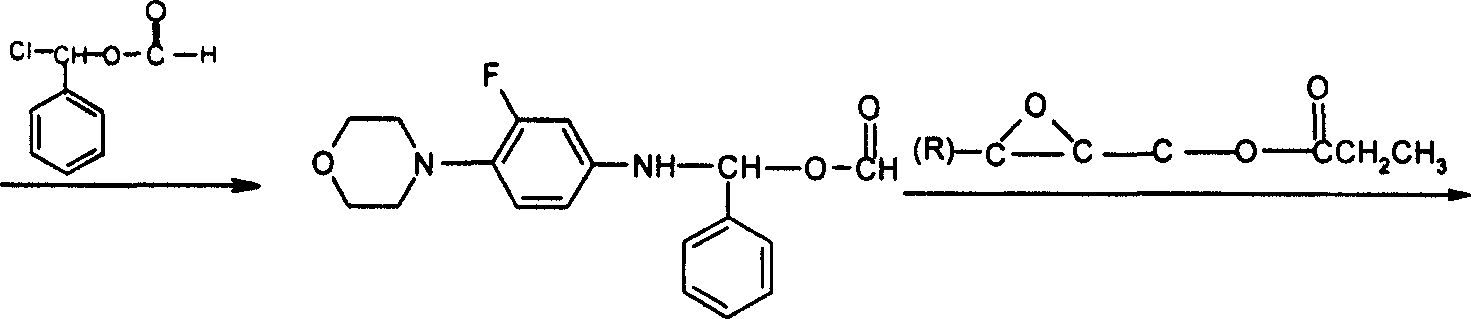
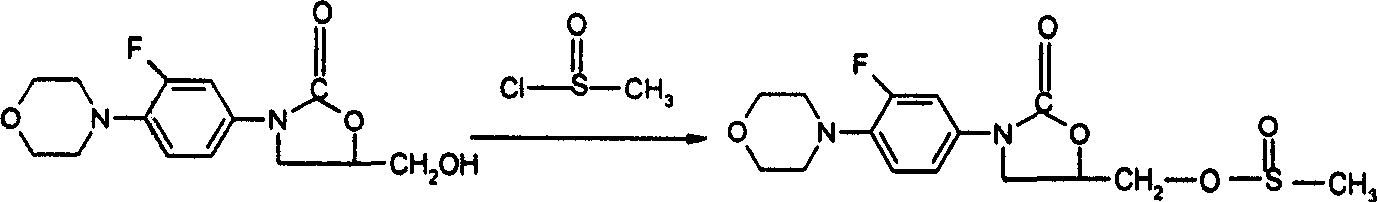
Prepn process of (R)-N-(3-fluoro-4-morpholinyl phenyl)-oxazolone-5-methyl alcohol
A technology of morpholine phenyl and preparation process is applied in the field of new synthesis process of important intermediate-N-oxazolone-5-methyl alcohol, and can solve the problem of rare raw materials, long process route and high price of the original process problem, to achieve the effect of simple process, low price and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0049] (1) Synthesis of 3-fluoro-4-morpholine nitrobenzene:
[0050] Dissolve 20ml 3,4-difluoronitrobenzene (0.18mol) in 50mL C 2 h 5 OH and add 0.5 g of anhydrous Na to the solution 2 CO 3 . Dissolve 19ml (0.20mol) morpholine in 50mL C 2 h 5Add the above solution after diluting with OH, and react for 6 hours under reflux (t=79°C), and the reaction solution is slightly orange-yellow. After cooling, it was filtered to obtain a yellow solid. The mother liquor was distilled to obtain a small amount of orange solid. The solid phases were combined, washed twice with 120 ml of water, and vacuum-dried for 50 min (vacuum degree 0.95, t=85° C.) to obtain 33.1 g of a yellow solid. After the above solid was recrystallized with ethyl acetate, it was stored in a dark place to prevent discoloration. The yield was 81.11%. It was observed under a microscope as a yellow flaky transparent crystal, and its melting point was mp111.7- 112.5°C. (Literature value mp 111-112°C)
[0051] Yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com