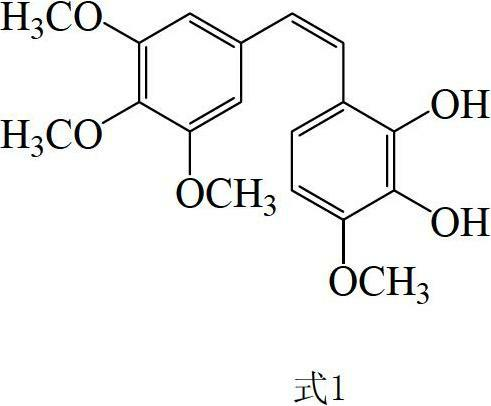
Preparation method and application of Z-3,4,4',5-tetramethoxy-2',3'-dihydroxy diphenylethylene
A technology of dihydroxystilbene and tetramethoxy, applied in ether preparation, organic chemistry and other directions, can solve the problems of expensive raw materials and palladium catalysts, difficult to prepare intermediates, difficult to obtain industrial applications, etc., and achieves low cost , The effect of high cis-selectivity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
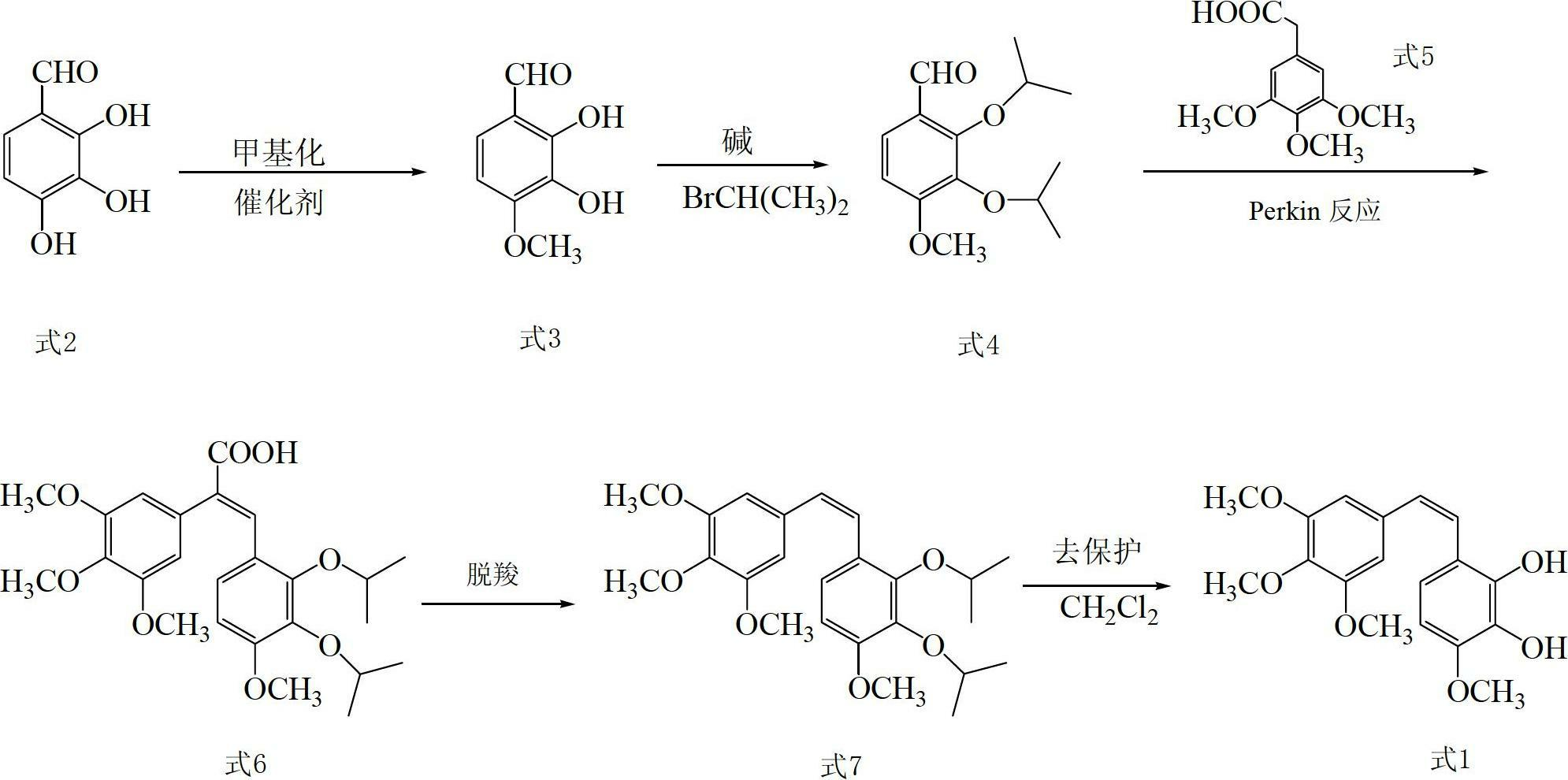
[0075] (1) Add 82.38g (216mmol) of borax and 500ml of purified water into a 1000ml three-neck flask, add 16.70g (108mmol) of 2,3,4-trihydroxybenzaldehyde while vigorously stirring, stir at 20°C for 30min, and add dropwise Dimethyl sulfate 21ml (216mmol) and NaOH solution (8.86g NaOH+90mlH 2 O), continue to stir for 15 hours, then add concentrated hydrochloric acid (37% by mass) to adjust to pH = 2, extract 3 times with chloroform, take the chloroform extraction layer, wash with saturated brine and wash with anhydrous Na 2 SO 4 After drying, a light yellow solid was obtained, which was recrystallized from chloroform / petroleum ether to obtain 11.79 g of 2,3-dihydroxy-4-methoxybenzaldehyde as a bright yellow needle-like solid, with a yield of 65.0%;
[0076] The characterization parameters of 2,3-dihydroxy-4-methoxybenzaldehyde are as follows:
[0077] EI-Ms m / z:168(M + ),150(M + -H 2 O);
[0078] 1 HNMR (400Hz, CDCl 3 ): δ11.08(s,1H,CHO),9.71(s,1H,2-OH,D 2 Oexchangeable...
Embodiment 2
[0100] (1) Add 80.09g (210mmol) of borax and 500ml of purified water into a 1000ml three-necked flask, and add 15.46g (100mmol) of 2,3,4-trihydroxybenzaldehyde while vigorously stirring, stir at 30°C for 30min, and add dropwise Dimethyl sulfate 20.4ml (216mmol) and NaOH solution (8.90g NaOH+90mlH 2 O), continue to stir for 16 hours, then add concentrated hydrochloric acid with a mass percentage of 37% to pH = 3, extract 3 times with chloroform, take the chloroform layer, wash it with saturated saline and wash it with anhydrous Na 2 SO 4 After drying, a light yellow solid was obtained, which was recrystallized from chloroform / petroleum ether to obtain 11.85 g of bright yellow needle-like solid 2,3-dihydroxy-4-methoxybenzaldehyde, with a yield of 65.3%;
[0101] (2) Add 6.72g (40mmol) of 2,3-dihydroxy-4-methoxybenzaldehyde, 70ml of DMF, anhydrous K 2 CO 3 11.04g (80mmol), heat to 60°C, add 7.5ml (80mmol) of bromoisopropane dropwise, keep the temperature in the reaction bottle...
Embodiment 3
[0106] (1) Add 82.38g (216mmol) of borax and 500ml of purified water into a 1000ml three-neck flask, add 16.70g (108mmol) of 2,3,4-trihydroxybenzaldehyde while vigorously stirring, stir at 20°C for 30min, and add dropwise Dimethyl sulfate 26ml (270mmol) and NaOH solution (8.86g NaOH+90mlH 2 O), continue to stir for 17 hours, then add concentrated hydrochloric acid with a mass percentage of 37% to pH = 3, extract 3 times with chloroform, take the chloroform layer, wash it with saturated saline and wash it with anhydrous Na 2 SO 4 After drying, a light yellow solid was obtained, which was recrystallized from chloroform / petroleum ether to obtain 11.91 g of 2,3-dihydroxy-4-methoxybenzaldehyde as a bright yellow needle-like solid, with a yield of 65.6%;
[0107] (2) Add 6.72g (40mmol) of 2,3-dihydroxy-4-methoxybenzaldehyde, 70ml of DMF, anhydrous K 2 CO 3 13.80g (100mmol), heat to 60°C, add 11.2ml (120mmol) of bromoisopropane dropwise, keep the temperature in the reaction bottle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com